Summary
Development of novel therapeutic agents is needed to address the problems of locally recurrent, metastatic, and advanced hormone-refractory prostate cancer. We have constructed a novel complex adenovirus (Ad) vector regulation system that incorporates both the prostate-specific ARR2PB promoter and a positive feedback loop using the TRE promoter to enhance gene expression. This regulation strategy involves the incorporation of the TRE upstream of the prostate-specific ARR2PB promoter to enhance its activity with Tet-regulation. The expressions of both GFP and tTA were placed under the control of these TRE-ARR2PB promoters, so that in the cells of prostate origin, a positive feedback loop would be generated. This design greatly enhanced GFP reporter expression in prostate cancer cells, while retaining tight control of expression in non-prostate cancer cells, even at MOI as high as 1000. This novel positive feedback loop with prostate specificity (PFLPS) regulation system we have developed may have broad applications for expressing not only high levels of toxic proteins in cancer cells but alternatively could be manipulated to regulate essential genes in a highly efficient conditionally replicative adenovirus (CRAd) vector specifically directed to prostate cancer cells. The PFLPS regulation system, therefore, serves as a promising new approach in the development of both a specific and effective vector for cancer gene therapy.
Keywords: prostate cancer, gene therapy, adenovirus, positive feedback loop, Tet responsive element (TRE), ARR2PB promoter
Introduction
For 2003, it was estimated that 220,900 new cases of prostate cancer would be diagnosed, and 28,900 men would die from this disease.1 Although the five-year relative survival rate for patients with diagnoses in the local and regional stages is 100%,1 approximately 30% of patients treated for localized disease relapse.2 In addition, current treatments of localized prostate cancer are not without complications.3–6 Radical prostatectomy involves undergoing major surgery and often results in temporary to permanent complications such as incontinence and impotence.3,4,6 In addition, not all cases of local disease can be treated by the traditional local curative approaches due to local invasion of nearby tissues and a loss of differentiation. Locally advanced tumor growth can lead to bladder outlet obstruction, base of bladder invasion, urethral obstruction, and local pain and discomfort in these patients.7 Therefore, there is clearly a need to investigate alternative treatment strategies to expand the arsenal of locally advanced prostate cancer treatment options.
One such treatment alternative is the use of gene therapy vectors to specifically eliminate prostate cancer cells utilizing pro-apoptotic genes including Fas ligand (FasL), tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL), or Bax. Because many of these cancer gene therapy strategies involve the induction of a toxic gene product to eliminate the cancer cells, it is important to localize that transgene expression to target cells only. Incorporation of tissue-specific promoters to localize transgene expression has been utilized for several cytotoxic cancer gene therapy vectors that have been investigated in clinical trials.8–10
One tissue-specific promoter that has shown promise as a candidate promoter for driving cytotoxic transgenes for the development of a prostate cancer gene therapy vector is the ARR2PB promoter. This synthetically derived, prostate-specific promoter was developed from regulatory elements from the rat probasin promoter.11–13 This promoter demonstrated good prostate-specific regulation both in vitro and in transgenic mice.13–16 Although the ARR2PB promoter includes two androgen response regions that greatly enhance prostate-specific transgene expression, induced transgene expression from ARR2PB, like that from most mammal-derived tissue-specific promoters, still tends to be significantly weaker than that induced by virus-derived promoters, such as the human cytomegalovirus intermediate/early (hCMVie) promoter.16
Previously in our lab, we attempted to enhance the transcriptional activity of the ARR2PB promoter by combining the ARR2PB promoter with elements of the tetracycline (Tet) regulatory system17–19 in a single complex adenoviral vector.16 While this complex vector, known as the Ad/FasL-GFPPS/TR vector, was successful in enhancing the induced levels of a Fas ligand-green fluorescent protein (FasL-GFP) fusion protein in prostate cancer cells, this combination of regulatory elements also resulted in a decrease in prostate specificity. This reduction in specificity may be the result of an inherent limitation of the tetracycline responsive element (TRE) promoter from which some transgene expression still occurs even under uninduced conditions (i.e., presence of excess doxycycline (dox, a tetracycline analog) in the case of the Tet activator (tTA); or the absence of dox in the case of the reverse Tet activator (rtTA)) in transient cell transduction systems like adenovirus; this has been observed by our group as well as by others.18–20 Such leaky expression could be quite detrimental in terms of a cancer gene therapy vector. If the transgene expression is not tightly regulated, toxic protein could be non-specifically expressed in several non-target cells, leading to unwanted destruction of non-cancerous tissues.
The ideal Ad vector for prostate cancer gene therapy would be one that is both prostate-specific yet still elicits highly induced expression of a toxic protein. We attempt to achieve this goal by developing a novel prostate-specific regulatory system known as the positive feedback loop with prostate specificity, or PFLPS, system. This system, incorporated into a replication-incompetent Ad vector deleted in E1, E3, and E4 (except for E4orf6), is composed of a unique arrangement of the Tet-off regulatory system elements in combination with the ARR2PB promoter. It is our belief that the low but significant non-specific expression induced in our Ad/FasL-3 GFPPS/TR vector may have been as a result of basal activity of the minimal CMV promoter incorporated in the TRE promoter. To circumvent this possible source of “leaky” expression, we have incorporated both a Tet responsive element upstream of the ARR2PB promoter along with a positive feedback loop to enhance prostate-specific transgene expression. This design ensures prostate-specific expression of both tTA and the gene of interest (GOI) while establishing a positive feedback loop in which prostate-specific expression of tTA further induces expression of itself as well as the GOI.
In this study, we develop and characterize our new PFLPS regulation system both in the context of plasmid constructs as well as in a single complex Ad vector. The PFLPS system, when cloned into plasmid vectors, demonstrated both prostate-specificity and high levels of induced expression of GFP. The GFP expressed from the PFLPS plasmids was found to be even greater than that induced by the non-specific Tet-regulated control. When incorporated into a complex Ad vector, the PFLPS system once more demonstrated highly induced, prostate-specific expression. These levels could be induced with dihydrotestosterone (DHT) to levels higher than that induced by the non-specific Tet-regulated control. In addition, preservation of prostate-specificity by PFLPS was demonstrated by analysis of A549, HeLa, and HepG2 cell lines infected with Ad/GFPPFLPS vector by both RT-PCR as well as by analysis of GFP expression at high multiplicities of infection (MOI). Prostate-specificity of the PFLPS system was retained even at MOI 1000, thereby demonstrating a high level of control in the PFLPS vector. The PFLPS regulation system, therefore, serves as a promising new approach in the development of both a specific and effective vector for future applications in the field of cancer gene therapy.
