Abstract
BACKGROUND
Subacute combined degeneration is an acquired myelopathy caused by vitamin B12 deficiency. Therapy with B12 leads to improvement in most but to complete recovery in only a few patients. Prognostic indicators in subacute combined degeneration are unknown; therefore, predicting complete recovery of neurologic deficits is challenging.
PURPOSE
To identify potential correlates of outcome and to generate hypotheses concerning predictors of complete resolution of neurologic deficits in subacute combined degeneration.
DATA SOURCE
We searched EMBASE (1974 to October 2005), MEDLINE (1968 to October 2005), and references from identified reports.
REPORTS SELECTION
Reports of patients with subacute combined degeneration containing results of magnetic resonance imaging (MRI) and description of outcome and 1 patient treated by the authors.
DATA EXTRACTION, SYNTHESIS
We extracted data from 45 reports and 57 patients (36 males, 21 females; age range: 10 to 81) with a diagnosis of subacute combined degeneration, and estimated the strength of association between clinical, laboratory, and radiological factors and complete resolution of signs and symptoms.
RESULTS
Eight patients (14%) achieved clinical resolution and 49 (86%) improved with B12 therapy. The absence of sensory dermatomal deficit, Romberg, and Babinski signs were associated with a higher complete resolution rate. Patients with MRI lesions in ≤7 segments and age less than 50 also appear to have higher rates of complete resolution.
CONCLUSIONS
B12 therapy is reported to stop progression and improve neurologic deficits in most patients with subacute combined degeneration. However, complete resolution only occurs in a small percentage of patients and appears to be associated with factors suggestive of less severe disease at the time of diagnosis.
Keywords: subacute combined degeneration, SCD, nitrous oxide, cobalamin, vitamin B12, combined system degeneration
Vitamin B12 deficiency can lead to serious neurologic complications including peripheral neuropathy, bilateral cerebral dysfunction, optic neuropathy, memory loss, personality changes, impaired recall, and subacute combined degeneration of the spinal cord.1,2 Subacute combined degeneration is a rapidly progressive myelopathy that can be associated with profound neurologic deficits including progressive sensory abnormalities, ascending paresthesias, weakness, ataxia, loss of sphincter control, and gait impairment.1–7 In some patients with vitamin B12 deficiency, subacute combined degeneration can be triggered by brief exposure to nitrous oxide during anesthesia8–10 and in others after prolonged recreational use of the drug.11 Pathologically, the disease is characterized by astrogliosis, normal oligodendroglia, and normal neurons.12 Subacute combined degeneration used to be a common devastating disease but the elucidation of its etiology has led to therapeutic and prophylactic use of B12, which likely has decreased its prevalence.13–15
It is surprising, however, that despite improvement in diagnostic tools, such as magnetic resonance imaging (MRI), and effective therapy, data on prognostic indicators and predictors of complete resolution of neurologic deficits in subacute combined degeneration are sorely lacking. When treating the disease, physicians are unable to predict whether a patient will have complete resolution or only improvement of the disabling neurologic deficits. Therefore, in this study, we sought to identify clinical, laboratory, and radiological factors present at the time of diagnosis that could potentially correlate with resolution of neurologic deficits in patients with subacute combined degeneration.
METHODS
Data Source
We searched EMBASE (1974 to October 2005) and MEDLINE (1968 to October 2005), scanned references from all included reports using MeSH headings (vitamin B12 deficiency, pernicious anemia, nitrous oxide), subheadings (complications, pathology, diagnosis, classification), and keywords (subacute combined degeneration and cobalamin). We also included data from a patient treated by the authors.
Reports Selection
We selected reports of patients with16 subacute combined degeneration that described results of MRI and patient's outcome after B12 therapy. We included patients who met the following criteria16: (1) clinical picture of myelopathy, i.e., anatomical localization of neurological findings to the spinal cord confirmed by MRI studies, (2) evidence of vitamin B12 deficiency, and (3) absence of identifiable pathology of the central or peripheral nervous system that could account for the clinical picture. Patients with preexistent or concomitant neurologic pathology (multiple sclerosis, tumors) described in the history or suggested by MRI were excluded.
Data Extraction
We extracted demographic, clinical, laboratory, and radiologic data. Potential predictive factors (variable) and outcome (complete resolution or improvement only) were reviewed independently by 2 neurologists (O.M.V. and E.H.P.) using preformatted coded case report forms. Discrepancies between data fields occurred in 2 instances and were related to reports written in a foreign language. These discrepancies were addressed with a translator and joint review of the manuscripts. As all reports identified in the literature only described patients who improved with B12 therapy, clinical outcomes were defined as improvement (arrest of disease progression followed by improvement but persistence of neurologic deficits) or complete resolution (arrest of disease progression followed by complete resolution of neurologic deficits). We evaluated the quality of the reports by assessing the accuracy of diagnosis, appropriateness of therapy, and report of clinical, laboratory, and radiological variables, and clinical outcome.
