Abstract
BACKGROUND
Major depression is common in older adults and is associated with increased health care costs. Depression often remains unrecognized in older adults, especially in primary care.
OBJECTIVE
To evaluate the cost-effectiveness of a disease management program for major depression in elderly primary care patients compared with usual care.
DESIGN
Economic evaluation alongside a cluster randomized-controlled trial.
PARTICIPANTS
Consecutive patients of 55 years and older were screened for depression using the Geriatric Depression Scale and the PRIME-MD was used for diagnosis.
INTERVENTIONS
General practitioners in the intervention group received training on how to implement the disease management program consisting of screening, patient education, drug therapy with paroxetine, and supportive contacts. General practitioners in the usual care group were blind to the screening results. Treatment in this group was not restricted in any way.
MEASUREMENTS
Severity of depression, recovery from depression, and quality of life. Resource use measured over a 12-month period using interviews and valued using standard costs.
RESULTS
Differences in clinical outcomes between the intervention and usual care group were small and statistically insignificant. Total costs were $2,123 in the intervention and $2,259 in the usual care group (mean difference −$136, 95% confidence interval: −$1,194; $1,110). Cost-effectiveness planes indicated that there were no statistically significant differences in cost-effectiveness between the 2 groups.
CONCLUSIONS
This disease management program for major depression in elderly primary care patients had no statistically significant relationship with clinical outcomes, costs, and cost-effectiveness. Therefore, based on these results, continuing usual care is recommended.
Keywords: depression, disease management program, elderly, primary care
The 1-month prevalence of major depression in elderly people ranges from 2.0% in the community to 8.7% in primary care.1,2 Major depression in the elderly is associated with increased physical disability, impaired well-being, and increased health service use and health care costs.3–7 Treatment with antidepressants and psychotherapy has proven to be efficacious in older adults with major depression.8 Nevertheless, major depression often remains unrecognized and undertreated in elderly people, especially in primary care.9,10 Recognition and treatment is complicated by comorbid physical and psychiatric illnesses, life-stress, and social and financial problems.9,10 Furthermore, symptoms of depression may be attributed to aging by both the physician and the patient.11
Screening for depression in primary care may improve outcomes, particularly when screening is followed by adequate treatment.12 A recent review concluded that disease management programs can improve quality of care and outcomes for patients with depression and that research is needed to assess the cost-effectiveness of such programs.13
In this study, we evaluated the cost-effectiveness of a disease management program consisting of screening, diagnosis, and treatment of major depression in elderly primary care patients in comparison with usual care.
METHODS
Design and Setting
A cluster randomized-controlled trial was performed in 34 general practices in the Netherlands. Randomization took place at practice level. The Medical Ethical Committee of the VU University Medical Center in Amsterdam approved the study protocol. The design of the study has been described extensively.14
Patient Selection
In the participating general practices, all consecutive patients 55 years and older visiting their general practitioner (GP), were requested by the practice assistant to complete the Geriatric Depression Scale (GDS-15).15 In the intervention group, patients with a GDS-15 score of 5 or higher were further evaluated by their GP using the mood module of the PRIMary care Evaluation of Mental Disorders (PRIME-MD).16 In the usual care group, the PRIME-MD was administered by a research assistant in order to keep the GP blind to the screening results. Patients who were diagnosed with major depression according to the PRIME-MD were eligible for the study. Exclusion criteria were as follows: current use of antidepressants, current psychosis, bipolar disorder, or alcohol or drugs abuse, severe social dysfunction, inability to communicate in Dutch, and impaired cognitive functioning. General practitioners of intervention patients had to agree with the diagnosis of major depression and to be willing to prescribe an antidepressant. Written informed consent was obtained from the patients both after screening and after the diagnostic interview.
Usual Care Treatment
General practitioners in the usual care group remained blind to the results of the screening process and did not receive any training. Treatment of depression in the usual care group depended on whether the GP recognized the patient as being depressed and was not restricted in any way. Dutch GPs are encouraged to work according to the depression guideline issued by the Dutch college of GPs, which recommends that treatment of depression primarily consists of education and coaching. Antidepressant treatment and/or referral for psychotherapy can be added, depending on the duration and severity of the depressive symptoms, the limitations in daily functioning, and the patient's preference.17 General practitioners are free to deviate from these guidelines and to organize care according to their own views.
