Abstract
When a patient with diabetes mellitus presents with worsening polyuria and polydipsia, what is a sensible, cost-effective approach? We report the unique coincidence of type 2 diabetes mellitus and diabetes insipidus. A 46-year-old woman with poorly controlled type 2 diabetes complained of polyuria with a daily output of 5 L. Although urinalysis demonstrated significant glucosuria, diabetes insipidus was suspected owing to a low urine specific gravity (1.008). The low specific gravity persisted during a water deprivation test. Ultimately, diabetes insipidus was confirmed when urine specific gravity and urine osmolality normalized following desmopressin administration. This case emphasizes the importance of accurately interpreting the urine specific gravity in patients with polyuria and diabetes mellitus to detect diabetes insipidus.
Keywords: urine specific gravity, diabetes mellitus, diabetes insipidus
Diabetes insipidus is a disorder that is characterized by complete or partial deficiency of antidiuretic hormone or by unresponsiveness to this hormone, such that patients present with polyuria and polydipsia. When a patient presents with polyuria and polydipsia, both diabetes mellitus and diabetes insipidus are the diseases that should be part of the differential diagnosis. Except for the well-defined inherited disorder, the DIDMOAD syndrome, which is characterized by diabetes insipidus (DI), type 1 diabetes mellitus (DM), optic atrophy (OA), and deafness (D),1 the concurrence of diabetes insipidus and diabetes mellitus, either type 1 or 2, has not been previously reported. In 1 isolated case, the association of diabetes insipidus and type 2 diabetes mellitus was described as a rare entity.2 This case emphasizes the importance of accurately interpreting the urine specific gravity in patients with polyuria and diabetes mellitus, to detect diabetes insipidus.
CASE REPORT
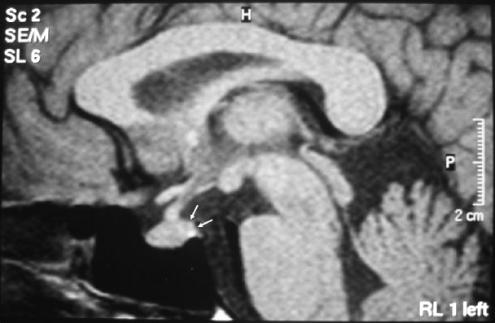
A 46-year-old woman was referred to our hospital because of worsening polyuria and polydipsia of 2 months duration. She had a 10-year history of type 2 diabetes mellitus, with poor glycemic control that required insulin. The patient drank an average of 5 L/d and her urine output was almost the same. Blood sampling for routine laboratory values were: hemoglobin (Hb), 8.8 mmol/L; platelet count (Plt), 230,000 per mm3; white blood cells (WBC), 6,320 per mm3; creatinine, 98 μmol/L; urea nitrogen, 5.2 mmol/L; uric acid, 351 μmol/L; aspartate transaminase (AST), 0.35 μkat/L; alanine transaminase ALT, 0.47 μkat/L; Na, 132 mmol/L; K, 4.5 mmol/L; glucose, 19.5 mmol/L (351 mg/dL); Ca, 2.3 mmol/L; albumin, 43 g/L; erythrocyte sedimentation rate, 19 mm/h; C-reactive protein 2.1 mg/L; and an HbA1C of 0.0107 Hb fraction. Urine analysis revealed +3 for glucose. A spot urine analysis revealed a urine specific gravity of 1.008 and a 24-hour urine collection revealed a urine specific gravity of 1.007 [reference range: 1.010 to 1.025].3,4 On repeated urine analysis, a 24-hour urine collection revealed a urine specific gravity of 1.008. Therefore, we suspected that diabetes insipidus might be the underlying cause, owing to the presence of a persistently low urine specific gravity. A urine and plasma osmolality was subsequently performed, which were 138 and 285 mmol/kg, respectively. These findings were consistent with diabetes insipidus. A water deprivation test was then performed. No significant increase occurred, either in urine specific gravity or urine osmolality. After the administration of desmopressin, urine specific gravity increased to 1.019 (in a 24-hour urine collection) and urine osmolality increased to 488 mmol/kg. In view of these results, a diagnosis of central diabetes insipidus was made. The patient's family history was unremarkable. The patient did not receive any medications except for insulin. The patient had no history of neurosurgery or trauma and no evidence of systemic illness such as weight loss and fever. The patient was not pregnant and during her last pregnancy, which was 10 years ago, she did not experience peripartum complications. A complete physical examination was normal, as were a PA chest radiograph and an electrocardiogram (ECG). An magnetic resonance imaging (MRI) revealed a decrease of hypophysis intensity (Fig. 1); however, this was considered a nonspecific finding.5 P-antineutrophil cytoplasmic antibody (P-ANCA), C-ANCA, and purified protein derivative (PPD) tests were negative. An angiotensin converting enzyme (ACE) level was normal. A repeat MRI scan at 6 months revealed similar findings. Thus, based on these results a diagnosis of idiopathic central diabetes insipidus was made. Hormonal values for other hypophysial hormones were in the normal range (adrenocorticotropin hormone [ACTH], 12.1 pmol/L; follicle stimulating hormone [FSH], 10.7 IU/L; leutenizing hormone [LH], 12.3 IU/L; prolactin, 12 μg/L; thyroid stimulating hormone [TSH], 3.2 μU/L). Complaints of polyuria and polydipsia resolved after treatment targeting diabetes insipidus was initiated. At the last followup visit, the patient was well with no complaints of polyuria. Further tests revealed a urine specific gravity of 1.015 and blood glucose of 8.3 mmol/L (151 mg/dL).
