Abstract
The antibacterial effect of lactoferrin (Lf) was tested on isolates of Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and coagulase-negative staphylococci (CNS) as well as on Pseudomonas aeruginosa (P. aeruginosa) and Klebsiella pneumoniae (K. pneumoniae), originally isolated from bovine mastitis. Concentrations of Lf used were 0.67 mg/ml, 1.67 mg/ml, and 2.67 mg/ml. Growth of udder pathogens was monitored by turbidometry either in broth culture or in whey prepared from normal milk. We focused on 3 different growth variables: lag time, slope, and maximum absorbance of bacterial growth curves. Growth inhibition was seen in the broth but hardly at all in whey. The isolates of E. coli and CNS did not grow sufficiently well in whey to draw any conclusions. The most effective inhibitory activity of Lf was seen against E. coli and P. aeruginosa. All 5 E. coli isolates had similar growth patterns. Inhibition of growth by Lf was concentration-dependent. The concentration of 0.67 mg/ml in broth and whey was generally too low for a significant inhibitory effect.
Keywords: mastitis pathogens, growth inhibition
Introduction
Lactoferrin (Lf) is an iron-binding glycoprotein found in milk, other external secretions, and the granules of neutrophilic polymorphonuclear leukocytes [3,17,18]. Lf has been shown to be bacteriostatic in vitro, and this inhibitory activity is believed to be the result of the powerful iron-chelating ability of Lf, making iron unavailable to bacteria [22,25]. Lactoferrin has a broad-spectrum antimicrobial activity against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, Streptococcus mutans and Candida albicans, among others [2,15]. Lf has also been shown to enhance the activity of some antimicrobials in vitro [7,23].
Evidence from a number of studies indicates that the antimicrobial activity of Lf is more complex than simple Fe chelation. Lf has bactericidal activity and can kill susceptible bacteria by a mechanism distinct from sequestering of Fe [2,4,6,9,10] established that the antimicrobial domain is near the N-terminus of Lf in a region distinct from its iron-binding sites. Apo-Lf (iron-free Lf) was shown to increase bacterial cell membrane permeability and directly damage the outer membrane of Gram-negative bacteria [9,10].
Normal bovine milk contains low concentrations of Lf, approximately 0.1 mg/ml or less, but in dry udder secretion Lf concentration is markedly higher and can reach a level of 20 mg/ml or higher [24,26]. During the dry period, the udder is very resistant to coliform infections, mostly due to the high Lf content of the secretion [20]. In mastitic cows, Lf concentrations of the milk have been shown to increase dramatically and can range from 0.3 mg/ml to 2.3 mg/ml [11,13].
Lf may have therapeutic potential in mastitis [7,14]. It could partly replace the use of antimicrobials, which cause problems due to residues in milk and the risk for emergence of resistance. Studies on the in vitro susceptibility of udder pathogens to Lf are, however, scant. The aim of this study was to determine the antibacterial activity of Lf against bovine udder pathogens in vitro.
Materials and methods
Lactoferrin
Bovine Lf was purified from cheese whey or concentrated cheese whey by the expanded bed absorption chromatography method [12]. Iron content of native Lf was approximately 8%–15% [12]. Lf was stored frozen at -20°C and sterile-filtered (32 mm Acrodisc PT Syringe Filters 0.8/0.2 μm, Gelman Laboratory REF: 4658) before use. The final concentration of Lf in the product was 35.5 mg/ml [12]. Purity of Lf was tested in SDS-page (sodium dodecyl sulphate polyacrylamide gel electrophoresis) [12]. Apo-Lf was prepared from Lf by citrate dialyzing [8], and its iron content was approximately 4%.
Growth media
Two growth media were used for bacterial cultures: the commercial Iso Sensitest-Broth (ISB CM473, Oxoid Ltd., Basingstoke, Hampshire, England), and whey. Whey was prepared from 3 liters of fresh raw milk obtained from the university dairy herd by high-speed centrifugation of defatted milk (32600 g for 60 min at 4°C). Aliquots of 40-ml whey were sterile-filtered and frozen immediately after preparation for later use.