Intervention Treatment
General practitioners in the intervention group attended a 4-hour training session that focused on the screening for, and diagnosis and treatment of late-life depression. The treatment offered by the GPs consisted of education and information, drug therapy (20 mg of paroxetine once daily), and supportive contacts and was based on the Dutch depression guideline.17 Two treatment phases were distinguished: an acute treatment phase during which patients were seen every 2 weeks by their GP for a period of 2 months, and a continuation phase during which patients were seen monthly for a period of 4 months.
Clinical Outcome Measures
Trained interviewers, who were unaware of the allocation status of the participating GPs, measured outcomes and resource use during interviews at the patients' home. Recovery from depression was defined as absence of a PRIME-MD diagnosis of major depression at 12 months. The Montgomery Asberg Depression Rating Scale (MADRS) was used to assess changes in severity of depressive symptoms during the 12 months of the study.18 Quality of life was measured using the EuroQol (EQ-5D).19 Quality Adjusted Life Years (QALYs) were calculated by multiplying the utility based on EuroQol scores with the amount of time a patient spent in a particular health state.20 Transitions between health states were linearly interpolated. All clinical outcome measures were measured shortly after the screening (T0) and at 2 (T1), 6 (T2), and 12 (T3) months of follow-up, with the exception of the PRIME-MD, which was not measured at 2 months of follow-up. Because the first interview was conducted shortly after the screening, treatment in the intervention group had already started at T0.
Cost Measures
Costs were measured at T0, T2, and T3 from a health care perspective using interviews. Patients were asked whether they had visited a specific health care provider in the past 6 months and if so, how many times. Because the interviews were conducted at the patients' home, it was possible for patients to get a diary to check their health care utilization. All direct health care and nonhealth care costs were considered, because it is very hard to discern which costs are depression-related and which are not. Of medication costs, only costs of psychotropic medication were included in the analyses. Intervention costs consisted of the total costs of the training sessions for the GPs and were equally allocated to the included intervention patients. Indirect costs of production losses were not measured, because it was assumed that a substantial proportion of the included patients had already retired. If available, Dutch guideline prices were used to value resource use.21,22 The cost categories and prices used are listed in the (online Appendix). Medication costs were valued using prices of the Royal Dutch Society for Pharmacy.23 Costs of complementary medicine visits were based on patients' estimates. All costs were adjusted to the year 2002 using consumer price indices. Discounting was unnecessary, because neither costs nor benefits were recorded beyond 12 months.
Statistical and Economic Analyses
We estimated that 68 patients in each group would be needed (2-sided α=0.05, β=0.20) to detect moderate clinical effects (Cohen's d = 0.50). Moderate clinical effects were considered to be clinically relevant and convincing for GPs.14 All analyses were intention-to-treat and limited to patients completing all follow-up assessments. Differences between both groups in improvement in severity of depression and QALYs gained at 12 months were tested using t tests and differences in recovery using χ2 tests.
To compare costs between the 2 groups, confidence intervals (CIs) for the mean differences in costs were calculated using bias-corrected and accelerated bootstrapping with 2,000 replications.24 For the cost-effectiveness analyses, the difference in total costs between the treatment groups was compared with the difference in improvement in the MADRS score, the difference in recovery rate based on the PRIME-MD, and for the cost-utility analysis with the difference in QALYs. Uncertainty around the cost-effectiveness and cost-utility ratios was calculated using the bias-corrected percentile bootstrapping method (5,000 replications).25 The bootstrapped cost-effect pairs were plotted on a cost-effectiveness plane.
RESULTS
Of the 34 participating general practices, 18 were allocated to the intervention group and 16 to the usual care group. There were no significant differences between GPs in the intervention and usual care group with regard to age, gender, and experience of GPs, and type and size of practice (data not shown).