Results
Design of the Positive Feedback Loop Prostate-Specific (PFLPS) regulation system
In order to augment the activity of the ARR2PB promoter, we have developed a novel positive feedback loop prostate-specific (PFLPS) regulation system that incorporates both the components of the Tet-off regulation system as well as the ARR2PB promoter, in a positive feedback loop. The design of the PFLPS regulation system as cloned into Ad5 vector deleted in E1, E3, and E4 (except for E4orf6) is depicted in Fig. 1 along with control Ad vectors, Ad/GFPTET, which expresses GFP under the control of the traditional Tet-off regulatory system and Ad/C.LacZ, which expresses β-galactosidase under the control of the hCMVie promoter.
Figure 1. Structures of adenovirus vectors.
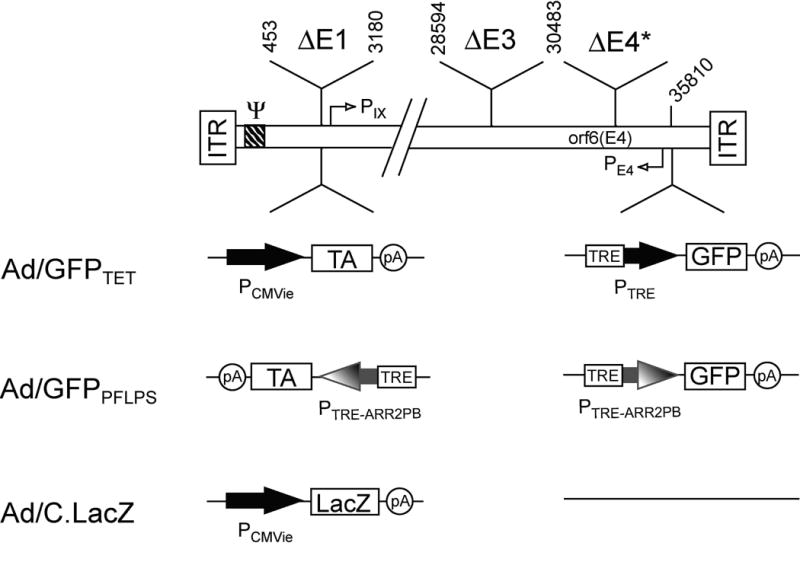
We assembled vector genomes in vitro using pLAd and pRAd shuttle vectors as described previously 22,23. The resulting rAd vectors are E1-deleted and have a deletion in the E3 region (E3 promoter is retained). They also lack all of the E4 ORFs, except orf6, which is expressed from the E4 promoter.
The PFLPS components were first cloned into plasmid vectors, incorporating the reporter gene, green fluorescent protein (GFP), as the gene of interest (Fig. 2a). Special consideration was made in the construction of this complex rAd vector based upon our previous findings regarding interference from the E1a enhancer. Because we previously had found that the basal activity of both the TRE and the ARR2PB promoters were significantly affected by interference from the E1a enhancer,21 we designed the Ad/GFPPFLPS vector so that the TRE-ARR2PB.GFP cassette was away from the E1 region by placing it near the right ITR (see the right end plasmid, pRAd2T2Pb.GFP.B; Fig. 2a). Meanwhile we also placed the TRE-ARR2PB.tTA cassette in reverse orientation near the left ITR so that its promoter was also away from the E1a enhancer region (see the left end plasmid, pLAd(T2Pb.tTA.S)r; Fig. 2a). The theoretical mechanism of action of the PFLPS regulation system is diagrammed in Figs. 2b and 2c. By having both tTA and GFP under the control of the TRE-ARR2PB promoter, this not only initiates a positive feedback loop in prostate cancer cells but also maintains the PFLPS system’s prostate-specificity in cells of non-prostate origin.
Figure 2. Schematic drawing of PFLPS regulation system.
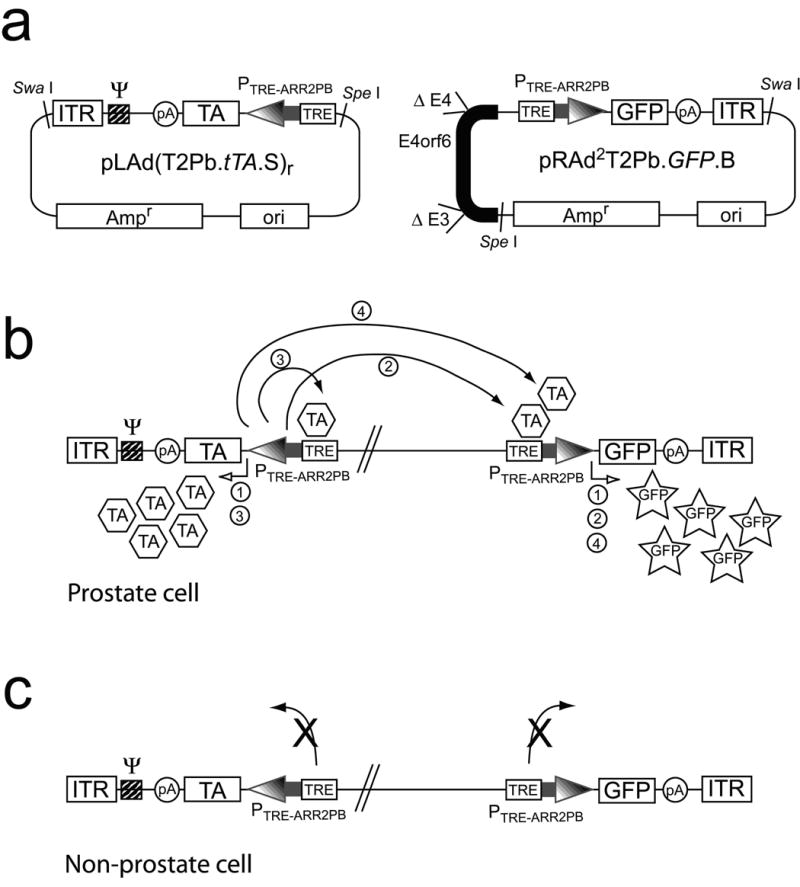
(a) PFLPS plasmid vectors. The components of the PFLPS regulation system were first cloned into pLAd (left-end Ad plasmid) and pRAd (right-end Ad plasmid) shuttle vectors (see Methods). (b) Mechanism of action of the Ad/GFPPFLPSvector when transduced into a prostate cell. We hypothesize that the following sequence of events would occur in the prostate cell: (1) both ARR2PB promoters would be induced, therefore inducing expression of both tTA and GFP; (2) tTA could then bind to the TRE within the TRE-ARR2PB promoter upstream of GFP and induce additional GFP expression; (3) tTA could also bind to its own TRE-ARR2PB promoter and further induce expression of itself—thereby initiating a positive feedback loop; and (4) highly induced expression of tTA that results from the feedback loop could then induce expression of GFP further. (c) Mechanism of action in non-prostate cells. In this case, since both tTA and GFP gene expressions are controlled by the TRE-ARR2PB promoter, neither gene would be induced in non-prostate cells because the ARR2PB promoter would not be induced in these cell types.