Data Synthesis and Analysis
Data from 57 patients were used to estimate the strength of association between 18 clinical, laboratory, and radiologic factors (Table 1) and resolution of signs and symptoms in patients with subacute combined degeneration. We calculated rates of complete resolution for patients with and without each of the study factors, complete clinical resolution rate differences between patients with and without factors, and their 95% confidence limits. The strength of the association is indicated by the magnitude of the difference in complete resolution rates between patients with and without a given factor. Whether the association is statistically different from 0 is indicated by whether the 95% confidence interval includes 0 or not. Statistical analyses were conducted using MIX version 1.1 software.17
Table 1.
Complete Clinical Resolution Rate of Signs and Symptoms in Patients with Subacute Combined Degeneration by Clinical, Laboratory, and Radiological Findings Identified at the Time of Diagnosis
| Findings Identified at Time of Diagnosis | Complete Clinical Resolution n/N (%)* | Difference (%) | 95% Confidence Interval of Difference |
|---|---|---|---|
| Clinical characteristics and physical findings | |||
| Gender | |||
| Male | 8/36 (22.2) | 22.2 | 8.6 to 35.8 |
| Female | 0/21 (0.0) | ||
| Age | |||
| Less than 50 y | 5/18 (27.8) | 20.1 | −2.2 to 42.4 |
| More than 50 y | 3/39 (7.6) | ||
| Etiology | |||
| Vegetarian | 1/3 (33.3) | 20.3 | −34 to 74.2 |
| Other | 7/54(13) | ||
| Lhermitte sign | |||
| Absent | 0/3 (0.0) | 21.4 | 0.0 to 42.9 |
| Present | 3/14 (21.4) | ||
| Sensory level | |||
| Normal | 1/1 (100) | 91.7 | 80.6 to 103.7 |
| Abnormal | 2/24 (8.3) | ||
| Romberg sign | |||
| Absent | 2/2 (100) | 91.4 | 82.2 to 100.7 |
| Present | 3/35 (8.6) | ||
| Babinski sign | |||
| Absent | 5/15 (33.3) | 29.3 | 4.3 to 54.4 |
| Present | 1/25 (4) | ||
| Laboratory findings | |||
| Anemia | |||
| No | 2/6 (33.3) | 21.7 | −17.2 to 60.6 |
| Yes | 5/43 (11.6) | ||
| Macrocytosis | |||
| No | 1/6 (16.7) | 4.2 | −27.1 to 35.4 |
| Yes | 6/48 (12.5) | ||
| Schilling test | |||
| Normal | 0/2 (0.0) | 18.2 | 2.1 to 34.3 |
| Abnormal | 4/22 (18.2) | ||
| Vitamin B12 levels | |||
| Normal | 1/4 (25) | 11.3 | −32.2 to 54.7 |
| Decreased | 7/51 (13.7) | ||
| Antiparietal antibody | |||
| Negative | 0/7 (0.0) | 7.7 | −6.8 to 22.2 |
| Positive | 1/13 (7.7) | ||
| Anti-intrinsic factor antibody | |||
| Negative | 1/8 (12.5) | 7.5 | −23.1 to 38.1 |
| Positive | 3/15 (20) | ||
| Gastric atrophy | |||
| No | 0/3 (0.0) | 5.3 | −4.8 to 15.3 |
| Yes | 1/19 (5.3) | ||
| MRI findings | |||
| Spinal cord atrophy | |||
| Absent | 2/7 (28.6) | 28.6 | −4.9 to 62 |
| Present | 0/2(0.0) | ||
| Spinal cord abnormality | |||
| In ≤7 segments | 7/29 (24.1) | 19.9 | 2.4 to 37.5 |
| In >7 segments | 1/24 (4.2) | ||
| Spinal cord edema | |||
| Absent | 3/13 (23) | 14.5 | −26.2 to 55 |
| Present | 3/8 (37.5) | ||
| MRI contrast enhancement | |||
| Absent | 3/20 (15) | 13.6 | −23.4 to 50.5 |
| Present | 2/7 (28.6) | ||
N represents the number of patients reported to have the findings as described at the time of diagnosis and n, the number of patients who had those findings and achieved complete resolution of signs and symptoms after treatment with B12. Anemia was defined as a hemoglobin level below and macrocytosis as a mean corpuscular volume above the normal reference range of the reporting institution. The strength of association between a given finding and complete clinical resolution is indicated by the magnitude of the difference in complete resolution rates between patients with and without a given factor.