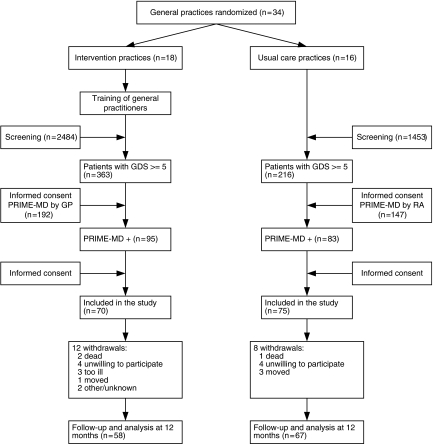
Between June 2000 and October 2002, of 3,937 patients screened for depressive symptoms with the GDS-15, 579 screened positive and were evaluated further using the PRIME-MD, resulting in 178 patients with major depression according to the PRIME-MD. Of the detected patients, 145 (81%) gave informed consent. Sixteen otherwise eligible intervention patients were not included, because their GP disagreed with the depression diagnosis or refused to prescribe antidepressants. Complete follow-up was available for 125 (86%) patients (Fig. 1). Patients who were lost to follow-up were older and more severely depressed than patients with complete follow-up.
FIGURE 1.
Flow of participants through the trial; GDS, Geriatric Depression Scale, PRIME-MD, PRIMary care Evaluation of Mental Disorders.
Except for marital status, there were no significant differences at T0 in patient characteristics between the intervention and the usual care group (Table 1), nor did these patient characteristics differ between general practices (data not shown).
Table 1.
Characteristics of Patients Allocated to the Intervention or Usual Care Group
| Intervention (n = 58) | Usual Care (n = 67) | |
|---|---|---|
| Mean (SD) age in years | 66.4 (8.6) | 64.7 (7.5) |
| Female | 38 (66) | 36 (54) |
| Married/living together | 34 (59) | 52 (78) |
| Previous depression | 49 (85) | 55 (82) |
| Mean (SD) MADRS score | 19.3 (8.7) | 18.7 (7.7) |
Values are numbers (percentages) of patients unless stated otherwise.
MADRS, Montgomery Asberg depression rating scale.
Clinical Effectiveness
Twenty-five (43%) and 32 (48%) of the patients in the intervention and usual care group, respectively, had recovered based on the PRIME-MD after 12 months. This difference in recovery rate was not statistically significant. The differences in improvement in severity of depressive symptoms and QALYs gained between the 2 treatment groups were small and not statistically significant (Table 2).
Table 2.
Clinical Outcomes After 12 Months
| Outcome measure | Intervention (n = 58) | Usual Care (n = 67) | Intervention Versus Usual Care* | P Value |
|---|---|---|---|---|
| % (No.) Recovered (PRIME-MD) | 43.1 (25) | 47.8 (32) | −4.7 (−22.5; 13.1) | .60 |
| Mean (SD) improvement in MADRS score | −7.8 (9.0) | −7.2 (9.0) | −0.6 (−3.8; 2.6) | .70 |
| Mean (SD) QALYs gained (EQ-5D) | 0.65 (0.19) | 0.70 (0.21) | −0.04 (−0.11; 0.03) | .20 |
Difference (95% confidence interval).
PRIME-MD, PRIMary care Evaluation of Mental Disorders, MADRS=Montgomery Asberg Depression Rating Scale, EQ-5D, EuroQol.
Health Care Utilization
Table 3 presents the utilization of health care resources in the 2 groups over 12 months. During the study period, 95% and 94% of the intervention and usual care patients, respectively, had at least 1 contact with their GP. Forty-six (79%) intervention and only 8 (12%) usual care patients received some form of mental health care (antidepressant medication or referral) during the follow-up period. At all 3 measurements, intervention patients were more likely to use an antidepressant. Twenty-eight (48%) intervention patients received antidepressant treatment for at least 6 months as recommended by the Dutch depression guidelines.17
Table 3.
