Abstract
OBJECTIVE
To validate and implement a computer module for the management of uncomplicated urinary tract infections (UTI).
PARTICIPANTS
Women age 18 to 64 years, with a previous UTI, voiding symptoms, and absence of complicating features (comorbidities, vaginal discharge, back pain, emesis, and fever/chills).
MEASUREMENTS
The computer module was validated against clinician diagnosis and urine culture. Following validation, the module was implemented in the urgent care clinic as a management option for women with suspected UTI; computer-directed therapy (CDT)-eligible women received antibiotic treatment without a clinician examination. Patient satisfaction with the module and return visits for UTI-related complaints were assessed.
RESULTS
In the validation study, 18 of 68 women (26%) were CDT-eligible. Clinicians diagnosed 17/18 CDT-eligible women with uncomplicated UTI. Sixty-seven percent of CDT-eligible women had a positive urine culture. Since implementation, 162 women have accessed the module, and 35% have received CDT. Ninety-eight percent (95% confidence interval: 95% to 100%) found the program easy to use and 95% (89% to 100%) would recommend it to friends/family. Two (4%) CDT-treated women had a return visit to our institution for a UTI-related illness within 2 weeks.
CONCLUSIONS
A computer module accurately identifies women with culture-confirmed, uncomplicated UTIs. Patients are highly satisfied with the module.
Keywords: urinary tract infection, cystitis, computer-assisted therapy, computer-assisted diagnosis, patient satisfaction
Uncomplicated urinary tract infections (UTIs)1 afflict approximately 1 in 2 women over their lifetime, and account for approximately 8 million visits each year in the United States.2,3 The cost of care for uncomplicated UTIs is estimated to be over 1.6 billion dollars.2,3 Because the predictive value of symptoms alone is equivalent to or exceeds the performance of urinalysis for the diagnosis of uncomplicated UTI (sensitivity =75%, specificity =82%),1 some health care delivery systems have established telephone management algorithms that have been shown to be safe, effective, and highly rated by patients.4–6
Telephone algorithms have generally required a preexisting relationship between the patient and clinician or system of care. Many women, however, either do not have a primary care clinician or adequate access to them for urgent telephone management of UTIs. Therefore, many of these women present to urgent care centers and emergency departments (EDs) for treatment. In fact, 1 in 10 women diagnosed with UTI in the United States each year are managed in EDs.3 To facilitate management of women who do not have access to telephone management of UTIs, we developed an interactive computer kiosk module based on previously validated telephone management algorithms. In this report, we describe the accuracy and acceptability of the computer module, and its subsequent implementation into clinical practice.
METHODS
A convenience sample of female patients (age 18 to 64 years) presenting to the urgent care center at the University of California, San Francisco from October 2003 to March 2004 and complaining of painful urination, increased urinary frequency or urgency, were invited to participate. Patients were seen by the triage nurse and referred to the computer kiosk (Kiosk Information Systems [KIS], Inc [http://www.kis-kiosk.com/standard-thinman.html].) The computer program module was developed by the investigators and constructed using Macromedia Authorware. Neither party had any role in the design, implementation or evaluation of the study. Discretionary funds of one of the investigators (R.G.) paid for all study-related expenses. Informed consent and patient information were obtained by the computer kiosk using touch-screen, audiovisual formats in English.
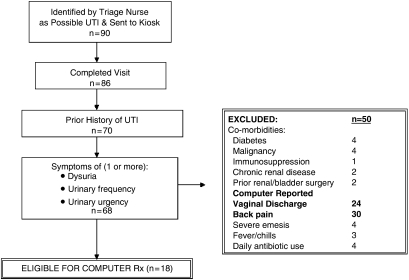
The computer kiosk required each patient to answer 29 simply phrased questions. Based on the answers to these questions, each patient was determined to be either eligible or ineligible for computer-directed treatment (CDT) of her UTI. Eligibility for CDT required: (a) history of prior UTI, (b) symptoms of a UTI (dysuria, urgency or frequency) or symptoms similar to the patient's previous UTI, and (c) absence of complicating factors (Fig. 1).
FIGURE 1.
Flow chart of patient eligibility during validation phase.
