Abstract
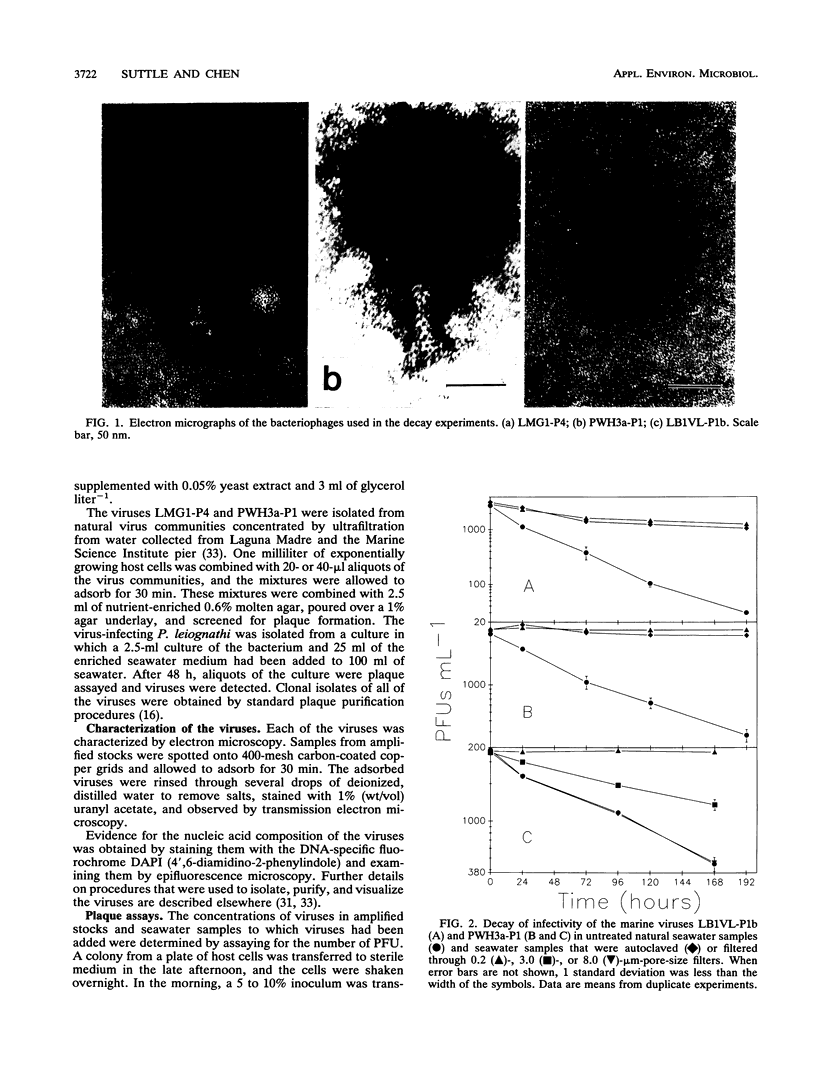
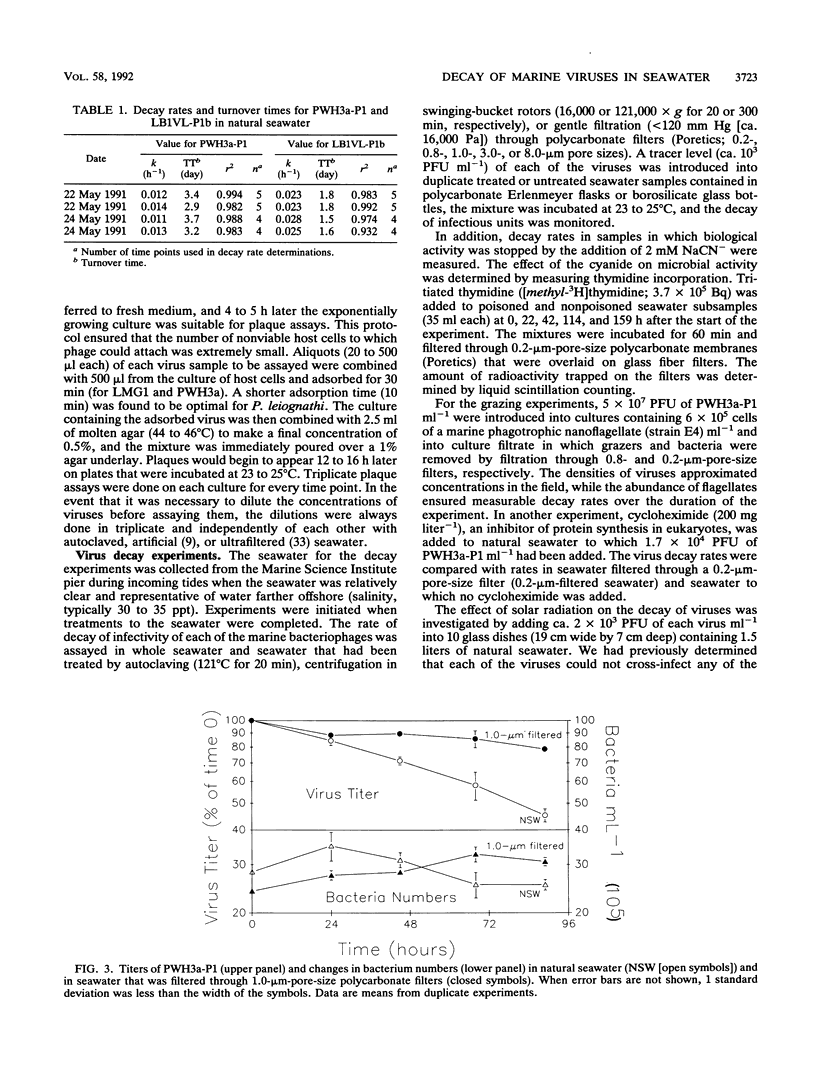
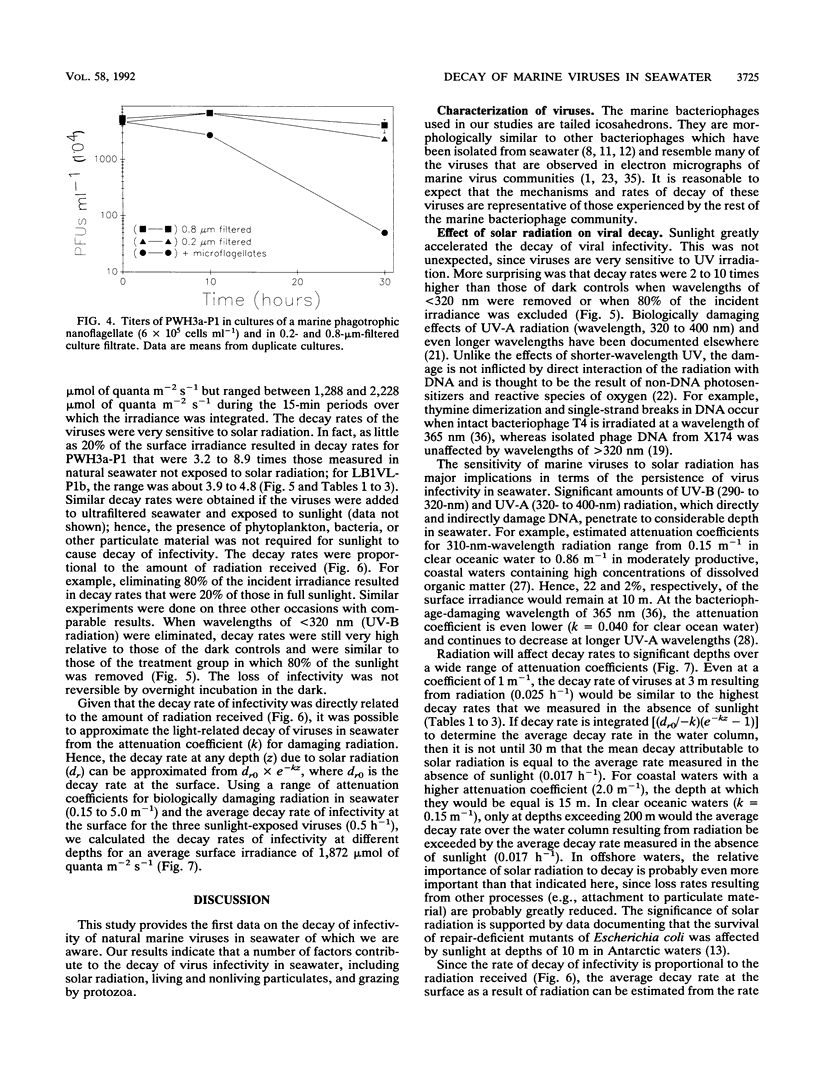
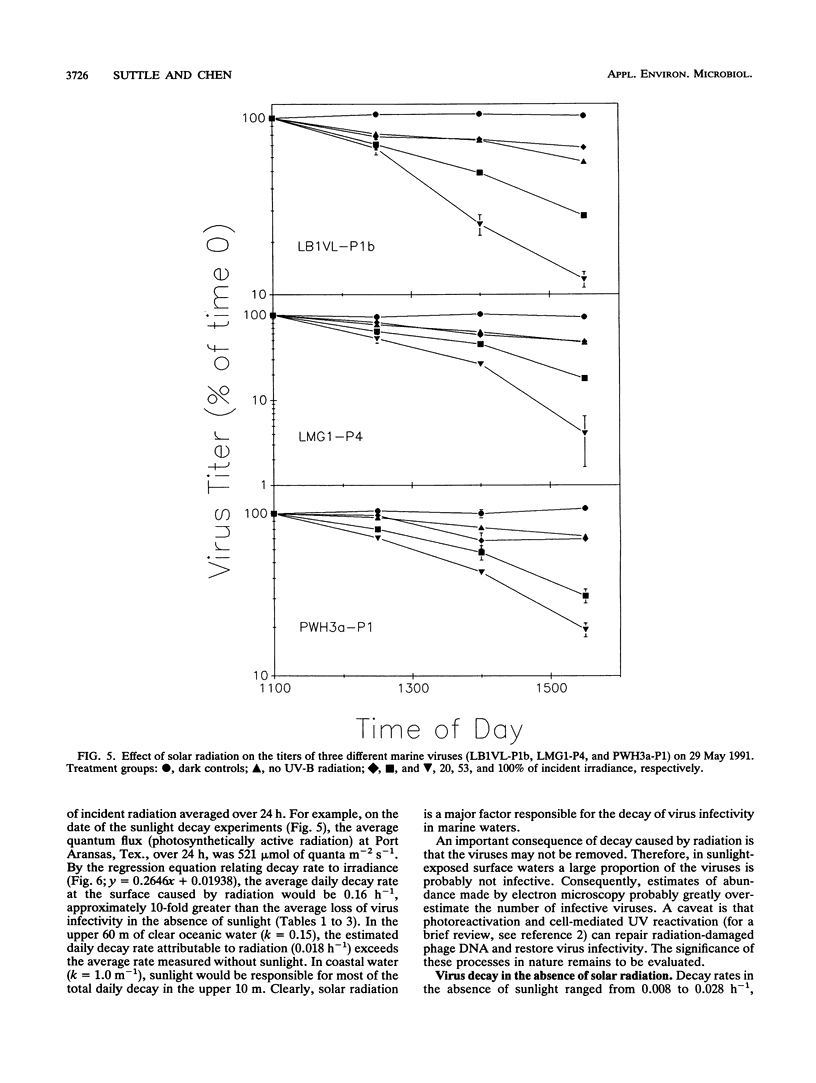
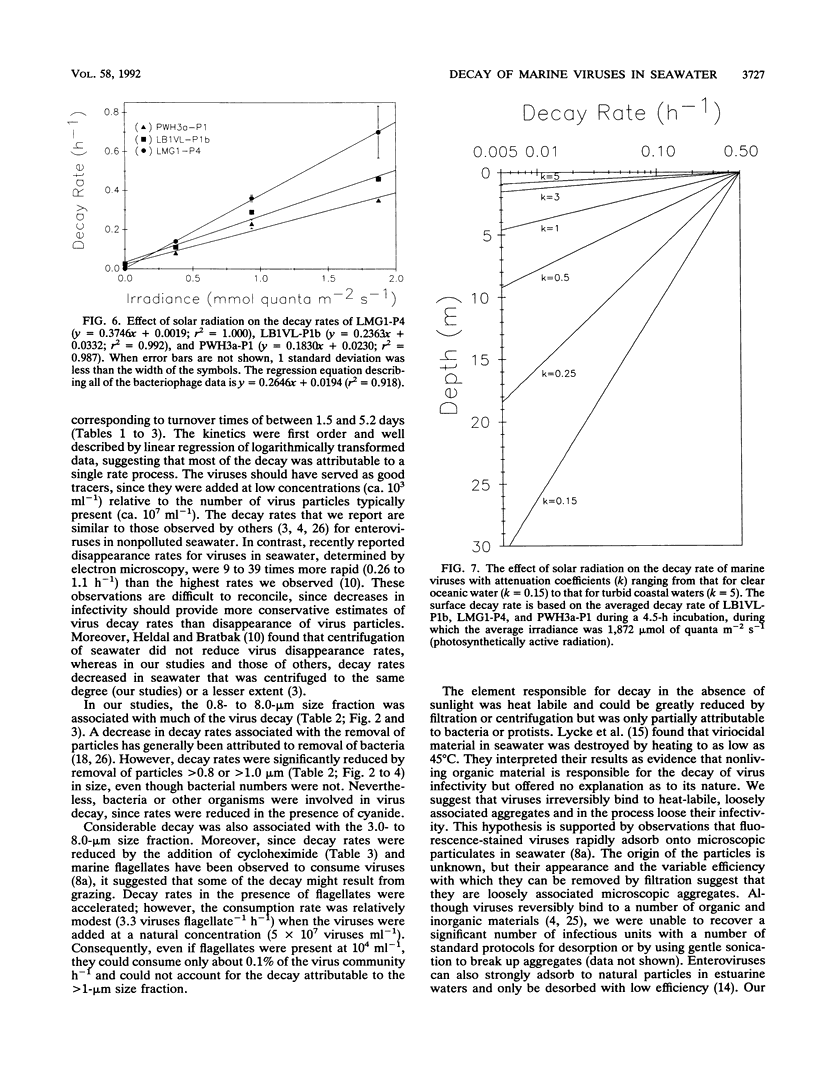
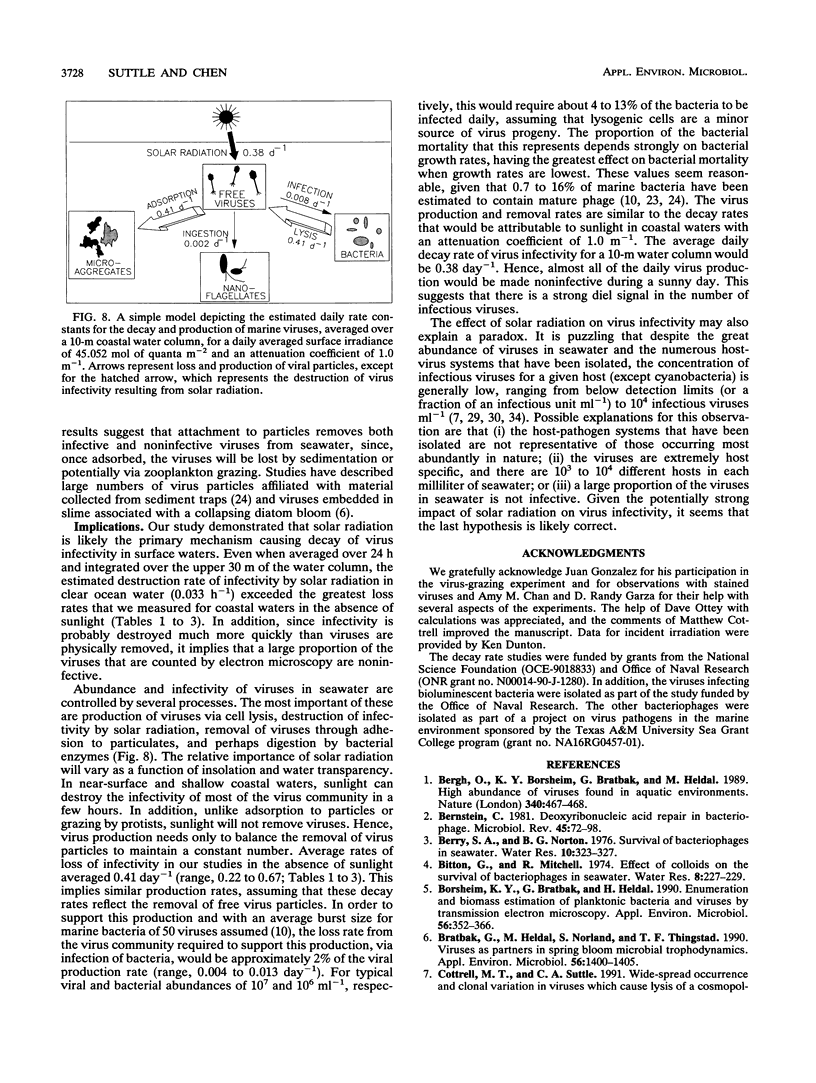
Loss rates and loss processes for viruses in coastal seawater from the Gulf of Mexico were estimated with three different marine bacteriophages. Decay rates in the absence of sunlight ranged from 0.009 to 0.028 h-1, with different viruses decaying at different rates. In part, decay was attributed to adsorption by heat-labile particles, since viruses did not decay or decayed very slowly in seawater filtered through a 0.2-μm-pore-size filter (0.2-μm-filtered seawater) and in autoclaved or ultracentrifuged seawater but continued to decay in cyanide-treated seawater. Cyanide did cause decay rates to decrease, however, indicating that biological processes were also involved. The observations that decay rates were often greatly reduced in 