RESULTS
Literature Search
We identified 239 potentially relevant reports and eliminated 194 lacking case studies, confirmed diagnosis, patient outcome, or MRI data. Although we searched the literature since 1968, the earliest report containing MRI data was from 1991.18 Forty-five reports described 57 patients with subacute combined degeneration who met inclusion criteria.9–11,18–59
Patient Treated by the Authors
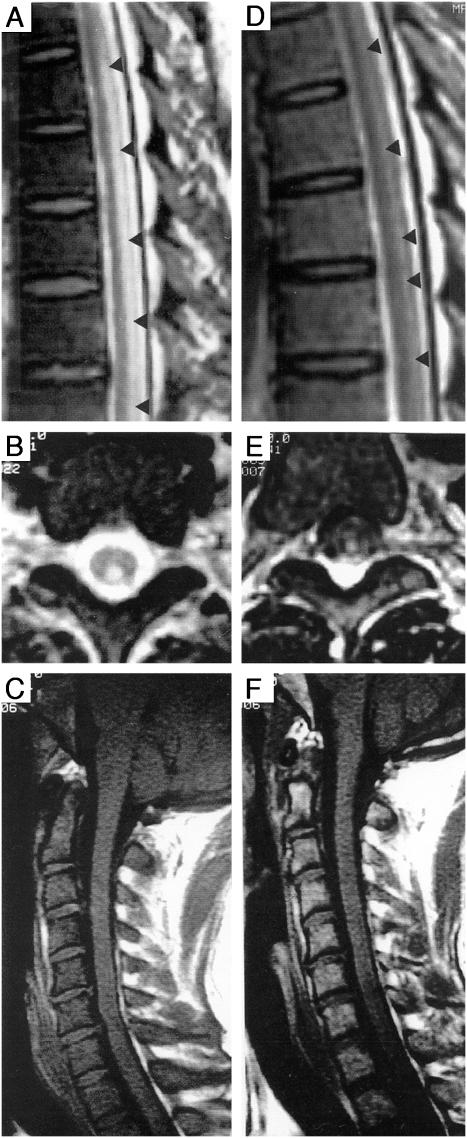
A 36-year-old male developed paresthesias and gait disturbance after general anesthesia including nitrous oxide. Symptoms and signs included leg weakness, erectile dysfunction, impaired bowel and bladder control, spastic quadriparesis, diffuse hypereflexia, extensor plantar responses, ataxic-paretic gait, Romberg sign, proprioceptive and vibratory sensory loss in the legs, and sensory deficit to T5. Laboratory studies showed macrocytic anemia and low serum B12. Spine MRI showed increased T2-weighted signal intensity of posterior columns and low T1-weighted signal intensity in the bone marrow (Fig. 1, available online, A, B, and E). Treatment with B12 yielded clinical and MRI improvement (Fig. 1, C, D, and F) and at 21-month follow-up, distal sensory deficits persisted and spine MRI showed residual patches of high T2-weighted signal intensity of posterior columns.
FIGURE 1.
Admission sagittal (A) and axial (B) spine MR sequences of a 36-year-old male showing an increased T2-weighted cord signal involving the posterior columns. Follow-up scan obtained 4 months after initiation of therapy showing significant resolution of the signal changes, correlating with clinical improvement (C) and (D). T1-weighted sequences on admission revealed a low signal in the bone marrow of the vertebral bodies (E). Rescue of bone marrow signal intensity was seen after 4 months of therapy (F).
Clinical, Laboratory, and Radiologic Profile
The mean age of patients who had complete resolution of signs and symptoms after B12 therapy was 39.8±18.8 and that of patients who only improved was 55.9±15.5 years (mean±SD). The median duration of illness at the time of diagnosis was 6 weeks (range 2 to 24 weeks) in patients who had complete resolution and 12 (range 1 to 84 weeks) in patients who only had improvement with B12 therapy. Of 57 patients, 43 (75%) had pernicious anemia, 3 were vegans (5%), and 10 (17.5%) had other identifiable risk factors for B12 deficiency. Only 1 patient had no reported risk factor for B12 deficiency. In 15 patients, exposure to nitrous oxide preceded the development of subacute combined degeneration (during surgical procedures or chronic recreational use). Reported signs and symptoms included symmetric ascending paresthesias, sensory loss, muscle weakness, ataxia, and gait abnormalities. Sensory deficit in a stocking glove distribution pattern and sharp spinal level (from C2 to T11) were reported. Tendon reflex abnormalities were ubiquitous and ranged from generalized hypereflexia with clonus to absent reflexes. Dissociation between upper (hyperactive) and lower (decreased or absent) extremity reflexes was reported frequently. In lower limbs, coexistence of hyperactive knee responses and hypoactive or absent ankle jerks was commonly observed. Sphincter dysfunction was present in a few patients. Of 57 patients, over 85% had anemia, macrocytosis, and decreased vitamin B12 levels (data not shown). On MRI, several patterns were described: focal or small cord lesions involving few contiguous levels (≤7 segments, N = 29); extensive linear abnormalities (≥8 segments, n = 22), and scattered small multi-focal lesions (N = 2). Increased T2-weighted signal intensity in dorsal columns was the most common finding.