After completing the computer module, all patients in the validation study were examined and treated as usual by a clinician who was blinded to the answers provided by the patient to the computer module. Clinicians were asked if they thought the patient could be appropriately diagnosed and treated by the computer algorithm. All study patients had urine cultures performed; positive cultures were defined as exhibiting at least 102 CFU/mL of a known uropathogen.7 A telephone follow-up interview approximately 1 week after the visit assessed symptom resolution and subsequent medical care related to the UTI episode. The validation study was approved by the UCSF Committee on Human Research.
Following validation of the computer module, the UTI Self-Management Kiosk Program was launched in December 2004. Women meeting criteria for CDT without a clinician examination receive a printout that includes their symptoms, current medications, medication allergies, an antibiotic prescription with options for 1 of 3 antimicrobial agents, and instructions regarding when and why to return for further medical care. The printout is reviewed by the next available clinician, who selects a specific antibiotic based on the recorded medications and allergies of the patient, signs the prescription, and returns the form to the patient (no exam is performed). Antibiotic choices provided on the prescription reflect the specific antibiotic sensitivites at our institution. A copy of the completed printout is placed in the medical record. Women completing the module also answered yes/no questions regarding ease of use, interest in other similar programs, and willingness to refer family and friends to use the program. The UCSF clinical information database was accessed to determine whether patients accessing the kiosk returned to UCSF for another visit, and if so, the reason for their visit.
Descriptive and bivariate statistics were performed using the SAS statistical application software (version 8.2; Cary, NC). Predetermined comparisons included the proportion of patients eligible and ineligible for CDT of uncomplicated UTI who had culture-confirmed UTI in the validation study, and the proportion of CDT eligible and ineligible patients with a return visit within 14 days of the index visit. Comparisons were performed with the chi-square and Fisher's Exact test.
RESULTS
In the validation phase, 90 women were referred to the computer kiosk and 86 completed a clinician visit (Fig. 1). Seventeen were CDT-ineligible due to no prior history of UTI, and 2 because they lacked UTI symptoms. Of the 68 remaining women, 18 (26%) met criteria for CDT of uncomplicated UTI; among whom clinicians diagnosed 17 with an uncomplicated UTI and 1 with pyelonephritis (Table 1). Review of the medical chart for the patient diagnosed with pyelonephritis documented no fever, tachycardia, or costovertebral angle tenderness, and urine culture grew 10,000 CFU of Escherichia coli. Clinicians reported that they believed the computer could adequately diagnose and treat all 17 of the CDT-eligible patients diagnosed with UTI. Among the 50 CDT-ineligible women, 30 reported back pain to the computer and 24 vaginal discharge (Fig. 1). Two thirds of these women were subsequently diagnosed with uncomplicated UTI by the clinician. Clinicans reported that they believed the computer could adequately diagnose 29 (58%) of these patients (Table 1).
Table 1.
Patient Characteristics by Computer-Eligibility for Treatment of Uncomplicated Urinary Tract Infections During Validation Phase
| Eligible for Computer Treatment (n = 18), N (%) | Not Eligible For Computer Treatment (N = 50), N (%) | |
|---|---|---|
| Age (y) | ||
| 18 to 24 | 2 (12%) | 10 (21%) |
| 25 to 44 | 12 (70%) | 29 (60%) |
| 45 to 64 | 3 (18%) | 9 (19%) |
| Missing | 1 | 2 |
| Oral temperature (100 F) | 0 | 0 |
| Heart rate >100 beats/min | 0 | 0 |
| Costovertebral angle tenderness | 0 (0%) | 5 (10%) |
| “Looks ill” | 1 (6%) | 1 (2%) |
| Physician noted vaginitis by history or physical exam | 0 (0%) | 9 (19%) |
| Physician diagnosis | ||
| UTI/cystitis | 17 (94%) | 33 (66%) |
| Pyelonephritis | 1 (6%) | 4 (8%) |
| Vaginitis | 0 | 6 (12%) |
| Other | 0 | 4 (8%) |
| Missing | 0 | 3 (6%) |
| Physician judgment | ||
| Computer can diagnose | 17 (94%) | 29 (58%) |
| Computer can treat | 17 (94%) | 32 (64%) |
| Culture positive | 12 (67%) | 13 (26%) |
Based on urine culture results, 12 of 18 (67%, 95% confidence interval [CI]: 45% to 89%) women CDT-eligible women had a confirmed infection (Table 1), representing 48% of all culture confirmed UTIs (total n = 25). The following organisms were isolated: E. coli (n = 20), Staphylococcus saprophyticus (n = 1), group B streptococcus (n = 2), enteric Gram-negative rods (n = 1),8–11 salmonella12,13 (n = 1).