Bacterial isolates and the preparation of inoculums
Five isolates of E. coli, S. aureus, and coagulase negative-staphylococci (CNS), 2 isolates of P. aeruginosa and 2 isolates of K. pneumoniae originally isolated from subclinical or clinical cases of bovine mastitis were used. These isolates were received from the mastitis laboratory of the Faculty of Veterinary Medicine and the National Veterinary and Food Research Institute, Helsinki. One of the S. aureus isolates was the reference isolate M60 kindly provided by Dr. A. J. Guidry (Immunology and Disease Resistance Laboratory USDA, Beltsville, USA). During the experiment, bacteria were maintained on blood agar plates at 8°C. To adapt the bacterial isolates to grow in whey, they were grown overnight at 37°C in a growth medium consisting of 2/3 Iso Sensitest-Broth and 1/3 sterile whey. The cultures were tested using Gram-staining for purity. Bacteria were harvested by centrifugation (5000 g for 10 min) and washed twice between centrifugations using sterile saline (0.9% NaCl, 20°C). A suspension containing approximately 109colony-forming units (CFU) in 0.9% NaCl was prepared according to the McFarland standard (bioMérieux sa, 69280 Marcy I'Etoile, France) by spectrophotometry (550 nm, Hitatchi U-2000, Hitachi, Ltd., Tokyo, Japan). Bacterial suspension was diluted to the final concentration of 1.5 × 103CFU/ml used in each well.
Analysis of bacterial growth by turbidometry
Bacterial growth was measured using turbidometry (Bioscreen instrument, Labsystems, Helsinki, Finland). The instrument is a fully automated analyzing system for measuring bacterial growth using the vertical light bath with wide band absorption principle; 200 individual samples can be run simultaneously. Each well contained 100 μl of ISB broth or 150 μl of whey as the growth medium, 50 μl of bacterial suspension, and 50 μl of Lf concentrate. Physiological saline was added to bring the final volume to 300 μl: 50 μl and 100 μl in ISB and whey wells, respectively. Tested amounts of Lf were 200 μg (the final Lf concentration in the well was 0.67 mg/ml), 500 μg (1.67 mg/ml), and 800 μg (2.67 mg/ml). Control wells contained all components except Lf, which was replaced by 50 μl of 0.9% saline. Five parallel wells were used. Wells between whey and ISB wells were filled with 0.9% NaCl to prevent cross-contamination. The wells on 2 100-well plates were covered and preincubated in the Bioscreen instrument for 30 min at 37°C. The change in turbidity was monitored automatically every hour for 20 h at 37°C. The plates were shaken 10 min before each measurement. Two E. coli and 2 S. aureus isolates were also tested with Apo-Lf. The lag time (time from beginning of incubation until the time-point when absorbance began to increase), slope (slope of the growth curve in logarithmic growth phase), and maximum absorbance (highest absorbance value measured during the 20-h period) were used as variables describing the bacterial growth. After the 20-h incubation period, bacterial survival and bactericidal effect of Lf in the wells were confirmed by culturing aliquots of 10 ml on blood agar plates and incubating the plates overnight at 37°C.
Statistical methods
The effect of different Lf concentrations on lag time, slope, and maximum absorbance was tested by repeated measures analysis of variance with concentration as a within factor. The significance of concentration was evaluated by Greenhouse-Geisser adjusted p-values. Concentrations of 0.67, 1.67, and 2.67 mg/ml of Lf were further compared with a negative control.
Results
Results of growth inhibition by Lf in ISB for E. coli, S. aureus, and CNS are shown in Table 1. The best inhibitory activity of Lf in the ISB was seen against E. coli and P. aeruginosa (data not shown for the latter). The typical growth curves of E. coli in the ISB broth with different concentrations of Lf are shown in Fig. 1. The inhibitory effect of Lf for E. coli was concentration-dependent (Fig. 2), and variation between the 5 isolates of E. coli was small. None of the isolates was totally resistant to Lf. The effect of Lf on the maximum absorbance and the slope of E. coli in ISB was statistically significant (p = 0.025 and p < 0.001, respectively), whereas the effect on the lag time was not significant (p = 0.065).
Table 1.