Mean (Standard Deviation) Health Care Utilization of Patients Allocated to the Intervention or Control Group During 12 Months
| Type of utilization | Intervention (n = 58) | Usual Care (n = 67) |
|---|---|---|
| Direct health care | ||
| Primary care | ||
| General practitioner (no. visits) | 7.8 (5.5) | 6.3 (5.5) |
| Dentist (no. visits) | 1.3 (1.8) | 0.8 (1.1) |
| Dietitian (no. visits) | 0.3 (1.4) | 0.6 (1.8) |
| Physical therapist (no. visits) | 4.8 (14.3) | 11.0 (20.6) |
| Social worker (no. visits) | 0.2 (0.8) | 0.0 (0.3) |
| Secondary care | ||
| Psychiatrist (no. visits) | 0.0 (0.3) | 0.4 (2.9) |
| Medical specialist (no. visits) | 2.9 (3.1) | 3.5 (5.1) |
| Hospital admission (no. d) | 1.3 (3.1) | 1.2 (3.1) |
| Hospital admission (no. d on ICU) | 0 (0) | 0.1 (0.3) |
| Psychiatric hospital admission (no. d) | 0 (0) | 0 (0) |
| Regional institute for mental welfare (no. visits) | 0.1 (0.4) | 0.3 (1.4) |
| Psychogeriatric services (no. visits) | 0 (0) | 0.1 (0.6) |
| Rehabilitation center (no. h) | 0 (0) | 2.7 (19.3) |
| Nursing home —temporary admission (no. d) | 1.3 (9.8) | 0 (0) |
| Nursing home—outpatients' treatment (no. d) | 0.4 (3.4) | 0 (0) |
| Home for the elderly—temporary admission (no. d) | 0.5 (3.7) | 0.2 (1.7) |
| Home for the elderly—outpatients' care (no. daily periods) | 0.9 (6.8) | 0 (0) |
| Supportive care | ||
| District nurse (no. h) | 0.9 (4.1) | 1.0 (3.4) |
| Home care (no. h) | 9.4 (32.9) | 14.0 (38.2) |
| Home help (no. h) | 16.1 (45.6) | 10.5 (35.7) |
| Home for the elderly—meal supply (no. meals) | 3.1 (23.9) | 0 (0) |
| Meal supply at home (no. meals) | 1.3 (10.2) | 3.9 (31.8) |
| Direct nonhealth care | ||
| Primary care | ||
| Alternative therapist (no. visits) | 0.2 (1.2) | 1.8 (8.5) |
| Memory training (no. contacts) | 0 (0) | 0.0 (0.1) |
Costs
At 12 months, total direct costs were somewhat lower in the intervention group, but this difference was not statistically significant. Psychotropic medication costs in the intervention group were significantly higher than in the usual care group (Table 4). However, almost all point estimates of the differences in costs indicated small savings. Moreover, the higher GP and medication costs in the intervention group seem to be substituted by higher physiotherapy costs in the usual care group.
Table 4.
Mean (Standard Deviation) Total Costs in Dollars, Differences in Mean (95% Confidence Intervals)* Total Costs in Dollars, and P value† During Follow-up of 12 mo
| Intervention (n = 58) | Usual Care (n = 67) | Intervention Versus Usual Care | P Value | |
|---|---|---|---|---|
| Primary care costs | 336 (375) | 494 (739) | −157 (−313;56) | .13 |
| GP costs | 170 (120) | 137 (120) | 33 (−20;68) | .12 |
| Physiotherapy costs | 115 (340) | 263 (490) | −148 (−276;19) | .05 |
| Secondary care costs | 885 (2,161) | 1,021 (2,513) | −136 (−902;727) | .75 |
| Outpatient costs | 156 (165) | 189 (274) | −32 (−107;46) | .42 |
| Admission costs | 664 (2,047) | 466 (1317) | 199 (−418;729) | .79 |
| Supportive care costs | 575 (1,289) | 663 (1,482) | −88 (−565;414) | .72 |
| Medication costs | 220 (162) | 82 (333) | 139 (4;407) | .003 |
| Intervention costs | 106 | — | — | |
| Total direct costs | 2,123 (2,661) | 2,259 (3,922) | −136 (−1,194;1,110) | .82 |
95% confidence intervals obtained by bias-corrected and accelerated bootstrapping.
P value obtained by applying the t distribution on the t value that was obtained during bootstrapping.