FIG. 1.
Arrows disclose the decreased intensity in the posterior hypophysis
DISCUSSION
When a diabetic patient presents with polyuria and polydipsia, the first etiology that should be considered is poor glycemic control. Achieving glycemic control remains the first course of action; however, the urine specific gravity should not be overlooked as it may provide evidence of concurrent diabetes insipidus.
Few conditions result in discordance between urine specific gravity and osmolality. These include administration of radiocontrast media, mannitol, or, high-dose carbenicillin, and the conditions of uremia and poorly controlled diabetes mellitus, the latter resulting in glucosuria.1–3 It is important to note that, even in these situations, urine osmolality does not change, while urine specific gravity increases.
Theoretically, in a patient with no significant renal disorder, all glucose present in the plasma passes through the glomerulus and is then absorbed by the tubular transport system at a maximum rate of 1.7 mmol/min (320 mg/min).6,7 However, maximum absorption is never achieved as all nephrons do not have the same capacity. Secondly, several nephrons can secrete glucose while not reaching their own maximum capacity. As a result, a level of 1.1 to 1.2 mmol/min (200 to 220 mg/min) is the threshold level observed in clinical practice.5 Blood glucose above this level is secreted in the urine; and, every 35 to 40 mmol/kg increment in urine osmolality increases the urine specific gravity by 0.001; consequently, every 0.05 mmol (10 mg) glucose/liter increases the urine specific gravity by 0.004.3,4,7 Thus, in uncontrolled diabetic patients, the urine specific gravity might reach 1.045 to 1.050 as a result of the above-mentioned loss of glucose in the urine.4
Specifically, in a patient with poor glycemic control elevated blood glucose levels should result in an increased urine specific gravity. Secondly, when diabetes insipidus occurs in a poorly controlled diabetic patient, the urine specific gravity might not be as low as expected as in diabetes insipidus. Consequently, urine specific gravity should be interpreted thoroughly in diabetic patients especially in poorly controlled situations, as it may be low, low normal, or normal owing to the degree of glucosuria.
Paulose and Padmakumar2 reported that the concurrence of type 2 diabetes mellitus and diabetes insipidus is a rare entity. United States census statistics have revealed that the incidence of diabetes insipidus is 1 in 6,666 (0.01%), while the incidence of type 2 diabetes mellitus is 1 in 340 (0.29%). Based upon these figures, we would expect that more than 100 people in the United States alone may have both diseases.
In general, distinguishing between diabetes mellitus and diabetes insipidus is not a challenging task. In most cases, a blood glucose measurement and urinalysis are sufficient. When a patient has diabetes mellitus, the urine specific gravity should be analyzed to avoid missing the diagnosis of concurrent diabetes insipidus.
In summary, in a poorly controlled diabetic patient, the urine specific gravity should exhibit a mild or moderate increase. When a patient with diabetes mellitus presents with worsening polyuria and a low or low normal urinary specific gravity, a diagnosis of diabetes insipidus must be considered. A routine urinalysis with specific gravity is a simple and inexpensive test that should not be overlooked, particularly in a diabetic patient with worsening polyuria. We suspect that the coexistence of type 2 diabetes mellitus and diabetes insipidus may not be as rare an entity as previously suggested.
REFERENCES
- 1.Lin CH, Lee YJ, Huang CY, et al. Wolfram (DIDMOAD) syndrome: report of two patients. J Pediatr Endocrinol Metab. 2004;17:1461–4. doi: 10.1515/jpem.2004.17.10.1461. [DOI] [PubMed] [Google Scholar]
- 2.Paulose KP, Padmakumar N. Diabetes insipidus in a patient with diabetes mellitus. J Assoc Physicians India. 2002;50:1176–7. [PubMed] [Google Scholar]
- 3.Fogazzi GB. Urinalysis and microscopy. In: Cameron JS, Davison AM, Grunfeld JP, Kerr O, Ritz E, editors. Oxford Textbook of Clinical Nephrology. New York: Oxford University Press; 1992. pp. 16–24. [Google Scholar]
- 4.Miller RB. Urinalysis. In: Massry SG, Glassock RJ, editors. Textbook of Nephrology. 2. Baltimore: Williams and Wilkins; 1989. pp. 1587–609. [Google Scholar]
- 5.Larsen PR. Williams Textbook of Endocrinology. 10. Philadelphia: Saunders; 2003. pp. 290–1. [Google Scholar]
- 6.Guyton AC, Hall JE. Textbook of Medical Physiology. 9. Philadelphia: W.B. Saunders; 1995. pp. 298–300. [Google Scholar]
- 7.Rose BD, Post TW. Clinical Physiology of Acid-Base and Electrolyte Disorder. 5. New York: McGraw Hill; 2001. pp. 410–2. [Google Scholar]