Abstract
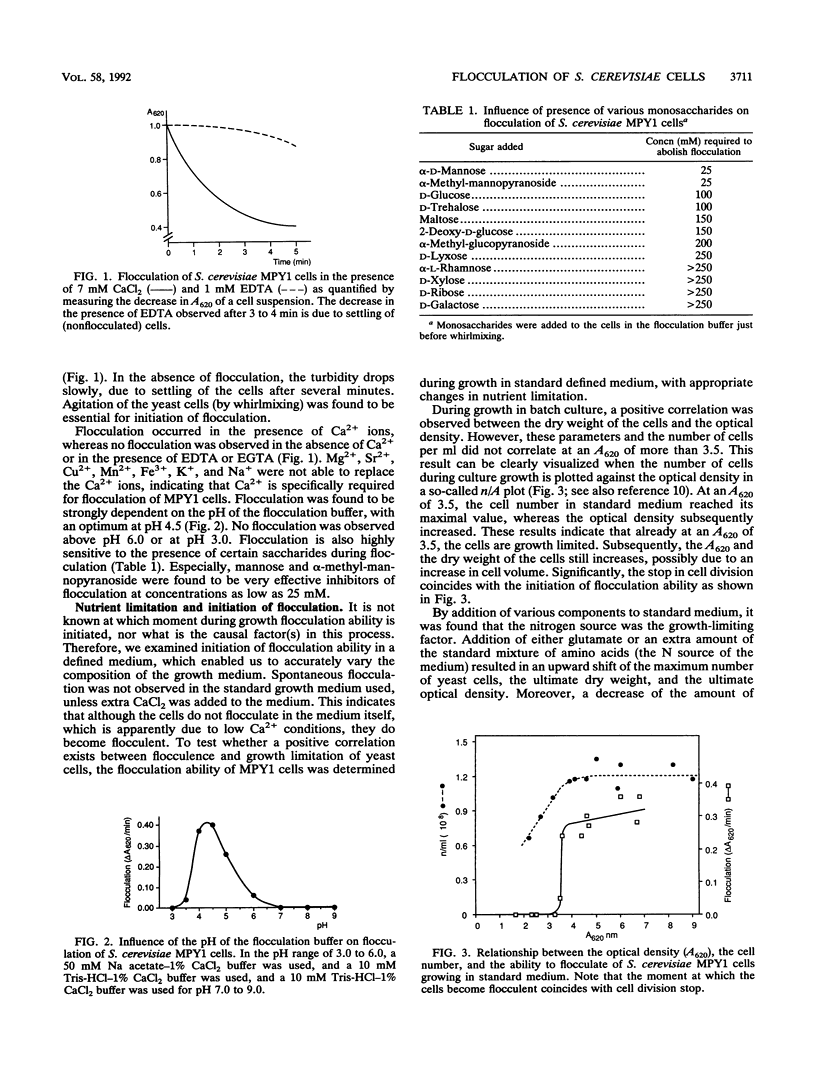
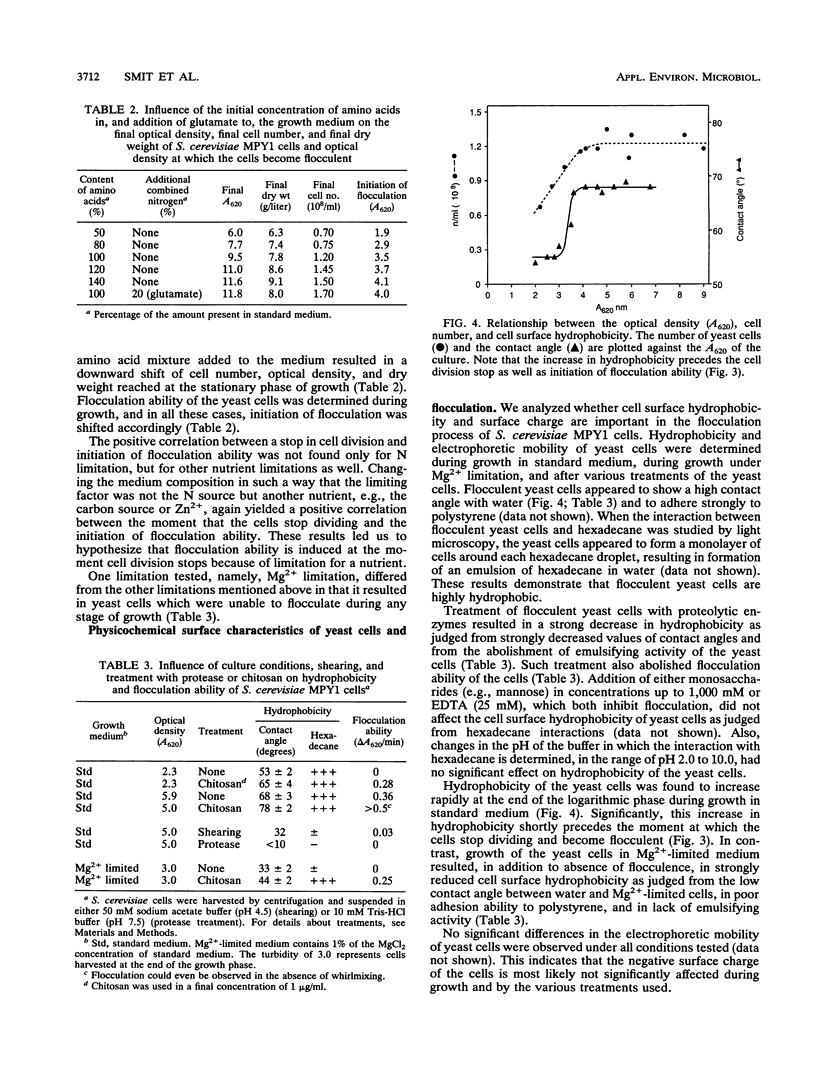
Initiation of flocculation ability of Saccharomyces cerevisiae MPY1 cells was observed at the moment the cells stop dividing because of nitrogen limitation. A shift in concentration of the limiting nutrient resulted in a corresponding shift in cell division and initiation of flocculence. Other limitations also led to initiation of flocculence, with magnesium limitation as the exception. Magnesium-limited S. cerevisiae cells did not flocculate at any stage of growth. Cell surface hydrophobicity was found to be strongly correlated with the ability of the yeast cells to flocculate. Hydrophobicity sharply increased at the end of the logarithmic growth phase, shortly before initiation of flocculation ability. Treatments of cells which resulted in a decrease in hydrophobicity also yielded a decrease in flocculation ability. Similarly, the presence of polycations increased both hydrophobicity and the ability to flocculate. Magnesium-limited cells were found to be strongly affected in cell surface hydrophobicity. A proteinaceous cell surface factor(s) was identified as a flocculin. This heat-stable component had a strong emulsifying activity, and appears to be involved in both cell surface hydrophobicity and in flocculation ability of the yeast cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavan M. J., Belk D. M., Stewart G. G., Rose A. H. Changes in electrophoretic mobility and lytic enzyme activity associated with development of flocculating ability in Saccharomyces cerevisiae. Can J Microbiol. 1979 Aug;25(8):888–895. doi: 10.1139/m79-132. [DOI] [PubMed] [Google Scholar]
- Busscher H. J., Weerkamp A. H., van der Mei H. C., van Pelt A. W., de Jong H. P., Arends J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl Environ Microbiol. 1984 Nov;48(5):980–983. doi: 10.1128/aem.48.5.980-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nobel J. G., Klis F. M., Munnik T., Priem J., van den Ende H. An assay of relative cell wall porosity in Saccharomyces cerevisiae, Kluyveromyces lactis and Schizosaccharomyces pombe. Yeast. 1990 Nov-Dec;6(6):483–490. doi: 10.1002/yea.320060605. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Doyle R. J., Rosenberg M. Mechanism of enhancement of microbial cell hydrophobicity by cationic polymers. J Bacteriol. 1990 Oct;172(10):5650–5654. doi: 10.1128/jb.172.10.5650-5654.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihn J. C., Masy C. L., Mestdagh M. M., Rouxhet P. G. Yeast flocculation: factors affecting the measurement of flocculence. Can J Microbiol. 1988 Jun;34(6):779–781. doi: 10.1139/m88-132. [DOI] [PubMed] [Google Scholar]