Levels of prostate-specific expression induced by PFLPS is greater than that induced by the non-specific, Tet-regulated control
In order to determine the activity of the PFLPS system, the system was first tested by transfection of plasmid vectors into prostate and non-prostate cell lines. LNCaP, a prostate cancer cell line, and U343MG, a brain tumor cell line (serving as non-prostate control cell line), were seeded in 24-well plates one day prior to being co-transfected with either Tet plasmids (pUHD 15-1 plus pRAd2T.GFP.B), ARR2PB plasmid (pRAd22Pb.GFP plus empty vector), PFLPS plasmids (pLAd(T2Pb.tTA.S)r plus pRAd2T.GFP.B) or empty vector. Three days post-transfection, media was aspirated and cells were lysed with 0.5% Triton x-100 solution and assayed for GFP expression as described in the Materials and Methods. As shown in Fig. 3, the PFLPS plasmids demonstrated high levels of prostate-specific induction of GFP expression in LNCaP cells while the levels of GFP expression in U343MG cells were similar to empty vector control. In addition, GFP induction in LNCaP cells was significantly higher than that induced by the non-specific, Tet-regulated positive control (Fig. 3) and at least 3.5 times higher than the ARR2PB promoter alone. The level of GFP expression from the ARR2PB plasmid in LNCaP cells was approximately 78.4% that of the Tet plasmid pair. This activity from the ARR2PB plasmid is likely higher than expected because positive GFP induction only requires transfection with a single plasmid whereas positive GFP expression induction by the Tet plasmids (and the PFLPS plasmids, as well) requires simultaneous transfection of a cell by two separate plasmids. U343MG cells were chosen as the non-prostate control cell line for these studies because both U343MG’s optimal transfection conditions and its transfection efficiency are similar to that of LNCaP.
Figure 3. Levels of prostate-specific expression induced by the PFLPS plasmid vectors are greater than that induced by the non-specific, Tet-regulated control.
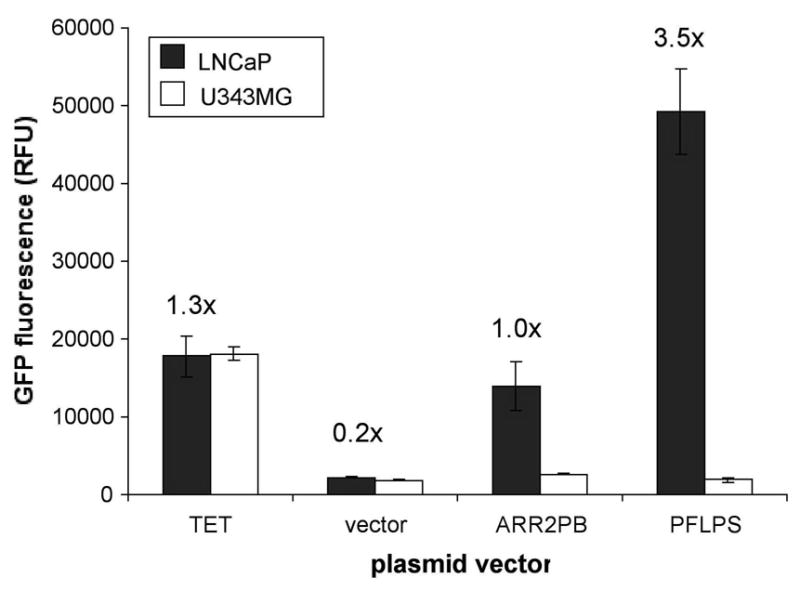
LNCaP and U343MG cells were seeded in 24-well plates and co-transfected with either Tet plasmids (pUHD 15-1 plus pRAd2T.GFP.B), ARR2PB plasmid (pRAd22Pb.GFP plus empty vector), PFLPS plasmids (pLAd(T2Pb.tTA.S)r plus pRAd2T2Pb.GFP.B) or empty vector using SuperFect reagent according to manufacturer’s instructions in the presence of 30μM DHT. 3 days post-transfection, cell lysates were assayed for GFP fluorescence on FLUOstar fluorescence plate reader. RFU: relative fluorescence units. Numbers above LNCaP data bars indicate fold-induction relative to transfection with ARR2PB plasmid (set as 1.0x).
The positive results obtained in Fig. 3 demonstrated the potential of the PFLPS system at the plasmid level and therefore justified the cloning of the PFLPS components into an Ad5 vector utilizing methods described previously.22–24 The resulting complex Ad vector, known as Ad/GFPPFLPS, was propagated and purified for characterization in in vitro studies. To determine the prostate-specificity of Ad/GFPPFLPS vector, its GFP expression was first characterized in two prostate cancer cell lines, LNCaP and 22RV1 (mouse prostate cancer cell line) and a non-prostate cell line, U251MG. Since the ARR2PB promoter is inducible by androgen (e.g., dihydrotestosterone; DHT), both LNCaP and 22RV1 cell lines were chosen specifically because they are two of the few prostate cancer cell lines available that express functional androgen receptor.
Infection of LNCaP, 22RV1, and U251MG with Ad/GFPPFLPS demonstrated high levels of GFP induction in the prostate cancer cell lines without a loss in prostate specificity, as the levels of GFP expression in the U251MG cells were similar to background (Fig. 4a and 4b). These data were represented in both raw units, as taken from the fluorescence plate reader (FLUOstar; BMG Labtechnologies) (Fig. 4a), as well as in percent GFP expression, setting infection with the Tet-regulated Ad/GFPTET vector at 100% (Fig. 4b). Both representations of the infection demonstrated that GFP induction in LNCaP cells by Ad/GFPPFLPS was significantly higher than that seen with the non-specific Ad/GFPTET vector; this supports the transfection results seen in Fig. 3.
Figure 4. Levels of prostate-specific expression induced by the Ad/GFPPFLPS vector is greater than that induced by the non-specific, Tet-regulated control.