$1=€0.80.
GP, general practitioner.
Total costs of visits to mental health care providers and psychotropic medication in the intervention group ($243) were higher than in the usual care group ($165), but this difference was not statistically significant (P = .22).
Cost-Effectiveness and Cost-Utility Analyses
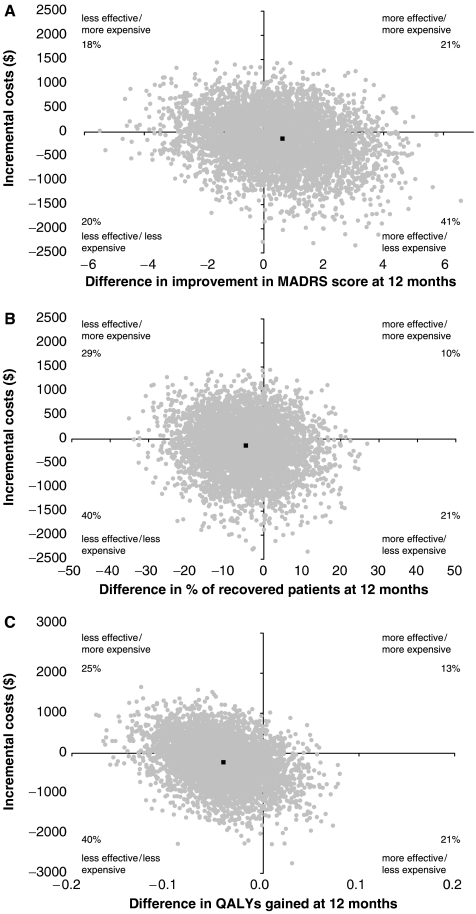
Figure 2 shows the cost-effectiveness planes for the intervention group in comparison with the usual care group for recovery, improvement in severity of depression, and QALYs gained at 12 months. In all 3 cost-effectiveness planes, all cost-effect pairs are located near the origin of the plane, suggesting neither large nor significant differences in costs and effects. This shows that the intervention is not cost-effective in comparison with usual care.
FIGURE 2.
(A) Cost-effectiveness plane for the difference in improvement in Montgomery Asberg Depression Rating Scale score over 12 months (point estimate: −219; 95% confidence interval [CI]: −17,054 to 1,065); (B) Cost-effectiveness plane for the difference in recovery rates based on the PRIMary care Evaluation of Mental Disorders after 12 months (point estimate: 29; 95% CI: −152 to 4,086); (C) Cost-effectiveness plane for the difference in Quality Adjusted Life-Years (QALYs) gained at 12 months (point estimate: 5,397; 95% CI: −28,108 to 450,977).
DISCUSSION
We evaluated whether a disease management program for major depression in elderly primary care patients would be cost effective in comparison with usual care. Although antidepressant treatment rates were significantly higher in the intervention group, we found no statistically significant difference in total costs, effects, and cost-effectiveness between the 2 treatment groups.
Our findings are in line with 2 randomized trials that also found that disease management programs for elderly primary care patients with depression had no significant effect on depression severity.26,27 In contrast, Katon et al.28 found a significant effect, accompanied by a modest and insignificant increase in total health care costs. However, patients in that study had access to a depression care manager, which makes the intervention program much more intensive.
Several American observational studies reported much higher health care costs in depressed elderly patients than we do.4,6,28–30 These studies used computerized databases to estimate costs, whereas our cost estimates were based on patient reports of resource use. Also, our study population was on average about 10 years younger than the study populations in the abovementioned studies.