After initiation of B12 therapy, all patients reported in the literature showed, at a minimum, arrest of progression of neurologic deficits. Of 57 patients included in the analysis, 49 (86%) improved and 8 (14%) had complete resolution of signs and symptoms. Resolution of radiological abnormalities on follow-up scans occurred in 5 of 8 subjects who had complete resolution of deficits. Conversely, in a few patients, follow-up MRI normalized despite persistence of neurologic deficits (paresthesias and sensory loss). In 2 subjects, clinical resolution preceded normalization of spine MRI findings. In these 2 cases, follow-up scan was obtained 12 to 24 weeks after the first study. In 2 subjects with segmental atrophy of the thoracic spinal cord, B12 therapy led to symptomatic improvement but no appreciable functional gain. Among 10 patients with extensive linear cord injury (≥20 segments), only 1 (10-year-old male) had complete resolution of deficits after B12 therapy.
Possible Correlates of Outcome
Possible correlates of outcome in patients with subacute combined degeneration and their respective magnitude of association are shown in Table 1. As indicated by the magnitude of differences in clinical resolution rates, factors showing a strong association with complete resolution of signs and symptoms include absence of sensory level, Romberg and Babinski sign, and spinal cord atrophy. In addition, the male gender, absence of anemia, presence of Lhermitte's sign, and age <50 years were also strongly associated with complete resolution of signs and symptoms in subacute combined degeneration.
DISCUSSION
While several medical specialties are likely to evaluate patients with subacute combined degeneration, little information on predictors of outcome is available.1,2,60–62 In this investigation, we used published data to generate hypotheses regarding potential correlates of outcomes and found that clinical, laboratory, and MRI findings at the time of diagnosis may correlate with complete resolution of signs and symptoms in subacute combined degeneration. However, the fact that we found no reports of patients with subacute combined degeneration who failed to improve after therapy limits our conclusions to only those patients who respond to B12 therapy. We acknowledge that patients who do not respond to therapy, and have not been reported in the literature, may have similar characteristics.
Our findings suggest that absence of sensory level, Romberg and Babinski signs may be associated with clinical resolution of signs and symptoms in patients with subacute combined degeneration. It is known that the presence of sensory level reflects spinal cord damage that interrupts transmission of sensory impulses to higher centers in the central nervous system, Romberg sign reflects blockade of deep sensory impulses from lower extremities and severe compromise of heavily myelinated sensory axons along the posterior columns, and that Babinski sign reflects damage to corticospinal tract fibers traveling in the lateral funiculi of the spinal cord. Therefore, it is conceivable that patients without sensory level, Romberg, and Babinski signs have less severe disease and therefore a higher likelihood of achieving complete resolution of neurologic deficits with B12 therapy.
Our study suggests that age <50 years is associated with a higher complete resolution rate of signs and symptoms in subacute combined degeneration. This is not surprising as younger patients have greater nervous system plasticity. The report of a 10-year-old who recovered despite extensive spinal cord involvement further supports this hypothesis.23 Also, at the time of diagnosis, patients who had complete resolution of signs and symptoms with B12 therapy had a shorter duration of illness compared with those who only improved. Therefore, our findings support the notion that younger age and, as previously suggested by others,3 shorter duration of illness positively impact on the likelihood of reaching complete resolution with B12 in subacute combined degeneration.