Effect of Lf on bacterial growth in ISB culture, measured by lag time and maximum absorbance.
| Lag time (h) | Maximum absorbance | |||||
| Bacteria | Mean | Min-max | SD | Mean | Min-max | SD |
| Escherichia coli (n = 5) | ||||||
| Control | 3.8 | 3.0–4.0 | 0.45 | 0.91 | 0.70–1.05 | 0.15 |
| 0.67 mg/ml Lf | 6.6 | 5–9 | 1.52 | 0.67 * | 0.57–0.73 | 0.06 |
| 1.67 mg/ml Lf | 9.0 | 5–15 | 4.1 | 0.52 * | 0.25–0.70 | 0.18 |
| 2.67 mg/ml Lf | 10.6 | 6.0–20.0 | 6.0 | 0.42 * | 0.11–0.68 | 0.24 |
| Staphylococcus aureus (n = 5) | ||||||
| Control | 4.8 | 4.0–5.0 | 0.45 | 0.60 | 0.49–0.65 | 0.07 |
| 0.67 mg/ml Lf | 7.2 | 5.0–11 | 2.4 | 0.55 | 0.42–0.69 | 0.11 |
| 1.67 mg/ml Lf | 8.4 * | 6.0–12 | 2.2 | 0.52 | 0.40–0.66 | 0.10 |
| 2.67 mg/ml Lf | 8.4 * | 6.0–12 | 2.2 | 0.49 * | 0.40–0.64 | 0.10 |
| Coagulase-negative staphylococci (n = 5) | ||||||
| Control | 6.4 | 5.0–10 | 2.2 | 0.56 | 0.53–0.60 | 0.03 |
| 0.67 mg/ml Lf | 12.2 | 6.0–20 | 7.2 | 0.36 | 0.10–0.56 | 0.23 |
| 1.67 mg/ml Lf | 12.2 | 6.0–20 | 2.2 | 0.34 | 0.11–0.52 | 0.21 |
| 2.67 mg/ml Lf | 12.4 | 7.0–20 | 7.0 | 0.34 | 0.10–0.55 | 0.21 |
Lag time = time from the beginning of incubation until the time-point when the absorbance began to increase; maximum absorbance = highest absorbance value measured during the 20-h incubation period.
Statistically significant difference when compared with negative control: * p ≤ 0.05
Figure 1.
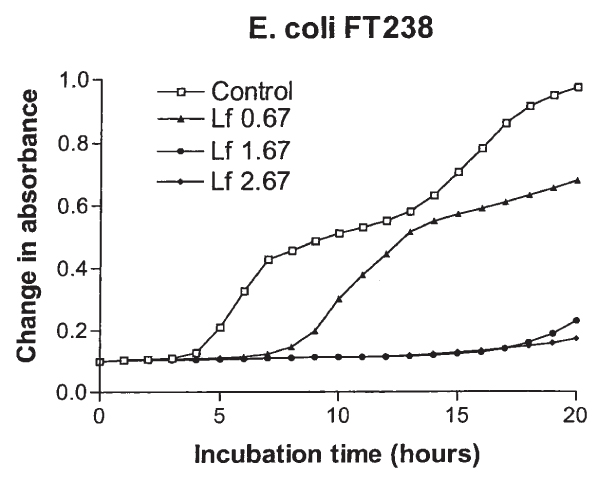
Growth curves of E. coli FT238 in Iso Sensitest-Broth (ISB) with concentrations of 0.67, 1.67, and 2.67 mg/ml lactoferrin (Lf) and without Lf.
Figure 2.
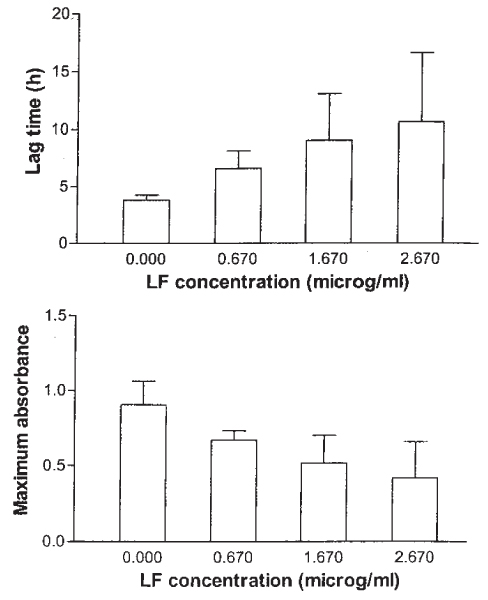
Mean and SD of lag time and maximum absorbance of five E. coli isolates in ISB with concentrations of 0.67, 1.67, and 2.67 mg/ml Lf and without Lf.