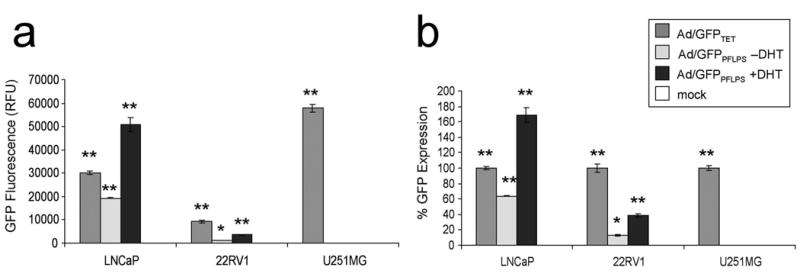
LNCaP, 22RV1, and U251MG cells were seeded in 24-well plates and infected with Ad vectors at MOI 50 in the presence or absence of 30nM DHT. 2 days post-infection, cell lysates were assayed for GFP fluorescence. Infection with HBS served as mock infection control. (a) GFP fluorescence as measured in relative fluorescence units (RFU). (b) GFP fluorescence calculated as percent GFP expression, setting GFP fluorescence as a result of Ad/GFPTET infection at 100% for each cell line. *p < 0.05 compared to mock. **p < 0.01 compared to mock.
GFP induction from PFLPS is restricted to only cells of prostate origin
Because a positive feedback loop has been incorporated in the PFLPS vector design, minute levels of non-specific transgene expression could initiate the positive feedback loop in non-target cells. Therefore, it is important to verify that prostate-specificity has been retained in the PFLPS regulation system. In order to determine if the Ad/GFPPFLPS vector not only is highly induced in prostate cancer cells but also retains prostate-specificity when transduced into non-prostate cells, LNCaP and U251MG were infected at MOI 0, 10, 100, and 1000 in the presence of 30nM DHT and assayed for GFP expression. Both the Ad/GFPPFLPS vector and the positive control Ad/GFPTET vector demonstrated dose-dependent GFP expression in LNCaP cells (Fig. 5a), and in addition further supported the data shown in Figs. 3 and 4 that PFLPS-induced GFP expression is significantly higher than that induced by the Tet-regulatory system. On the other hand, only the Ad/GFPTET vector demonstrated dose-dependent GFP expression in U251MG cells. In addition, GFP induction by Ad/GFPPFLPS vector was undetectable in U251MG, even at MOI 1000 (Fig. 5b), demonstrating the transgene expression from the PFLPS regulatory system is tightly controlled.
Figure 5. Transduction with Ad/GFPPFLPS vector induces dose-dependent GFP expression in LNCaP cells while retaining prostate-specificity in U251MG cells.
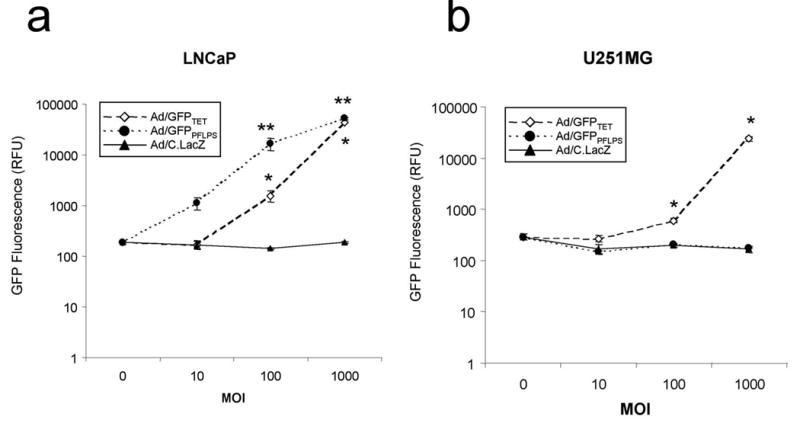
LNCaP and U251MG cells were seeded in 96-well plates and infected with Ad vectors at MOI 0, 10, 100, or 1000 in the presence of 30nM DHT. 2 days post-infection, cell lysates were assayed for GFP fluorescence. Infection with Ad/C.LacZ served as vector infection control. GFP fluorescence of (a) LNCaP cells and (b) U251MG cells. *p < 0.05 compared to Ad/C.LacZ. **p < 0.01 compared to Ad/C.LacZ.
To characterize the prostate-specificity, Ad/GFPPFLPS vector was transduced into three additional non-prostate cell lines (A549, HeLa, and HepG2). Since LNCaP cells tend to be highly transducible and in addition more efficiently express GFP when compared to the other cell lines tested, the non-prostate cell lines were infected at MOI 1000 while LNCaP was transduced at MOI 100. These cells were then assayed for GFP expression. Remarkably, even in the presence of DHT and at MOI 1000, GFP expression from the non-prostate cell lines was undetectable following transduction with Ad/GFPPFLPS vector while the same vector induced higher than Ad/GFPTET levels of GFP expression in LNCaP cells (Fig. 6a).
Figure 6. Transduction with Ad/GFPPFLPS vector retains prostate-specificity in non-prostate cell lines.
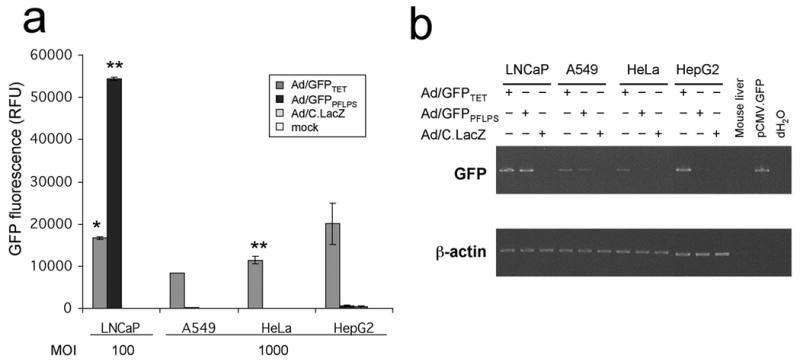
(a) LNCaP, A549, HeLa, and HepG2 cell lines were seeded in 96-well plates and infected at MOI 100 or 1000 in the presence of 30nM DHT. 2 days post-infection, cell lysates were assayed for GFP fluorescence. Infection with HBS served as mock infection control. *p < 0.05 compared to Ad/C.LacZ. **p < 0.01 compared to Ad/C.LacZ. (b) LNCaP, A549, HeLa, and HepG2 cell lines were seeded in 100 mm2 plates and infected at MOI 10 in the presence of 30nM DHT. 2 days post-infection, cells were harvested in TRI reagent for RNA extraction. GFP and β-actin transcripts were amplified by RT-PCR. Mouse liver RNA (from Ambion’s RETROscript™ kit) served as a GFP negative control while 10 ng of pCMV.GFP plasmid served as a GFP positive control.
As an additional, more stringent test of specificity, RT-PCR for GFP expression was also conducted on the same cell lines assayed in Fig. 6a to determine relative mRNA transcription following Ad/GFPPFLPS infection. In this analysis, total mRNA was purified from vector-transduced cells two days post-infection and levels of reporter gene induction were determined by PCR analysis of cDNA for GFP. Relative levels of GFP induction among different cell lines were standardized in relation to β-actin transcription levels. The RT-PCR analyses demonstrated that although the transduction efficiency tended to vary from cell line to cell line (as seen in Fig. 6a), the data still indicated retention in prostate-specificity of the PFLPS regulatory system (Fig. 6b).