Eighty-four percent (42/50) of the CDT-ineligible women and 89% (16/18) of CDT-eligible women had telephone follow-up. Among CDT-ineligible women, 11 (26%) had persistant symptoms and 6 (14%) had a return visit or call to a clinician for this issue. By comparison, 2 (13%) CDT-eligible women had persistent symptoms at 1 week, and none sought further care.
Since the validation study, 162 women have accessed the UTI kiosk, and 56 (35%) received CDT for uncomplicated UTI. No women referred to the kiosk have refused to use it. Of the 106 CDT-ineligible women, the majority were ineligible because they either had no prior UTI (n = 18), had insufficient UTI symptoms (n = 23), had symptoms longer than 1 week (n = 34), or had new back/flank pain (n = 22). Other reasons for ineligibilty were less common, including new vaginal discharge (n = 10), fever (n =5), age ≥65 (n = 4), comorbid illnesses (n = 4), vomiting (n = 3), and pregnancy (n = 1). Among women treated by the UTI kiosk, 4% (2/56) had a return visit to our institution for a UTI-related illness within 2 weeks of the initial visit. Both presented with persistent symptoms. None returned with symptoms or signs suggestive of pyelonephritis.
Patients completing the module were highly satisfied with it; 98% (95% CI: 95% to 100%) found the program easy to use; 93% (95% CI: 87% to 100%) answered that there should be computerized pathways for other types of common illnesses; and 95% (89% to 100%) stated that they would recommend this program to friends or other family members with bladder symptoms.
DISCUSSION
An interactive computer module identifies approximately half of all women presenting to the urgent care clinic with culture-confirmed, uncomplicated UTIs, and safely distinguishes women who require further evaluation and treatment. We believe this is the first example described in the medical literature of a patient-directed computerized application to evaluate and treat patients with an acute illness.
Our computer module had an excellent true negative rate in that only 1 of 18 CDT-eligible women received a diagnosis other than uncomplicated UTI, and all 18 women were treated with antibiotics by the clinician. The isolated case not diagnosed as uncomplicated UTI received a diagnosis of pyelonephritis—despite the absence of clinical features supporting this diagnosis. It is also reassuring that 67% of CDT-eligible women also had urine culture results compatible with UTI. In addition, while the majority of CDT-ineligible women were ultimately diagnosed as having an uncomplicated UTI by the clinician (67%), only 26% of these women had a culture-confirmed UTI. These findings, in aggregate, demonstrate that the computer criteria effectively distinguish patients with high and low probability of culture-confirmed UTI. In both the validation and implementation phases of this program, it is reassuring that estimates of treatment failure (based on telephone follow-up and administrative data, respectively) were similar between CDT-eligible and CDT-ineligible patients, and are comparable with those reported in other studies.14–17
A UTI-Self Care Kiosk such as the one described in this study may offer significant benefits to health care maintenance organizations, providers, and patients. The total cost of the kiosk, including 2 years of 24-hour tech support is approximately $5,000. Computer programming for the initial development of this module was approximately $2,500. From a health care organization perspective, the kiosk allows patients to receive timely care for their acute medical problem at a reduced overall cost compared with ED or even standard physician urgent care costs, while maintaining or even improving patient satisfaction with their care. Providers benefit from the use of this computer module in that it frees up time to see other patients. From a patient perspective, the program reduces individual wait times. Given that the kiosk uses evidence-based principles including antibiotic choices offered, it provides an opportunity to increase quality of care.
There are several limitations to consider in interpreting the results of this study. First, the program was tested among English-speaking patients and at a single study site. There is no physiologic reason that the technology could not be used for non-English-speaking patients at other sites of care, however, appropriate translation and assessment of acceptability of this technology in non-English-speaking patients is necessary before implementation. In addition, because our validation study was performed using a convenience sample of patients presenting to the urgent care clinic with UTI symptoms, our results are subject to spectrum bias. However, our patient population and the incidence of culture-confirmed UTI are consistent with those reported in previous studies.1 As this was intended as a feasibility study, a randomized-controlled trial was not performed. It is also important to recognize that the estimates of failure rates in the implementation phase of the study are based only on return visits to UCSF. It is possible that these patients accessed care at other locations, thereby overestimating the safety of this intervention. Finally, because of our sample size, we are unable to ascertain rare outcomes such as urosepsis.