There may be several explanations for the nonsignificant results of this study. First, despite the fact that all patients were diagnosed as having major depression according to the PRIME-MD, most patients detected by our screening method were only mildly to moderately depressed according to their MADRS scores at T0.31,32 This may arise from the fact that screening in primary care typically leads to detection of mildly depressed patients.33 Because patients with milder forms of major depression are not likely to be included in trials evaluating the efficacy of antidepressants, it is unclear whether antidepressants are efficacious in these patients.34 Indeed, a recent review concluded that antidepressants have only moderate effects in older ambulatory patients with mild to moderate depression.35
Another partial explanation for the nonsignificant findings of this study may be the existence of a Hawthorne effect, although this seems inevitable in this type of research. The fact that GPs and patients knew they were participating in a study influences their behavior and perceptions and thereby may reduce any differences there might have been between the treatment groups. The fact that trained research assistants interviewed study patients at home may also have contributed to the Hawthorne effect. We tried to control for a Hawthorne effect by blinding usual care GPs to the screening results and the research assistants to the allocation status of the GPs. However, the Hawthorne effect may have been substantial, because, especially in the treatment of depression, attitudes, environment, time spent with patients, etc., will have an effect on the results. Related to the Hawthorne effect is that usual care patients frequently received GP care and physiotherapy for comorbid disorders. Treating comorbidity may improve outcomes of depression as well. This may also partly explain the relatively positive outcomes in usual care patients.
There are also 2 design aspects of our study that may have contributed to the nonsignificant findings of our study. First, we chose a pragmatic design, meaning that we tried to replicate everyday clinical practice as much as possible to enhance the generalizability of our findings. However, a disadvantage of a pragmatic design is that the contrast between the treatment groups may be diminished. Second, it was not possible to blind patients included in the usual care group. Although usual care patients were requested not to reveal to their GP that they were participating in this study, there is a risk that some patients informed their GP and subsequently received some kind of treatment for depression. However, as only a few usual care patients were prescribed antidepressants or referred to a mental health care provider, this problem seems to be small.
Initiation of mental health care treatment in both the intervention and the usual care group was associated with more severe depression at baseline. Thus, GPs use severity of depression as a criterion to initiate depression treatment, but this criterion alone seems insufficient to distinguish patients who will benefit from depression treatment from patients who will not benefit.
General practitioners of intervention patients had to agree with the depression diagnosis and had to be willing to prescribe antidepressants, which may have led to selection bias in the intervention group. In this case, it can be expected that intervention patients have more severe depression at baseline than usual care patients. Intervention patients were indeed somewhat more depressed than usual care patients at baseline, but the difference was small and not statistically significant. Therefore, we do not expect that this particular form of selection bias was very strong in our study.
The follow-up rate of 86% is very good for studies in elderly depressed populations. Patients who dropped out before the end of the study were older and more depressed than patients who completed all follow-up measurements. Moreover, although a sufficient number of patients was included in the study, the number of patients analyzed at 12 months was smaller than the required sample size to detect moderate clinical effects. However, the results of a missing value analysis using the Expectation Maximization algorithm36 did not differ from the complete case analysis (data not shown). Therefore, we do not expect that inclusion of patients who dropped out before the end of the study would have altered our conclusions.
Our study was underpowered to detect relevant differences in costs, which is reflected in wide confidence intervals for cost differences. This is a common problem in “piggy back” economic evaluations. Because the distribution of cost data is typically heavily skewed, very large numbers of study patients are needed to detect relevant cost differences.37 It is generally considered unethical to increase study sizes beyond the level needed to prove clinical effectiveness.
Another limitation to the economic evaluation presented in this article is the manner in which cost data were collected. In interviews, patients were asked about their health care utilization over the past 6 months. Subjects in general, and elderly subjects in particular may not be able to recall health care utilization reliably over such a long period. This may have introduced recall bias. Most likely, our estimates are an underestimate of the true utilization rates for frequently occurring resources such as visits to the GP. For more seldom occurring resources such as hospitalizations, we expect our estimates to be reasonably adequate.
A final limitation is that costs of production losses and informal care giving were not measured. As the differences in clinical outcomes and direct costs were small, we consider it unlikely that inclusion of lost productivity costs would have altered the results of our study. Because our study population consisted of elderly subjects, it is probable that many subjects received informal care. Future studies should attempt to measure these costs.