Discussion
When developing gene therapy vectors expressing toxic transgenes such as FasL, several factors must be considered in the vector design. First, in order to prevent transgene-related systemic cytotoxicity, toxic gene expression must be restricted to only the cancer cell targets. Secondly, while the safety of the vector is important, highly induced expression in the target cells must not be compromised in the process since oftentimes, a major limitation of gene therapy vectors is insufficient gene expression to initiate a therapeutic effect. Conversely, highly induced transgene expression must also retain specificity in order to preserve the safety of the vector. Finally, it is important to consider the possibility that the vector’s propagating cell line (e.g., HEK293 cells for rAd vectors) may also be susceptible to the effects of the toxic transgene. Therefore, it is necessary to also prevent transgene expression during the propagation and production of the gene therapy vector. For these reasons, it is important to consider regulation of transgene expression when designing cancer gene therapy vectors.
Strategies for restricting toxic gene expression both temporally and spatially include incorporating tissue- or cancer-specific promoters and drug-inducible or -repressible regulation systems.9,10,16,19,25,26 In the present study, we describe a strategy for inducing potent prostate-specific transgene expression incorporating elements of the Tet-off regulation system with the prostate-specific ARR2PB promoter. This regulation system demonstrates an enhancement of the transcriptional activity of the ARR2PB promoter without losing specificity. The PFLPS regulation system has three characteristics that make it unique: (1) the newly developed TRE-ARR2PB promoter; (2) induction of a prostate-specific positive feedback loop; and (3) the cloning of the entire system into a single complex recombinant Ad vector, thus preventing the need for co-infection with two separate Ad vectors. By combining the prostate specificity of the ARR2PB promoter with the Tet responsive element, we were able to establish a positive feedback loop with prostate specificity (PFLPS) regulatory system that demonstrated highly induced levels of prostate-specific expression. Interestingly, activity from the PFLPS regulation system was at least 1.5-fold higher than the highly induced Tet-regulated system.
This type of activity from a tissue-specific gene therapy vector is quite unusual and sets an exciting new precedent in the field of gene therapy. Normally one would expect that the consequence of such highly induced expression from a tissue-specific vector would be a loss in tissue-specificity. However, this was not the case. Even at MOI as high as 1000, the Ad/GFPPFLPS vector demonstrated a retention in prostate-specificity.
Notably, the Ad/GFPPFLPS vector demonstrated little GFP expression in HepG2 cells at MOI 1000. This lack of transgene expression in liver-derived cells is significant since Ad vectors typically accumulate in the liver following systemic injection. Therefore, liver toxicity due to non-specific transgene induction may become less of an issue when the PFLPS system is utilized to control the expression of toxic transgenes. We plan on confirming this in future studies.
In this study, we have investigated the activity of a novel transgene regulation system that, in future studies, will regulate the pro-apoptotic FasL gene. Our current studies were conducted utilizing GFP as a reporter; however, when designing gene therapy vectors that will be expressing toxic transgenes, it is important consider that production of such vectors involves special manipulations that may not be necessary when propagating vectors expressing non-toxic genes. For instance, merely expressing FasL, or any other stand-alone toxic transgene, from a constitutive promoter such as the hCMVie promoter is not a viable option. Since the toxic transgene would lethally damage the propagating cell line (HEK293) before any viral particles could be formed, the resultant vector would likely be either low in titer or favor selection for replication-competent mutant vectors. For this reason, our first generation FasL-GFP vector, Ad/FasL-GFPTET, incorporated the Tet-off regulation system to express the FasL-GFP fusion24 and was propagated in the absence of doxycycline in 293CrmA cells (HEK293 cells stably transfected with Cowpox virus (Chordopoxvirinae) cytokine response modifer A (CrmA)24) to minimize Fas-mediated apoptosis of the propagating cell line. Consequently, we have incorporated prostate-specificity into the PFLPS vector to not only reduce systemic toxicity in the gene therapy recipient but also to aid in the production of a high titer Ad vector expressing a toxic transgene.
Future applications of the PFLPS regulation system include organ-restricted cancer gene therapy applications, general gene therapy applications, recombinant vaccine technology and transgenic mouse models. As intimated with the discussion of our Ad/FasL-GFPTET vector, incorporating FasL-GFP into PFLPS may serve as a promising vector for prostate cancer gene therapy as we have previously demonstrated this transgene’s efficacy in eliminating Fas-resistant prostate cancer cells.27 Other candidate toxic transgenes for the gene therapy treatment of cancers include TRAIL,28–30 Bax,15,31–33 and suicide genes such as herpes simplex virus thymidine kinase (HSV-tk).34–37 Additionally, conditionally replicating adenovirus (CRAd) vectors have recently gained attention as a potential gene therapy vector for the treatment of cancers.38–40 Incorporation of prostate-specific regulation of Ad early genes using the PFLPS regulation system could potentially produce a potent yet safe vector for the treatment of prostate cancers.
The PFLPS regulation system is not restricted to only the gene therapy treatment of prostate cancers, however, as the positive feedback loop concept could be transferred to other tissue types by simply incorporating different tissue-specific promoters, thereby expanding its potential utility to include gene therapy of other cancers and molecular genetic applications other than cancer, such as the gene therapy treatment of genetic disorders, development gene-based vaccines expressing immunogenic bacterial or viral antigens, and development of new mouse models that require highly induced, organ-restricted expression of a particular gene of interest. Finally, the effective transcriptional regulation afforded by PFLPS could be combined with current methods of transductional regulation including manipulation of Ad fiber knob41–47 and use of bi-specific antibodies38,48–51 to further improve the targeting of gene therapy vectors to specific cell types and therefore increase the specificity and the safety of the vectors.
In conclusion, we have developed and characterized a novel transcriptional regulatory system that demonstrates highly induced, prostate-specific expression without a loss in specificity. Such a regulation system could be potentially altered to include tissue- or cancer-specific promoters in the place of the ARR2PB promoter as well as any desired transgene in place of GFP and thus is ideal for several molecular genetic and gene therapeutic applications that require highly induced, organ-restricted expression of a particular gene of interest. This transcriptional regulation can also be combined with transductional regulation systems to increase the specificity and the safety of current gene therapy vectors. The PFLPS regulation system, therefore, serves as an exciting new strategy for the transcriptional regulation of therapeutic genes for future molecular genetic and therapeutic applications.