In conclusion, we have developed, validated, and implemented a computerized pathway for the evaluation and management of acute uncomplicated UTI that appears to be accurate, safe, and highly acceptable to patients. Studies assessing this program in other populations and settings and studies to assess the cost-effectiveness of such interventions are needed. We believe that this and similar computerized pathways have the potential to provide safe and effective care for a variety of acute and chronic illnesses, while maintaining patient satisfaction and possibly reducing health care costs.
Acknowledgments
Financial support for this study was provided by departmental funds and the discretionary research funds of one of the authors (R.G.). The funding agreement ensured all of the authors' independence in designing the study, interpreting the data, writing, and publishing the report.
REFERENCES
- 1.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287:2701–10. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–15. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 4.Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9–16. doi: 10.7326/0003-4819-135-1-200107030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Saint S, Scholes D, Fihn SD, Farrell RG, Stamm WE. The effectiveness of a clinical practice guideline for the management of presumed uncomplicated urinary tract infection in women. Am J Med. 1999;106:636–41. doi: 10.1016/s0002-9343(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 6.Barry HC, Hickner J, Ebell MH, Ettenhofer T. A randomized controlled trial of telephone management of suspected urinary tract infections in women. J Fam Pract. 2001;50:589–94. [PubMed] [Google Scholar]
- 7.Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463–8. doi: 10.1056/NEJM198208193070802. [DOI] [PubMed] [Google Scholar]
- 8.Brook I. Urinary tract and genito-urinary suppurative infections due to anaerobic bacteria. Int J Urol. 2004;11:133–41. doi: 10.1111/j.1442-2042.2003.00756.x. [DOI] [PubMed] [Google Scholar]
- 9.Farrell DJ, Morrissey I, De Rubeis D, Robbins M, Felmingham D. A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J Infect. 2003;46:94–100. doi: 10.1053/jinf.2002.1091. [DOI] [PubMed] [Google Scholar]
- 10.Navaneeth BV, Belwadi S, Suganthi N. Urinary pathogens' resistance to common antibiotics: a retrospective analysis. Trop Doct. 2002;32:20–2. doi: 10.1177/004947550203200110. [DOI] [PubMed] [Google Scholar]
- 11.Ti TY, Kumarasinghe G, Taylor MB, et al. What is true community-acquired urinary tract infection? Comparison of pathogens identified in urine from routine outpatient specimens and from community clinics in a prospective study. Eur J Clin Microbiol Infect Dis. 2003;22:242–5. doi: 10.1007/s10096-003-0893-7. [DOI] [PubMed] [Google Scholar]
- 12.Allerberger FJ, Dierich MP, Ebner A, et al. Urinary tract infection caused by nontyphoidal Salmonella: report of 30 cases. Urol Int. 1992;48:395–400. doi: 10.1159/000282362. [DOI] [PubMed] [Google Scholar]
- 13.Ramos JM, Aguado JM, Garcia-Corbeira P, Ales JM, Soriano F. Clinical spectrum of urinary tract infections due on nontyphoidal Salmonella species. Clin Infect Dis. 1996;23:388–90. doi: 10.1093/clinids/23.2.388. [DOI] [PubMed] [Google Scholar]
- 14.Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA. 2000;283:1583–90. doi: 10.1001/jama.283.12.1583. [DOI] [PubMed] [Google Scholar]
- 15.Fihn SD. Clinical practice. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259–66. doi: 10.1056/NEJMcp030027. [DOI] [PubMed] [Google Scholar]
- 16.Bjerrum L, Dessau RB, Hallas J. Treatment failures after antibiotic therapy of uncomplicated urinary tract infections. A prescription database study. Scand J Prim Health Care. 2002;20:97–101. [PubMed] [Google Scholar]
- 17.Lawrenson RA, Logie JW. Antibiotic failure in the treatment of urinary tract infections in young women. J Antimicrob Chemother. 2001;48:895–901. doi: 10.1093/jac/48.6.895. [DOI] [PubMed] [Google Scholar]