The growth of 2 isolates of P. aeruginosa was clearly inhibited in ISB. In contrast, the growth of 2 K. pneumoniae isolates was hardly inhibited at all. In whey, K. pneumoniae showed variable susceptibility and the results were contradictory (data not shown).
The isolates of CNS and S. aureus in ISB showed more variation to Lf than E. coli. Lf had significant effects on lag time (p = 0.014) and maximum absorbance (p = 0.014) of S. aureus, while no significant effects were seen for CNS (Table 1). As regards the slope, a statistically significant difference was present between Lf concentration and the control in the growth of S. aureus (p = 0.001) and CNS (p = 0.002). The growth of four CNS isolates was somewhat inhibited by Lf, but one was totally resistant. Three isolates of S. aureus were more susceptible to Lf than the 2 other isolates at 0.67 mg/ml of Lf. The results for S. aureus in whey are presented in Table 2. Lf concentration had a significant inhibitory effect on lag time (p = 0.015), maximum absorbance (p = 0.011), and slope (p = 0.027) of S. aureus in whey. The initial absorbancies in whey wells were higher than in ISB cultures (~1.0) because whey is more turbid than ISB. The growth of E. coli, P. aeruginosa, and CNS isolates was so poor in normal whey that it was not possible to draw any conclusions about the effect of Lf in that medium.
Table 2.
Effect of Lf on the growth of S. aureus in whey culture, measured by lag time and maximum absorbance. The initial absorbancies in whey wells were higher than in ISB cultures (~1.0) because whey is more turbid than ISB. For explanations see Table 1
| Lag time (h) | Maximum absorbance | |||||
| Bacteria | Mean | Min-max | SD | Mean | Min-max | SD |
| Staphylococcus aureus (n = 5) | ||||||
| Control | 10.6 | 8.0–13 | 1.82 | 1.66 | 1.12–2.22 | 0.48 |
| 0.67 mg/ml Lf | 14.2 | 8.0–20 | 5.6 | 1.23 * | 0.95–1.59 | 0.27 |
| 1.67 mg/ml Lf | 16.8 * | 10–20 | 4.6 | 1.05 * | 0.87–1.28 | 0.16 |
| 2.67 mg/ml Lf | 20 ** | - * | ||||
- = no bacterial growth
Statistically significant difference when compared with negative control:
* p < 0.05 ** p < 0.01
Two isolates of E. coli and 2 isolates of S. aureus were also tested with Apo-Lf in ISB. The results were similar to those of native Lf (data not shown).
Discussion
Our results demonstrate that bovine Lf in vitro is bacteriostatic towards some udder pathogens. The most interesting finding was the clear inhibitory activity of Lf against E. coli, which is in agreement with many previous studies [8,19,21]. Unfortunately, the most important target pathogen, E. coli, was in our study unable to grow in whey prepared from bulk milk with low somatic cell count. [21] tested the in vitro susceptibility to Lf of 35 E. coli isolates, which had originally been isolated from clinical or subclinical bovine mastitis. He found that most of the isolates were completely inhibited by 0.1 mg/ml Apo-Lf at the end of the 16-h incubation period. A few isolates partially resisted the bacteriostatic action of Lf, but none was totally resistant. [19] reported bacteriostatic, but not bactericidal, activity of bovine Lf against Gram-negative mastitis-causing bacteria E. coli and Kl. pneumoniae. [8] reported that an Lf concentration of 1.0 mg/ml inhibited growth of all 19 isolates of enterotoxigenic E. coli isolated from porcine enteritis. The degree of inhibition was strain-dependent. Bacterial killing occurred at relatively high initial concentrations of bacteria (5 × 103 CFU/ml), but bacteriostatic effects were seen even at higher concentrations. Contradictory results have also been found; [23] did not see effect of Lf alone at concentrations from 0.5 to 3 mg/ml on three E. coli strains isolated from bovine mastitis.