The reports indicate that subacute combined degeneration is associated with heterogeneous patterns on MRI, suggesting that the disease may be a continuum—focal areas of demyelination gradually coalesce into larger spinal cord lesions as described in neuropathologic studies.12 We found that involvement of 7 or less spinal cord segments, presence of spinal cord edema, and/or contrast enhancement, but not cord atrophy, were associated with higher resolution rates in patients with subacute combined degeneration. These findings support the notion that patients with less severe spinal cord involvement on MRI are likely to have less severe disease and therefore higher resolution rates after B12 treatment. Another interesting observation was that in a few patients, resolution of clinical signs and symptoms preceded normalization of MRI abnormalities. This suggests that, in the absence of axonal injury, clinical resolution after B12 may precede normalization of increased T2-weighted signal on MRI, which reflects edema of myelin sheaths. Overall, our study raises the possibility that MRI findings at the time of diagnosis could be potential correlates of outcome and have a prognostic value in subacute combined degeneration.
While the majority of patients in our study had pernicious anemia, our findings suggest that the absence of anemia was associated with higher rates of resolution of neurologic deficits after B12 therapy. This finding was surprising as the severity of hematological changes in subjects with pernicious anemia does not appear to correlate with neurological complications.2,60 Also, among the factors analyzed, laboratory markers of pernicious anemia had weaker associations with resolution of signs and symptoms. In addition, all patients reported with subacute combined degeneration had some improvement with B12 therapy despite variability of regimens. These findings, along with MRI and the clinical factors described above, suggest that lesser disease severity and duration, rather than etiology, is associated with complete resolution of signs and symptoms of subacute combined degeneration.
Of interest, 15 of 57 patients included in this study developed subacute combine degeneration after exposure to nitrous oxide either during a general anesthetic or after chronic use as a recreational drug. It is known that nitrous oxide affects vitamin B12 metabolism by oxidizing its reduced form. In turn, as the reduced form of B12, a coenzyme for methionine synthase, is oxidized, methionine synthase is inhibited by deprivation of the reduced form of B12. Consequently, the transmethylation of homocysteine to methionine catalyzed by methionine synthase is impaired, which in turn can ultimately impact on DNA, myelin, and catecholamine synthesis.63 Given that recreational use of nitrous oxide appears to be gaining popularity, recognition that exposure to the drug can lead to subacute combined degeneration is important. In addition, in patients with newly diagnosed myelopathy, the possibility of nitrous oxide abuse should be explored as cessation of exposure is necessary for successful therapy.63
Although our study generates valuable information that should be further evaluated in future studies, it has limitations. They include its retrospective nature, uncontrollable variability of diagnostic criteria and treatment regimens, number of treating centers, and lack of standardized tools to measure outcomes and standard follow-ups. While informative and the first to suggest that clinical and radiological findings at the time of diagnosis can potentially correlate with resolution of neurologic deficits, our study simply generates hypotheses about the biology of subacute combined degeneration that warrant further testing. Nevertheless, our findings suggest that young patients with less severe neurologic deficits, less extensive spinal cord lesions on MRI, who are treated earlier in the course of the disease, are likely to have higher resolution rates of signs and symptoms caused by subacute combined degeneration.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, NIH Clinical Center, Department of Anesthesia and Surgical Services.
REFERENCES
- 1.Healton EB, Savage DG, Brust JC, Garrett TJ, Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore) 1991;70:229–45. doi: 10.1097/00005792-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood. 1990;76:871–81. [PubMed] [Google Scholar]
- 3.Shevell MI, Rosenblatt DS. The neurology of cobalamin. Can J Neurol Sci. 1992;19:472–86. [PubMed] [Google Scholar]
- 4.Licht DJ, Berry GT, Brooks DG, Younkin DP. Reversible subacute combined degeneration of the spinal cord in a 14-year-old due to a strict vegan diet. Clin Pediatr (Philadelphia) 2001;40:413–5. doi: 10.1177/000992280104000710. [DOI] [PubMed] [Google Scholar]
- 5.Scalabrino G. Subacute combined degeneration one century later. The neurotrophic action of cobalamin (vitamin B12) revisited. J Neuropathol Exp Neurol. 2001;60:109–20. doi: 10.1093/jnen/60.2.109. [DOI] [PubMed] [Google Scholar]
- 6.Stabler SP, Allen RH. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr. 2004;24:299–326. doi: 10.1146/annurev.nutr.24.012003.132440. [DOI] [PubMed] [Google Scholar]
- 7.Scalabrino G. Cobalamin (vitamin B(12)) in subacute combined degeneration and beyond: traditional interpretations and novel theories. Exp Neurol. 2005;192:463–79. doi: 10.1016/j.expneurol.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Kinsella LJ, Green R. ‘Anesthesia paresthetica’: nitrous oxide-induced cobalamin deficiency. Neurology. 1995;45:1608–10. doi: 10.1212/wnl.45.8.1608. [DOI] [PubMed] [Google Scholar]
- 9.Pant SS, Asbury AK, Richardson EP., Jr The myelopathy of pernicious anemia. A neuropathological reappraisal. Acta Neurol Scand. 1968;44(suppl 5):1–36. [PubMed] [Google Scholar]
- 10.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol. 1990;34:90–8. doi: 10.1002/ajh.2830340204. [DOI] [PubMed] [Google Scholar]
- 11.Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol. 1990;34:99–107. doi: 10.1002/ajh.2830340205. [DOI] [PubMed] [Google Scholar]
- 12.Naurath HJ, Joosten E, Riezler R, Stabler SP, Allen RH, Lindenbaum J. Effects of vitamin B12, folate, and vitamin B6 supplements in elderly people with normal serum vitamin concentrations. Lancet. 1995;346:85–9. doi: 10.1016/s0140-6736(95)92113-3. [DOI] [PubMed] [Google Scholar]
- 13.Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318:1720–8. doi: 10.1056/NEJM198806303182604. [DOI] [PubMed] [Google Scholar]
- 14.Bax L. MIX: Meta-analysis with Interactive eXplanations. 1.1 ed; 2005. Available at http://www.mix-for-meta-analysis.info/index.html.