Materials and methods
Cell lines
HEK293 (human embryonic kidney), LNCaP (human prostate cancer) and 22RV1 (mouse prostate cancer) cells were obtained from American Type Culture Collection (ATCC; Manassas, VA). U343MG and U251MG (brain tumor) cell lines were obtained from the Brain Tumor Research Center Tissue Bank (Dept. of Neurological Surgery, UCSF, San Francisco, CA). All cell lines were maintained in media supplemented with 10% cosmic calf serum (CCS; HyClone, Logan, UT), with HEK293 being maintained in DMEM, LNCaP and 22RV1 being maintained in RPMI, and U343MG and U251MG being maintained in MEM.
Construction of plasmids vectors
The pUHD10-3 (containing the TRE promoter) and pUHD15-1 (containing the tTA gene) were generously provided by Hermann Bujard (Center for Molecular Biology, University of Heidelberg, Heidelberg, Germany). The ARR2PB (0.45 kb) promoter was developed in the laboratory of Robert J. Matusik (Department of Cell Biology, Vanderbilt University Medical Center, Nashville, TN), who contributed the pARR2PB.PolI.TRZ-SK vector. ARR2PB is based on the minimal probasin promoter with a duplicated probasin androgen response region (ARR) upstream of it.12 Construction of pLAd-CMV, pLAd-mcs and pRAd-T.GFP vectors has been described previously.24 We excised the ARR2PB promoter from pARR2PB.PolI.TRZ-SK and the tTA gene from pUHD15-1 and cloned them into pLAd-mcs to generate pLAd-2Pb.tTA.16 We excised the ARR2PB.tTA cassette from pLAd-2Pb.tTA and cloned it back in but in reverse to generate pLAd(2Pb.tTA)r. We excised the TRE promoter from pUHD10-3 and cloned it upstream of the ARR2PB promoter in the pLAd(2Pb.tTA)r construct to generate pLAd(T2Pb.tTA.S)r (see Fig. 2a). We cloned the TRE-ARR2PB.tTA cassette in reverse orientation near the left ITR so that its promoter is away from the E1a enhancer region since we had previously found that the basal activity of both the TRE promoter and the ARR2PB promoter were significantly affected by interference from the E1a enhancer.21 To construct the pRAd2T2Pb.GFP.B plasmid (see Fig. 2a), we excised the TRE-ARR2PB promoter from pLAd-2Pb.tTA and cloned it in the place of the TRE promoter in pRAd2T.GFP.B (a plasmid closely related to pRAd-T.GFP).
Construction of recombinant adenoviral vectors
Construction of Ad/C.LacZ and Ad/GFPTET has been described previously.24 pLAd(T2Pb.tTA.S)r and pRAd2T2Pb.GFP.B plasmids were digested with Swa I and Spe I and ligated to an Ad5 genome backbone (Ad5sub360SR) digested on both ends with Xba I. The assembly of the Ad/GFPPFLPS vector genome was constructed as described previously.22,23 All Ad vectors were based on Ad5sub360SR, which contains deletions in E3 and all E4 ORFs with the exception of ORF6.
Propagation and titering of recombinant adenovirus vectors
All vectors were propagated in HEK293 cells, using standard procedures.22–24 Briefly, HEK293 cells, which provide Ad5 E1a and E1b functions in trans, were transfected with the ligation mixture containing the recombinant adenovirus (rAd) vector DNA using Fugene 6 transfection reagent (Roche, Indianapolis, IN) and manufacturer’s instructions. Transfected cells were maintained until adenovirus-related cytopathic effects (CPE) were observed (typically 7–14 days post-transfection), at which point the cells were collected. Vector propagation and amplification was then achieved by standard techniques. Briefly, adenoviral lysates from twenty-four 150 mm2 plates were banded twice on CsCl gradients and desalted twice with a PD-10 size exclusion column (Amersham Scientific, Piscataway, NJ) into HEPES buffered saline (HBS; 21 mM HEPES, 140 mM NaCl, 5 mM KCl, 0.75 mM Na2HPO4
 2H2O, and 0.1% (w/v) dextrose; adjust pH with NaOH to 7.5; and filter sterilize) containing 5% glycerol, and stored at −70°C. All vectors were titrated on HEK293 cells infected in serial dilution on triplicate columns of 96-well plates for either GFP fluorescence or X-gal staining. GFP fluorescence was monitored with Axiovert-25 fluorescent microscope (Carl Zeiss, Germany) and FITC excitation/emission filter set (Chroma Technology Corp, Rockingham, VT) two days post-infection. Cells infected with Ad/C.LacZ were fixed two days post-infection with fixative solution (2% formaldehyde, 0.05% glutaraldehyde in 1x PBS) for 5 minutes at room temperature and then stained overnight at 37°C in X-gal solution (1 mg/ml X-gal [5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside], 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2 in 1x PBS). The resulting titers were scored as infectious units (IU) per ml.
2H2O, and 0.1% (w/v) dextrose; adjust pH with NaOH to 7.5; and filter sterilize) containing 5% glycerol, and stored at −70°C. All vectors were titrated on HEK293 cells infected in serial dilution on triplicate columns of 96-well plates for either GFP fluorescence or X-gal staining. GFP fluorescence was monitored with Axiovert-25 fluorescent microscope (Carl Zeiss, Germany) and FITC excitation/emission filter set (Chroma Technology Corp, Rockingham, VT) two days post-infection. Cells infected with Ad/C.LacZ were fixed two days post-infection with fixative solution (2% formaldehyde, 0.05% glutaraldehyde in 1x PBS) for 5 minutes at room temperature and then stained overnight at 37°C in X-gal solution (1 mg/ml X-gal [5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside], 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2 in 1x PBS). The resulting titers were scored as infectious units (IU) per ml.
Transfections and Infections in vitro
For plasmid DNA transfections, 1.0–2.5 x 105 cells/well were seeded in 24-well plates and transfected 18 hr post-seeding using SuperFect reagent (Qiagen, Hilden, Germany) according to manufacturer’s instructions. Co-transfection with pUHD 15-1 and pRAd2T.GFP.B (designated as Tet in Fig. 3) served as a positive control for GFP expression. pLAd-CMV served as an empty vector control for transfections. Co-transfection of pRAd22Pb.GFP and pLAd-CMV served as a control for prostate-specific GFP expression (designated as ARR2PB in Fig. 3). For Ad vector infections, 1 x 104 cells/well were seeded in 96-well plates or 1x105 cells/well were seeded in 24-well plates. Seeded cells were infected 3 hours post-seeding at multiplicities of infection (MOI) of 0, 10, 50, 100, or 1000. MOI calculations were based on cell numbers at the time of seeding and on Ad vector titers based on IU/ml.