In conclusion, this disease management program for major depression in elderly primary care patients was not cost-effective in comparison with usual care. There were no significant differences in depressive symptoms, quality of life, and costs between the intervention and usual care group at 12 months. Therefore, based on these results, we recommend continuing usual care by GPs, which mainly consists of “watchful waiting.” In this situation, treatment for depression is initiated only when the GP diagnoses the patient as being depressed. We recommend that future research focuses on improvement of the detection of clinically important and treatable depression by GPs. Evidence is needed on indicators that help GPs in determining which patients may profit from depression treatment. Research is also needed on the (cost-) effectiveness of treatments for depression in elderly primary care patients, especially on the (cost-) effectiveness of treatments other than antidepressants, such as different forms of psychotherapy.
Acknowledgments
The Dutch Health Care Insurance Board (DHCIB) financed the trial (grant number OG 00-252).
Supplementary Material
The following supplementary material is available for this article online at www.blackwell-synergy.com
Costs applied in the economic evaluation.
REFERENCES
- 1.Beekman AT, Deeg DJ, van Tilburg T, Smit JH, Hooijer C, Van Tilburg W. Major and minor depression in later life: a study of prevalence and risk factors. J Affect Disord. 1995:65–75. doi: 10.1016/0165-0327(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 2.Barry KL, Fleming MF, Manwell LB, Copeland LA, Appel S. Prevalence of and factors associated with current and lifetime depression in older adult primary care patients. Fam Med. 1998:366–71. [PubMed] [Google Scholar]
- 3.Beekman AT, Deeg DJ, Braam AW, Smit JH, Van Tilburg W. Consequences of major and minor depression in later life: a study of disability, well-being and service utilization. Psychol Med. 1997:1397–409. doi: 10.1017/s0033291797005734. [DOI] [PubMed] [Google Scholar]
- 4.Luber MP, Meyers BS, Williams-Russo PG, et al. Depression and service utilization in elderly primary care patients. Am J Geriatr Psychiatry. 2001:169–76. [PubMed] [Google Scholar]
- 5.Callahan CM, Hui SL, Nienaber NA, Musick BS, Tierney WM. Longitudinal study of depression and health services use among elderly primary care patients. J Am Geriatr Soc. 1994:833–8. doi: 10.1111/j.1532-5415.1994.tb06554.x. [DOI] [PubMed] [Google Scholar]
- 6.Unutzer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997:1618–23. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- 7.Penninx BW, Deeg DJ, van Eijk JT, Beekman AT, Guralnik JM. Changes in depression and physical decline in older adults: a longitudinal perspective. J Affect Disord. 2000:1–12. doi: 10.1016/s0165-0327(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin RC, Anderson D, Black S, et al. Guideline for the management of late-life depression in primary care. Int J Geriatr Psychiatry. 2003:829–38. doi: 10.1002/gps.940. [DOI] [PubMed] [Google Scholar]
- 9.Lebowitz BD, Pearson JL, Schneider LS, et al. Diagnosis and treatment of depression in late life. Consensus statement update. JAMA. 1997:1186–90. [PubMed] [Google Scholar]
- 10.Unutzer J, Katon W, Sullivan M, Miranda J. Treating depressed older adults in primary care: narrowing the gap between efficacy and effectiveness. Milbank Q. 1999:225–56. doi: 10.1111/1468-0009.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NIH Consensus Conference. Diagnosis and treatment of depression in late life. JAMA. 1992:1018–24. [PubMed] [Google Scholar]
- 12.Pignone MP, Gaynes BN, Rushton JL, et al. Screening for depression in adults: a summary of the evidence for the U. S. Preventive Services Task Force. Ann Intern Med. 2002:765–76. doi: 10.7326/0003-4819-136-10-200205210-00013. [DOI] [PubMed] [Google Scholar]
- 13.Badamgarav E, Weingarten SR, Henning JM, et al. Effectiveness of disease management programs in depression: a systematic review. Am J Psychiatry. 2003:2080–90. doi: 10.1176/appi.ajp.160.12.2080. [DOI] [PubMed] [Google Scholar]