0.8- or 1.0-μm-filtered seawater, whereas bacterial numbers were not, suggested that most bacteria were not responsible for the decay. Decay rates were also reduced in 3-μm-filtered or cycloheximide-treated seawater but not in 8-μm-filtered seawater, implying that flagellates consumed viruses. Viruses added to flagellate cultures decayed at 0.15 h-1, corresponding to 3.3 viruses ingested flagellate-1 h-1. Infectivity was very sensitive to solar radiation and, in full sunlight, decay rates were 0.4 to 0.8 h-1. Even when UV-B radiation was blocked, rates were as high as 0.17 h-1. Calculations suggest that in clear oceanic waters exposed to full sunlight, most of the virus decay, averaged over a depth of 200 m, would be attributable to solar radiation. When decay rates were averaged over 24 h for a 10-m coastal water column, loss rates of infectivity attributable to sunlight were similar to those resulting from all other processes combined. Consequently, there should be a strong diel signal in the concentration of infectious viruses. In addition, since sunlight destroys infectivity more quickly than virus particles, a large proportion of the viruses in seawater is probably not infective.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bernstein C. Deoxyribonucleic acid repair in bacteriophage. Microbiol Rev. 1981 Mar;45(1):72–98. doi: 10.1128/mr.45.1.72-98.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G., Heldal M., Norland S., Thingstad T. F. Viruses as partners in spring bloom microbial trophodynamics. Appl Environ Microbiol. 1990 May;56(5):1400–1405. doi: 10.1128/aem.56.5.1400-1405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børsheim K. Y., Bratbak G., Heldal M. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol. 1990 Feb;56(2):352–356. doi: 10.1128/aem.56.2.352-356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Appl Environ Microbiol. 