- Kijne J. W., Smit G., Díaz C. L., Lugtenberg B. J. Lectin-enhanced accumulation of manganese-limited Rhizobium leguminosarum cells on pea root hair tips. J Bacteriol. 1988 Jul;170(7):2994–3000. doi: 10.1128/jb.170.7.2994-3000.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILL P. J. THE EFFECT OF NITROGENOUS SUBSTANCES ON THE TIME OF FLOCCULATION OF SACCHAROMYCES CEREVISIAE. J Gen Microbiol. 1964 Apr;35:53–60. doi: 10.1099/00221287-35-1-53. [DOI] [PubMed] [Google Scholar]
- Miki B. L., Poon N. H., James A. P., Seligy V. L. Possible mechanism for flocculation interactions governed by gene FLO1 in Saccharomyces cerevisiae. J Bacteriol. 1982 May;150(2):878–889. doi: 10.1128/jb.150.2.878-889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki B. L., Poon N. H., Seligy V. L. Repression and induction of flocculation interactions in Saccharomyces cerevisiae. J Bacteriol. 1982 May;150(2):890–899. doi: 10.1128/jb.150.2.890-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara H., Toraya T., Fukui S. Effect of chemical modification of cell surface components of a brewer's yeast on the floc-forming ability. Arch Microbiol. 1977 Oct 24;115(1):19–23. doi: 10.1007/BF00427840. [DOI] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Correlation between extracellular fibrils and attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1986 Nov;168(2):821–827. doi: 10.1128/jb.168.2.821-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Logman T. J., Boerrigter M. E., Kijne J. W., Lugtenberg B. J. Purification and partial characterization of the Rhizobium leguminosarum biovar viciae Ca2+-dependent adhesin, which mediates the first step in attachment of cells of the family Rhizobiaceae to plant root hair tips. J Bacteriol. 1989 Jul;171(7):4054–4062. doi: 10.1128/jb.171.7.4054-4062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford M., Coleman H. P., Keenan M. H. Yeast flocculation: a dynamic equilibrium. Yeast. 1988 Sep;4(3):199–208. doi: 10.1002/yea.320040305. [DOI] [PubMed] [Google Scholar]
- Stratford M., Keenan M. H. Yeast flocculation: kinetics and collision theory. Yeast. 1987 Sep;3(3):201–206. doi: 10.1002/yea.320030307. [DOI] [PubMed] [Google Scholar]
- Stratford M., Keenan M. H. Yeast flocculation: quantification. Yeast. 1988 Jun;4(2):107–115. doi: 10.1002/yea.320040204. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. Electrophoretic mobility and hydrophobicity as a measured to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