- 15.Savage DG, Lindenbaum J. Neurological complications of acquired cobalamin deficiency: clinical aspects. Baillieres Clin Haematol. 1995;8:657–78. doi: 10.1016/s0950-3536(05)80225-2. [DOI] [PubMed] [Google Scholar]
- 16.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159:1289–98. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 17.Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197–204. [PubMed] [Google Scholar]
- 18.Weimann J. Toxicity of nitrous oxide. Best Pract Res Clin Anaesthesiol. 2003;17:47–61. doi: 10.1053/bean.2002.0264. [DOI] [PubMed] [Google Scholar]
- 19.Marie RM, Le Biez E, Busson P, et al. Nitrous oxide anesthesia-associated myelopathy. Arch Neurol. 2000;57:380–2. doi: 10.1001/archneur.57.3.380. [DOI] [PubMed] [Google Scholar]
- 20.Sesso RM, Iunes Y, Melo AC. Myeloneuropathy following nitrous oxide anesthaesia in a patient with macrocytic anaemia. Neuroradiology. 1999;41:588–90. doi: 10.1007/s002340050812. [DOI] [PubMed] [Google Scholar]
- 21.Pema PJ, Horak HA, Wyatt RH. Myelopathy caused by nitrous oxide toxicity. Am J Neuroradiol. 1998;19:894–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Berger JR, Quencer R. Reversible myelopathy with pernicious anemia: clinical/MR correlation. Neurology. 1991;41:947–8. doi: 10.1212/wnl.41.6.947. [DOI] [PubMed] [Google Scholar]
- 23.Tracey JP, Schiffman FJ. Magnetic resonance imaging in cobalamin deficiency. Lancet. 1992;339:1172–3. doi: 10.1016/0140-6736(92)90773-v. [DOI] [PubMed] [Google Scholar]
- 24.Timms SR, Cure JK, Kurent JE. Subacute combined degeneration of the spinal cord: MR findings. Am J Neuroradiol. 1993;14:1224–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Murata S, Naritomi H, Sawada T. MRI in subacute combined degeneration. Neuroradiology. 1994;36:408–9. doi: 10.1007/BF00612130. [DOI] [PubMed] [Google Scholar]
- 26.Tajima Y, Mito Y, Owada Y, Moriwaka F, Tashiro K. MR appearance of subacute combined degeneration of the spinal cord. Jpn J Psychiatry Neurol. 1994;48:611–4. doi: 10.1111/j.1440-1819.1994.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolansky LJ, Goldstein G, Gozo A, Lee HJ, Sills I, Chatkupt S. Subacute combined degeneration of the spinal cord: MRI detection of preferential involvement of the posterior columns in a child. Pediatr Radiol. 1995;25:140–1. doi: 10.1007/BF02010329. [DOI] [PubMed] [Google Scholar]
- 28.Rosener M, Dichgans J. Severe combined degeneration of the spinal cord after nitrous oxide anaesthesia in a vegetarian. J Neurol Neurosurg Psychiatry. 1996;60:354. doi: 10.1136/jnnp.60.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duprez TP, Gille M, Vande Berg BC, et al. MRI of the spine in cobalamin deficiency: the value of examining both spinal cord and bone marrow. Neuroradiology. 1996;38:511–5. doi: 10.1007/BF00626083. [DOI] [PubMed] [Google Scholar]
- 30.Larner AJ, Zeman AZ, Allen CM, Antoun NM. MRI appearances in subacute combined degeneration of the spinal cord due to vitamin B12 deficiency. J Neurol Neurosurg Psychiatry. 1997;62:99–100. doi: 10.1136/jnnp.62.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng VW, Gross M, Clifton A. Case report: MR appearances in vitamin B12 neuropathy. Clin Radiol. 1997;52:394–6. doi: 10.1016/s0009-9260(97)80138-9. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Pena F, Repetto JA, Contreras A, Gomez-Cardenas E, Garcia-Vazquez F. Nuclear magnetic resonance in subacute combined degeneration of the spinal cord. A case report. Rev Neurol. 1997;25:2077–8. [PubMed] [Google Scholar]