[8] found no significant differences in the activity between native (32% Fe) and Apo-(<1% Fe) Lf. [5] pointed out that Apo- and Fe-saturated forms of bovine Lf were equally effective against experimental S. aureus in in vivo infections in mice; bovine Lf with different degrees of iron saturation (9%–97%) was found to be similar. We conducted limited testing using Apo-Lf, the results being comparable with those of native Lf. However, because Apo-Lf is not a realistic candidate for potential use in cows, we focused on native Lf.
We used whey as a growth medium because it simulates the environment of the milk compartment of the cow udder. Whey from milk of healthy cows is known to inhibit the growth of a number of bacterial species [16]. Whey prepared from milk of mastitic cows may have been a better medium for our studies, but it would have been difficult to standardize the medium and compare our results with those of other authors.
The mechanism by which Lf inhibits bacterial growth has not been fully elucidated. Early studies attributed such effects to the acquisition of essential Fe from the environment, but more recent findings have implicated wider cell interactions. Lf damages the outer membrane of bacteria, with a concomitant release of LPS from Gram-negative bacteria. The ultrastuctural alterations caused by Lf to the bacteria enhance the activity of some antimicrobial agents [7,23], and one approach could be to combine Lf with antibiotics in treating infections. We decided to test Lf alone to avoid the problems related to the use antibiotics and to see the real net effect of Lf against several bacteria species. [7] demonstrated a synergistic effect between Lf and penicillin against three S. aureus strains tested. Lf alone showed a weak inhibitory activity which agrees with our results. Lf can also bind LPS and at least partly block its detrimental effects [1,27] demonstrated in vitro and in an experimental mouse model that the E. coli endotoxin-neutralizing capability of human Lf was derived from a 33-mer synthetic peptide, lactoferricin. Lf or lactoferricin could potentially be used for the treatment of endotoxin-induced septic shock. Gram-negative bacteria, mainly E. coli, cause severe mastitis in lactating cows, which may result in endotoxin shock and death. Lf could be a potentially useful treatment for this condition, but its efficacy should be tested using in vivo studies.
Conclusion
The antibacterial effects of Lf could only be demonstrated in the ISB medium, as bacterial growth in whey was weak and variable. The best inhibitory activity of Lf was seen against Gram-negative E. coli and P. aeruginosa. The variation in the susceptibility of the 5 isolates of E. coli to Lf was small, and none of the isolates was totally resistant. The response of S. aureus and CNS isolates to Lf was more variable. Our findings confirm that bovine Lf in vitro is antibacterial towards some major pathogens.
Acknowledgments
Acknowledgements
We thank our cooperators Riitta Keiski, Liisa Myllykoski, Kimmo Vahtola, Carita Berg, and Päivi Anttila at the University of Oulu, Finland, who provided the Lf. We also thank Arto Ketola, M. Sc. (Soc.), for statistical analysis and Ilkka Saastamoinen (technician) and Susann Sunqvist (laboratory assistant) for their technical support.
References
- Appelmelk BJ, Yun-qing A, Geerts M, Thijs BG, Boer HA, Maclaren DM, Graaff J, Nuijens JH. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold RR, Brewer M, Gauthier JJ. Bactericidal activity of human lactoferrin: sensitivity of a variety of micro-organisms. Infect Immun. 1980;28:893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M, DeDuve C, Masson PL, Heremans JF. Association of lactoferrin with specific granules in rabbit heterophil leucocytes. J Exp Med. 1970;131:559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy W, Takase M, Yamauchi K, Wakabayshi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- Bhimani RS, Vendrov Y, Furmanski P. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J Appl Microb. 1999;86:135–144. doi: 10.1046/j.1365-2672.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- Dalmastri C, Valenti P, Vittoriosso P, Orsi N. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microb. 1988;11:225–230. [PubMed] [Google Scholar]