Quantification of GFP expression
In the transfection or infection experiments, GFP fluorescence in cells was visualized 72 or 48 hours post-transduction, respectively, using Axiovert-25 fluorescent microscope with FITC filter set. For quantitative analysis of GFP activity, cells were lysed with 0.5% Triton x-100 in 1x PBS. Cell lysates were transferred to 96-well black microtiter plates (BMG Labtechnologies, Offenburg, Germany) and relative GFP fluorescence was measured using FLUOstar™ dual fluorescence/absorbance plate reader (BMG Labtechnologies) at excitation 485 nm and emission 520 nm.
RT-PCR
Cells were seeded on 100 mm2 plates. When cells reached 80–90% confluency, cells were trypsinized and counted the day of infection. Cells were resuspended in 10ml serum-containing medium, infected at MOI 10, and plated onto 100 mm2 plates. Two days post-infection, media was aspirated and cells were harvested in 1 ml TRI reagent (Sigma-Aldrich, St. Louis, MO). RNA was purified according to manufacturer’s instructions. cDNA was synthesized from 1μg RNA/sample using the RETROscript™ kit (Ambion Inc., Austin, TX) according to manufacturer’s instructions. Following reverse transcription, cDNA were amplified for either GFP or β-actin using GoTaq DNA Polymerase (Promega, Madison, WI) according to manufacturer’s instructions. PCR was performed on the cDNA using the following sense and anti-sense primers: 5′-GCAAGGGCGAGGAGCTGTTCA-3′ and 5′-AAGTTCACCTTGATGCCGTTCTTC-3′ for GFP and 5′-GTGGGGCGCCCCAGGCACCA-3′ and 5′-CTCCTTAATGTCACGCACGATTTC-3′ for β-actin. PCR products were amplified by the following touchdown PCR program: 96°C for 2 min; 12 cycles of 96°C for 20 sec, 75°C decreasing 1.5°C/cycle for 20 sec; 72°C for 1 min; 13 cycles of 96°C for 20 sec, 58°C for 20 sec, 72°C for 1 min; 72°C for 10 min; hold at 4°C. PCR products were resolved on 1:1 mixture of 3% Synergel agarose clarifier additive (Diversified Biotech, Boston, MA) and 0.8% agarose (EM Science; Gibbstown, NJ) in 1x TAE buffer.
Acknowledgments
We would like to thank Robert J. Matusik for providing the pARR2PB.PolI.TRZ-SK plasmid and Hermann Bujard for providing the pUHD 10-3 and pUHD 15-1 plasmids. We would also like to thank the Brain Tumor Cell Core at UCSF for providing the U343MG and U251MG brain tumor cell lines. We would like to acknowledge the MUSC Viral Vector Core Facility for help in the propagation and purification of the Ad/GFPPFLPS and control vectors. This work was supported in part by a grant from the National Institutes of Health, R01 DK57997, and a startup fund provided to Jian-yun (John) Dong by MUSC.
References
- 1.Compiled by the American Cancer Society. 2003. Cancer Facts & Figures 2003. [Google Scholar]
- 2.Pound CR, et al. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 3.Meuleman EJ, Mulders PF. Erectile function after radical prostatectomy: a review. Eur Urol. 2003;43:95–101. doi: 10.1016/s0302-2838(02)00546-8. discussion 101–102. [DOI] [PubMed] [Google Scholar]
- 4.Hara I, et al. Comparison of quality of life following laparoscopic and open prostatectomy for prostate cancer. J Urol. 2003;169:2045–2048. doi: 10.1097/01.ju.0000063961.99940.6c. [DOI] [PubMed] [Google Scholar]
- 5.Dahm P, et al. A longitudinal assessment of bowel related symptoms and fecal incontinence following radical perineal prostatectomy. J Urol. 2003;169:2220–2224. doi: 10.1097/01.ju.0000065116.20997.a3. [DOI] [PubMed] [Google Scholar]
- 6.Kirschner-Hermanns R, Jakse G. Quality of life following radical prostatectomy. Crit Rev Oncol Hematol. 2002;43:141–151. doi: 10.1016/s1040-8428(02)00026-4. [DOI] [PubMed] [Google Scholar]
- 7.Klein EA, et al. Locally advanced prostate cancer. Curr Treat Options Oncol. 2001;2:403–411. doi: 10.1007/s11864-001-0045-1. [DOI] [PubMed] [Google Scholar]
- 8.Doehn C, Jocham D. Technology evaluation: CV-787, Calydon Inc. Curr Opin Mol Ther. 2001;3:204–210. [PubMed] [Google Scholar]
- 9.Kubo H, et al. Phase I dose escalation clinical trial of adenovirus vector carrying osteocalcin promoter-driven herpes simplex virus thymidine kinase in localized and metastatic hormone-refractory prostate cancer. Hum Gene Ther. 2003;14:227–241. doi: 10.1089/10430340360535788. [DOI] [PubMed] [Google Scholar]
- 10.Shirakawa T, et al. Tissue-specific promoters in gene therapy for the treatment of prostate cancer. Mol Urol. 2000;4:73–82. doi: 10.1089/10915360050138620. [DOI] [PubMed] [Google Scholar]
- 11.Snoek R, et al. Differential transactivation by the androgen receptor in prostate cancer cells. Prostate. 1998;36:256–263. doi: 10.1002/(sici)1097-0045(19980901)36:4<256::aid-pros7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Kasper S, et al. Selective activation of the probasin androgen-responsive region by steroid hormones. J Mol Endocrinol. 1999;22:313–325. doi: 10.1677/jme.0.0220313. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, et al. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141:4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 15.Andriani F, et al. Use of the probasin promoter ARR2PB to express Bax in androgen receptor-positive prostate cancer cells. J Natl Cancer Inst. 2001;93:1314–1324. doi: 10.1093/jnci/93.17.1314. [DOI] [PubMed] [Google Scholar]
- 16.Rubinchik S, et al. A complex adenovirus vector that delivers FASL-GFP with combined prostate-specific and tetracycline-regulated expression. Mol Ther. 2001;4:416–426. doi: 10.1006/mthe.2001.0478. [DOI] [PubMed] [Google Scholar]
- 17.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furth PA, et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994;91:9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kistner A, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howe JR, et al. The responsiveness of a tetracycline-sensitive expression system differs in different cell lines. J Biol Chem. 1995;270:14168–14174. doi: 10.1074/jbc.270.23.14168. [DOI] [PubMed] [Google Scholar]
- 21.Rubinchik S, et al. Creation of a new transgene cloning site near the right ITR of Ad5 results in reduced enhancer interference with tissue-specific and regulatable promoters. Gene Ther. 2001;8:247–253. doi: 10.1038/sj.gt.3301364. [DOI] [PubMed] [Google Scholar]