- 33.Felten D, Naccache L, Sarrazin J, Lapeyre E, Taillia H, Renard J. Pernicious anemia and subacute combined degeneration of the spinal cord: MR findings. Neurology. 1998;50(4 suppl 4):120. [Google Scholar]
- 34.Beltramello A, Puppini G, Cerini R, et al. Subacute combined degeneration of the spinal cord after nitrous oxide anaesthesia: role of magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 1998;64:563–4. doi: 10.1136/jnnp.64.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada K, Shrier DA, Tanaka H, Numaguchi Y. A case of subacute combined degeneration: MRI findings. Neuroradiology. 1998;40:398–400. doi: 10.1007/s002340050610. [DOI] [PubMed] [Google Scholar]
- 36.Giron JM, Munoz A, Caro P, Rodriguez F, Vila MJ. Anestesia parestesica: aportacion de un nuevo caso y estudio evolutivo por resonancia magnetica. Neurologia. 1998;13:307–10. [PubMed] [Google Scholar]
- 37.Karacostas D, Artemis N, Bairactaris C, Tsitourides I, Milonas I. Cobalamin deficiency: MRI detection of posterior columns involvement and posttreatment resolution. J Neuroimaging. 1998;8:171–3. doi: 10.1111/jon199883171. [DOI] [PubMed] [Google Scholar]
- 38.Katsaros VK, Glocker FX, Hemmer B, Schumacher M. MRI of spinal cord and brain lesions in subacute combined degeneration. Neuroradiology. 1998;40:716–9. doi: 10.1007/s002340050670. [DOI] [PubMed] [Google Scholar]
- 39.Hemmer B, Glocker FX, Schumacher M, Deuschl G, Lucking CH. Subacute combined degeneration: clinical, electrophysiological, and magnetic resonance imaging findings. J Neurol Neurosurg Psychiatry. 1998;65:822–7. doi: 10.1136/jnnp.65.6.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousry TA, Strupp M, Bruning R. Common variable immunodeficiency leading to spinal subacute combined degeneration monitored by MRI. J Neurol Neurosurg Psychiatry. 1998;64:663–6. doi: 10.1136/jnnp.64.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma S, Khilnani GC, Berry M. Case of the season. Megaloblastic anemia with subacute combined degeneration (SCD) of the spinal cord. Semin Roentgenol. 1999;34:2–4. doi: 10.1016/s0037-198x(99)80014-5. [DOI] [PubMed] [Google Scholar]
- 42.Bassi SS, Bulundwe KK, Greeff GP, Labuscagne JH, Gledhill RF. MRI of the spinal cord in myelopathy complicating vitamin B12 deficiency: two additional cases and a review of the literature. Neuroradiology. 1999;41:271–4. doi: 10.1007/s002340050746. [DOI] [PubMed] [Google Scholar]
- 43.Locatelli ER, Laureno R, Ballard P, Mark AS. MRI in vitamin B12 deficiency myelopathy. Can J Neurol Sci. 1999;26:60–3. [PubMed] [Google Scholar]
- 44.Kuker W, Hesselmann V, Thron A, de Simone A. MRI demonstration of reversible impairment of the blood-CNS barrier function in subacute combined degeneration of the spinal cord. J Neurol Neurosurg Psychiatry. 1997;62:298–9. doi: 10.1136/jnnp.62.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warmuth-Metz M, Naumann M, Solymosi L. MRT bei funikularer Myelose. Rofo: Fortschr Geb Rontgenstr Nuklearmed. 1999;170:591–3. doi: 10.1055/s-2007-1011097. [DOI] [PubMed] [Google Scholar]
- 46.Karantanas AH, Markonis A, Bisbiyiannis G. Subacute combined degeneration of the spinal cord with involvement of the anterior columns: a new MRI finding. Neuroradiology. 2000;42:115–7. doi: 10.1007/s002340050027. [DOI] [PubMed] [Google Scholar]
- 47.Yu MK, Rodgers GM. Detection of occult cobalamin deficiency by magnetic resonance imaging. Am J Hematol. 2000;65:83–4. doi: 10.1002/1096-8652(200009)65:1<83::aid-ajh16>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 48.Ravina B, Loevner LA, Bank W. MR findings in subacute combined degeneration of the spinal cord: a case of reversible cervical myelopathy. Am J Roentgenol. 2000;174:863–5. doi: 10.2214/ajr.174.3.1740863. [DOI] [PubMed] [Google Scholar]