- Diarra MS, Peticlerc D, Lacasse P. Effect of lacto-ferrin in combination with penicillin on the morphology and the physiology of Staphylococcus aureus isolated from bovine mastitis. J Dairy Sci. 2002;85:1141–1149. doi: 10.3168/jds.S0022-0302(02)74176-3. [DOI] [PubMed] [Google Scholar]
- Dionysius DA, Grieve PA, Milne JM. Forms of lactoferrin: their antibacterial effect on enterotoxigenic Escherichia coli. J Dairy Sci. 1993;76:2597–2606. doi: 10.3168/jds.S0022-0302(93)77594-3. [DOI] [PubMed] [Google Scholar]
- Ellison RT, III, Giehl TJ, LaForse FM. Damage of outer membrane of enteric gram negative bacteria by lactoferrin and transferrin. Infect Immun. 1988;56:2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison RT, III, LaForse FM, Giehl TJ, Boose DS, Dunn BE. Lactoferrin and transferrin damage of the gram negative outer membare is modulated by Ca2+ and Mg2+ J Gen Microbiol. 1990;136:1437–1446. doi: 10.1099/00221287-136-7-1437. [DOI] [PubMed] [Google Scholar]
- Harmon RJ, Schanbacher FL, Ferguson LC, Smith KL. Changes in lactoferrin, immunoglubulin G, bovine serum albumin, and alpha lactalbumin during acute experimental and natural coliform mastitis of cows. Infect Immun. 1976;13:533–542. doi: 10.1128/iai.13.2.533-542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomäki R. Licenciate thesis. University of Oulu; 1999. Separation of whey antimicrobial proteins and development of bovine lactoferrin immunoassays; p. 89. [Google Scholar]
- Kawai K, Hagiwara S, Anri A, Nagahata H. Lactoferrin concentration in milk of bovine clinical mastitis. Vet Res Commun. 1999;23:391–398. doi: 10.1023/A:1006347423426. [DOI] [PubMed] [Google Scholar]
- Lohuis JACM, Hensen SM, Beers H. Effect of lactoferrin and cephapirin, alone and in combination on growth of E. coli strains from mastitis. 3rd Int Mast Sem Proc. 1995;II:110–111. [Google Scholar]
- Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Ann Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- Maisi P, Mattila T, Sandholm M. Mastitis whey – a good medium for bacteria? Acta vet Scand. 1984;25:297–308. doi: 10.1186/BF03547272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson PL, Heremans JF, Dive C. An iron-binding protein common to many external secretions. Clin Chim Acta. 1966;14:735–739. doi: 10.1016/0009-8981(66)90004-0. [DOI] [Google Scholar]
- Masson PL, Heremans JF, Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leucocytes. J Exp Med. 1969;130:643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnecke BJ, Smith KL. Inhibition of mastitic bacteria by bovine milk apo-lactoferrin evaluated by in vitro microassay of bacterial growth. J Dairy Sci. 1984;67:606–613. doi: 10.3168/jds.S0022-0302(84)81345-4. [DOI] [PubMed] [Google Scholar]
- Oliver SP, Bushe T. Growth inhibition of Escherichia coli and Klebsiella pneumoniae during involution of the bovine mammary gland: relation to secretion composition. Am J Vet Res. 1987;48:1669–1973. [PubMed] [Google Scholar]
- Rainard P. Bacteriostatic activity of bovine milk lactoferrin against mastitic bacteria. Vet Microb. 1986;11:387–392. doi: 10.1016/0378-1135(86)90068-4. [DOI] [PubMed] [Google Scholar]
- Reiter B, Oram JD. Bacterial inhibitors in milk and other biological fluids. Nature (Lond) 1967;216:328–330. doi: 10.1038/216328a0. [DOI] [PubMed] [Google Scholar]
- Sanchez MS, Watts J. Enhancement of the activity of novobiocin against Escerichia coli by lactoferrin. J Dairy Sci. 1999;82:494–499. doi: 10.3168/jds.S0022-0302(99)75259-8. [DOI] [PubMed] [Google Scholar]
- Schanbacher FL, Talhouk RS, Murray FA. Biology and origin of bioactive peptides in milk. Livestock Prod Sci. 1997;50:105–123. doi: 10.1016/S0301-6226(97)00082-1. [DOI] [Google Scholar]
- Weinberg ED. Iron and infection. Mikrob Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty FK, Smith KL, Schanbacher FL. Lactoferrin concentration during involution of the bovine mammary gland. J Dairy Sci. 1976;59:224–231. doi: 10.3168/jds.s0022-0302(76)84188-4. [DOI] [PubMed] [Google Scholar]
- Zhang G-H, Mann DM, Tsai C-M. Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derived peptide. Infect Immun. 1999;67:1353–1358. doi: 10.1128/iai.67.3.1353-1358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