1979 Jul;38(1):93–101. doi: 10.1128/aem.38.1.93-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke E., Magnusson S., Lund E. Studies on the nature of the virus inactivating capacity of sea water. Arch Gesamte Virusforsch. 1965;17(3):409–413. doi: 10.1007/BF01241195. [DOI] [PubMed] [Google Scholar]
- Patrick J. R., Brabham D. E., Achey P. M. Photoreactivity of UV-b damage in bacteriophage phi X174 DNA. Photochem Photobiol. 1981 May;33(5):769–771. doi: 10.1111/j.1751-1097.1981.tb05489.x. [DOI] [PubMed] [Google Scholar]
- Paul J. H., Jiang S. C., Rose J. B. Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl Environ Microbiol. 1991 Aug;57(8):2197–2204. doi: 10.1128/aem.57.8.2197-2204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak J. G., Peak M. J., Sikorski R. S., Jones C. A. Induction of DNA-protein crosslinks in human cells by ultraviolet and visible radiations: action spectrum. Photochem Photobiol. 1985 Mar;41(3):295–302. doi: 10.1111/j.1751-1097.1985.tb03488.x. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Ito A., Foote C. S., Peak J. G. Photosensitized inactivation of DNA by monochromatic 334-nm radiation in the presence of 2-thiouracil: genetic activity and backbone breaks. Photochem Photobiol. 1988 Jun;47(6):809–813. doi: 10.1111/j.1751-1097.1988.tb01664.x. [DOI] [PubMed] [Google Scholar]
- Spencer R. INDIGENOUS MARINE BACTERIOPHAGES. J Bacteriol. 1960 Apr;79(4):614–614. doi: 10.1128/jb.79.4.614-614.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle C. A., Chan A. M., Cottrell M. T. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl Environ Microbiol. 1991 Mar;57(3):721–726. doi: 10.1128/aem.57.3.721-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, oregon: ecological and taxonomical implications. Appl Environ Microbiol. 1979 Apr;37(4):774–778. doi: 10.1128/aem.37.4.774-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell R. M. Repair of near (365 nm)- and far (254 nm)- UV damage to bacteriophage of Escherichia coli. Photochem Photobiol. 1979 May;29(5):963–970. doi: 10.1111/j.1751-1097.1979.tb07799.x. [DOI] [PubMed] [Google Scholar]