- 49.Coignard P, Lemesle M, Madinier G, et al. Interet de l'imagerie par resonance magnetique dans la sclerose combinee de la moelle par carence en vitamine B12. Rev Neurol (Paris) 2000;156:1000–4. [PubMed] [Google Scholar]
- 50.Jongen JC, Koehler PJ, Franke CL. Gecombineerde strengziekte door vitamine-B12-deficientie: eenvoudige diagnose, effectieve therapie. Ned Tijdschr Geneeskd. 2001;145:1229–33. [PubMed] [Google Scholar]
- 51.Cornejo W, Gonzalez F, Toro ME, Cabrera D. Degeneracion combinada subaguda. Descripcion de un caso en un nino vegetariano estricto. Rev Neurol. 2001;33:1154–7. [PubMed] [Google Scholar]
- 52.Ilniczky S, Jelencsik I, Kenez J, Szirmai I. MR findings in subacute combined degeneration of the spinal cord caused by nitrous oxide anaesthesia—two cases. Eur J Neurol. 2002;9:101–4. doi: 10.1046/j.1468-1331.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- 53.Beauchet O, Exbrayat V, Navez G, Blanchon MA, Quang BL, Gonthier R. Sclerose combinee medullaire revelatrice d'une carence en vitamine B12: particularites geriatriques a propos d'un cas evalue par imagerie par resonance magnetique nucleaire. Rev Med Intern. 2002;23:322–7. doi: 10.1016/s0248-8663(01)00558-6. [DOI] [PubMed] [Google Scholar]
- 54.Pittock SJ, Payne TA, Harper CM. Reversible myelopathy in a 34-year-old man with vitamin B12 deficiency. Mayo Clin Proc. 2002;77:291–4. doi: 10.4065/77.3.291. [DOI] [PubMed] [Google Scholar]
- 55.Srikanth SG, Jayakumar PN, Vasudev MK, Taly AB, Chandrashekar HS. MRI in subacute combined degeneration of spinal cord: a case report and review of literature. Neurol India. 2002;50:310–2. [PubMed] [Google Scholar]
- 56.Penas M, Blanco A, Villarejo A, Juntas R, Miranda P, Martinez A. Degeneracion combinada subaguda medular: hallazgos en la resonancia magnetica. Neurologia. 2002;17:447–8. [PubMed] [Google Scholar]
- 57.Krishna KK, Arafat AS, Ichaporia NR, Jain MM. MRI findings in cobalamin deficiency. J Clin Neurosci. 2003;10:84–5. doi: 10.1016/s0967-5868(02)00274-6. [DOI] [PubMed] [Google Scholar]
- 58.Fritschi J, Sturzenegger M. Spinal MRI supporting myelopathic origin of early symptoms in unsuspected cobalamin deficiency. Eur Neurol. 2003;49:146–50. doi: 10.1159/000069087. [DOI] [PubMed] [Google Scholar]
- 59.Berlit P, Ringelstein A, Liebig T. Spinal MRI precedes clinical improvement in subacute combined degeneration with B12 deficiency. Neurology. 2004;63:592. doi: 10.1212/01.wnl.0000133406.50055.41. [DOI] [PubMed] [Google Scholar]
- 60.Ahn SC, Brown AW. Cobalamin deficiency and subacute combined degeneration after nitrous oxide anesthesia: a case report. Arch Phys Med Rehabil. 2005;86:150–3. doi: 10.1016/j.apmr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Naidich MJ, Ho SU. Case 87: subacute combined degeneration. Radiology. 2005;237:101–5. doi: 10.1148/radiol.2371031757. [DOI] [PubMed] [Google Scholar]
- 62.Plotas AA, Stamoulis E, Kehagias D, Papadopoulos A, Lahanis SV. Subacute combined degeneration of the spinal cord. Eur Radiol. 2005;15:1775–8. [Google Scholar]
- 63.Morishita A, Tomita H, Takaishi Y, Nishihara M, Kohmura E. A case of subacute combined degeneration of the spinal cord diagnosed by characteristic findings of magnetic resonance imaging: case report and review of 22 cases. No Shinkei Geka. 2005;33:489–95. [PubMed] [Google Scholar]