- 14.Bijl D, van Marwijk HWJ, Beekman ATF, De Haan M, Van Tilburg W. A randomized controlled trial to improve the recognition, diagnosis and treatment of major depression in elderly people in general practice: design, first results and feasibility of the West Friesland Study. Primary Care Psychiatry. 2003:135–40. [Google Scholar]
- 15.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS); Recent evidence and development of a shorter version. Clin Gerontol. 1986:165–73. [Google Scholar]
- 16.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994:1749–56. [PubMed] [Google Scholar]
- 17.van Marwijk HWJ, Grundmeijer HGLM, Brueren MM, et al. Guidelines on Depression of the Dutch College of General Practitioners (NHG-Standaard Depressie) Huisarts Wet. 1994:482–90. [Google Scholar]
- 18.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 19.EuroQol—a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 20.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997:1095–108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Oostenbrink JB, Koopmanschap MA, Van Rutten FFH. Handleiding voor Kostenonderzoek. Methoden en Richtlijnprijzen voor Economische Evaluaties in de Gezondheidszorg. [Handbook for Cost studies, Methods and Guidelines for Economic Evaluation in Health Care] The Hague, the Netherlands: Health Care Insurance Council; 2000. [Google Scholar]
- 22.Oostenbrink JB, Koopmanschap MA, Rutten FF. Standardisation of costs: the Dutch Manual for Costing in economic evaluations. Pharmacoeconomics. 2002:443–54. doi: 10.2165/00019053-200220070-00002. [DOI] [PubMed] [Google Scholar]
- 23.Z-index. G-Standaard. The Hague, the Netherlands: Z-index; 2002. [Google Scholar]
- 24.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 25.Chaudhary MA, Stearns SC. Estimating confidence intervals for cost-effectiveness ratios: an example from a randomized trial. Stat Med. 1996:1447–58. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1447::AID-SIM267>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Callahan CM, Hendrie HC, Dittus RS, Brater DC, Hui SL, Tierney WM. Improving treatment of late life depression in primary care: a randomized clinical trial. J Am Geriatr Soc. 1994:839–46. doi: 10.1111/j.1532-5415.1994.tb06555.x. [DOI] [PubMed] [Google Scholar]
- 27.Whooley MA, Stone B, Soghikian K. Randomized trial of case-finding for depression in elderly primary care patients. J Gen Intern Med. 2000:293–300. doi: 10.1046/j.1525-1497.2000.04319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry. 2005:1313–20. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 29.Fischer LR, Wei F, Rolnick SJ, et al. Geriatric depression, antidepressant treatment, and healthcare utilization in a health maintenance organization. J Am Geriatr Soc. 2002:307–12. doi: 10.1046/j.1532-5415.2002.50063.x. [DOI] [PubMed] [Google Scholar]
- 30.Katon WJ, Lin E, Russo J, Unutzer J. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003:897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- 31.Kearns NP, Cruickshank CA, McGuigan KJ, Riley SA, Shaw SP, Snaith RP. A comparison of depression rating scales. Br J Psychiatry. 1982:45–9. doi: 10.1192/bjp.141.1.45. [DOI] [PubMed] [Google Scholar]
- 32.Mittmann N, Mitter S, Borden EK, Herrmann N, Naranjo CA, Shear NH. Montgomery-Asberg severity gradations. Am J Psychiatry. 1997:1320–1. doi: 10.1176/ajp.154.9.1320b. [DOI] [PubMed] [Google Scholar]
- 33.Coyne JC, Schwenk TL, Fechner-Bates S. Nondetection of depression by primary care physicians reconsidered. Gen Hosp Psychiatry. 1995:3–12. doi: 10.1016/0163-8343(94)00056-j. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman M, Mattia JI, Posternak MA. Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? Am J Psychiatry. 2002:469–73. doi: 10.1176/appi.ajp.159.3.469. [DOI] [PubMed] [Google Scholar]
- 35.McCusker J, Cole M, Keller E, Bellavance F, Berard A. Effectiveness of treatments of depression in older ambulatory patients. Arch Intern Med. 1998:705–12. doi: 10.1001/archinte.158.7.705. [DOI] [PubMed] [Google Scholar]
- 36.SPSS Inc. SPSS 10.1. 2000. Chicago, IL, SPSS Inc.
- 37.Briggs A. Economic evaluation and clinical trials: size matters. BMJ. 2000:1362–3. doi: 10.1136/bmj.321.7273.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Costs applied in the economic evaluation.