- 22.Rubinchik S, Norris JS, Dong JY. Construction, purification and characterization of adenovirus vectors expressing apoptosis-inducing transgenes. Methods Enzymol. 2002;346:529–547. doi: 10.1016/s0076-6879(02)46075-2. [DOI] [PubMed] [Google Scholar]
- 23.Rubinchik S, et al. Improving the transcriptional regulation of genes delivered by adenovirus vectors. Methods in Molecular Medicine. 2003;76:167–199. doi: 10.1385/1-59259-304-6:167. [DOI] [PubMed] [Google Scholar]
- 24.Rubinchik S, et al. Adenoviral vector which delivers FasL-GFP fusion protein regulated by the tet-inducible expression system. Gene Ther. 2000;7:875–885. doi: 10.1038/sj.gt.3301172. [DOI] [PubMed] [Google Scholar]
- 25.Kanai F. Transcriptional targeted gene therapy for hepatocellular carcinoma by adenovirus vector. Mol Biotechnol. 2001;18:243–250. doi: 10.1385/MB:18:3:243. [DOI] [PubMed] [Google Scholar]
- 26.Haviv YS, Curiel DT. Conditional gene targeting for cancer gene therapy. Adv Drug Deliv Rev. 2001;53:135–154. doi: 10.1016/s0169-409x(01)00225-3. [DOI] [PubMed] [Google Scholar]
- 27.Hyer ML, et al. Intracellular Fas ligand expression causes Fas-mediated apoptosis in human prostate cancer cells resistant to monoclonal antibody-induced apoptosis. Mol Ther. 2000;2:348–358. doi: 10.1006/mthe.2000.0139. [DOI] [PubMed] [Google Scholar]
- 28.Rubinchik S, et al. Enhanced apoptosis of glioma cell lines is achieved by co-delivering FasL-GFP and TRAIL with a complex Ad5 vector. Cancer Gene Ther. 2003;10:814–822. doi: 10.1038/sj.cgt.7700651. [DOI] [PubMed] [Google Scholar]
- 29.Seol JY, et al. Adenovirus-TRAIL can overcome TRAIL resistance and induce a bystander effect. Cancer Gene Ther. 2003;10:540–548. doi: 10.1038/sj.cgt.7700597. [DOI] [PubMed] [Google Scholar]
- 30.Voelkel-Johnson C, King DL, Norris JS. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Ther. 2002;9:164–172. doi: 10.1038/sj.cgt.7700420. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu K, et al. Cre-loxP-mediated bax gene activation reduces growth rate and increases sensitivity to chemotherapeutic agents in human gastric cancer cells. Cancer Gene Ther. 2000;7:885–892. doi: 10.1038/sj.cgt.7700181. [DOI] [PubMed] [Google Scholar]
- 32.Lee A, et al. Bax expressed from a herpes viral vector enhances the efficacy of N,N′-bis(2-hydroxyethyl)-N-nitrosourea treatment in a rat glioma model. Cancer Gene Ther. 2000;7:1113–1119. doi: 10.1038/sj.cgt.7700205. [DOI] [PubMed] [Google Scholar]
- 33.Shinoura N, et al. Adenovirus-mediated transfer of bax with caspase-8 controlled by myelin basic protein promoter exerts an enhanced cytotoxic effect in gliomas. Cancer Gene Ther. 2000;7:739–748. doi: 10.1038/sj.cgt.7700158. [DOI] [PubMed] [Google Scholar]
- 34.Fillat C, et al. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr Gene Ther. 2003;3:13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- 35.Nishihara E, et al. Treatment of thyroid carcinoma cells with four different suicide gene/prodrug combinations in vitro. Anticancer Res. 1998;18:1521–1525. [PubMed] [Google Scholar]
- 36.Yazawa K, Fisher WE, Brunicardi FC. Current progress in suicide gene therapy for cancer. World J Surg. 2002;26:783–789. doi: 10.1007/s00268-002-4053-5. [DOI] [PubMed] [Google Scholar]
- 37.Kirn D, et al. The emerging fields of suicide gene therapy and virotherapy. Trends Mol Med. 2002;8:S68–73. doi: 10.1016/s1471-4914(02)02318-3. [DOI] [PubMed] [Google Scholar]
- 38.van Beusechem VW, et al. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Ther. 2003;10:1982–1991. doi: 10.1038/sj.gt.3302103. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–1218. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 40.Wildner O. Comparison of replication-selective, oncolytic viruses for the treatment of human cancers. Curr Opin Mol Ther. 2003;5:351–361. [PubMed] [Google Scholar]
- 41.Volk AL, et al. Enhanced Adenovirus Infection of Melanoma Cells by Fiber-Modification: Incorporation of RGD Peptide or Ad5/3 Chimerism. Cancer Biol Ther. 2003;2:511–515. doi: 10.4161/cbt.2.5.440. [DOI] [PubMed] [Google Scholar]
- 42.Buskens CJ, et al. A genetically retargeted adenoviral vector enhances viral transduction in esophageal carcinoma cell lines and primary cultured esophageal resection specimens. Ann Surg. 2003;238:815–824. doi: 10.1097/01.sla.0000098622.47909.c0. discussion 825–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belousova N, et al. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol. 2002;76:8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wesseling JG, et al. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969–976. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- 45.Heideman DA, et al. Selective gene delivery toward gastric and esophageal adenocarcinoma cells via EpCAM-targeted adenoviral vectors. Cancer Gene Ther. 2001;8:342–351. doi: 10.1038/sj.cgt.7700313. [DOI] [PubMed] [Google Scholar]
- 46.Vigne E, et al. Genetic manipulations of adenovirus type 5 fiber resulting in liver tropism attenuation. Gene Ther. 2003;10:153–162. doi: 10.1038/sj.gt.3301845. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Sato K, Hamada H. Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber. J Virol. 2003;77:2512–2521. doi: 10.1128/JVI.77.4.2512-2521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jongmans W, et al. Targeting of adenovirus to human renal cell carcinoma cells. Urology. 2003;62:559–565. doi: 10.1016/s0090-4295(03)00378-9. [DOI] [PubMed] [Google Scholar]
- 49.Nettelbeck DM, et al. Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int J Cancer. 2004;108:136–145. doi: 10.1002/ijc.11563. [DOI] [PubMed] [Google Scholar]
- 50.Henning P, et al. Genetic modification of adenovirus 5 tropism by a novel class of ligands based on a three-helix bundle scaffold derived from staphylococcal protein A. Hum Gene Ther. 2002;13:1427–1439. doi: 10.1089/10430340260185067. [DOI] [PubMed] [Google Scholar]
- 51.Kashentseva EA, et al. Adenovirus targeting to c-erbB-2 oncoprotein by single-chain antibody fused to trimeric form of adenovirus receptor ectodomain. Cancer Res. 2002;62:609–616. [PubMed] [Google Scholar]


