Abstract
Aggregatibacter actinomycetemcomitansW is an oral bacterium that causes localized aggressive periodontitis (LAP) and extra-oral infections such as sub-acute infective endocarditis. As part of its array of virulence factors, A. actinomycetemcomitans produces leukotoxin (LtxA), a member of the RTX family of toxins. LtxA kills human leukocytes and we have recently shown that the toxin is required for β -hemolysis by A. actinomycetemcomitans on solid medium. In other RTX toxin-producing bacteria, an outer membrane channel-forming protein, TolC, is required for toxin secretion and drug export. We have identified an ORF in A. actinomycetemcomitans that encodes a putative protein having predicted structural properties similar to TolC. Inactivation of this ORF resulted in a mutant that was no longer β -hemolytic and did not secrete LtxA. This mutant was significantly more sensitive to antimicrobial agents compared to the wild type strain and was unable to export the antimicrobial agent berberine. Thus, this ORF was named tdeA for “toxin and drug export”. Examination of the DNA sequence surrounding tdeA revealed two upstream ORFs that encode proteins similar to the drug efflux proteins, MacA and MacB. Inactivation of macB in A. actinomycetemcomitans did not alter the drug sensitivity profile or the hemolytic activity of the mutant. The genes macA, macB and tdeA are organized as an operon and are constitutively expressed as a single transcript. These results show that A. actinomycetemcomitans indeed requires a TolC-like protein for LtxA secretion and that this protein, TdeA, also functions as part of a drug efflux system.
Keywords: leukotoxin, periodontitis, endocarditis, outer membrane protein, antibiotics
1. INTRODUCTION
Aggregatibacter (formerly Actinobacillus (Nørskov-Lauritsen and Kilian, 2006)) actinomycetemcomitans is the etiologic agent of localized aggressive periodontitis (LAP), a destructive disease of the oral cavity (Zambon, 1996). A. actinomycetemcomitans produces several putative virulence factors (Fives-Taylor et al., 1999; Henderson et al., 2003) including the RTX (repeats in toxin) protein toxin, leukotoxin (LtxA) (Kolodrubetz et al., 1989; Lally et al., 1989b). Other toxins in the RTX family include Escherichia coli α -hemolysin (HlyA), Bordetella pertussis adenylate cyclase, Vibrio cholerae RTX toxin, and Mannheimia haemolytica leukotoxin. It is known that LtxA kills specifically white blood cells of humans and Old World Primates (Tsai et al., 1979; Tsai et al., 1984; Taichman et al., 1987). In addition, we recently found that purified A. actinomycetemcomitans LtxA is also able to lyse erythrocytes (Balashova et al., 2006).
The ltx operon in A. actinomycetemcomitans shares features common to the RTX operons of other bacteria. This operon consists of four genes, ltxC, ltxA, ltxB and ltxD. The first gene of the operon, ltxC, encodes a protein similar to E. coli HlyC (Kraig et al., 1990). HlyC is an acyl transferase required for activation via fatty acid modification of HlyA (Stanley et al., 1998). Lally et al. (Lally et al., 1994) found that expression of ltxC is required for the production of active LtxA in E. coli. The second gene of the operon, ltxA, encodes the ~114 kDa toxin LtxA (Kolodrubetz et al., 1989; Lally et al., 1989a; Lally et al., 1989b). The third gene in the leukotoxin operon, ltxB, encodes a protein with 83% amino acid identity to E. coli HlyB (Guthmiller et al., 1990a). In E. coli, HlyB is an integral inner membrane protein and ATP binding cassette (ABC) transporter that is part of a type I secretion system required for export of α -hemolysin (Hardie et al., 1991; Holland and Blight, 1999). The last gene in the leukotoxin operon, ltxD, encodes a protein that shares 68% amino acid identity with HlyD of E. coli (Guthmiller et al., 1990b). Like HlyB, HlyD is part of the type I secretion system required for export of α -hemolysin from E. coli and has been referred to as a membrane fusion protein (MFP) since it allows the formation of a channel connecting the inner and outer membranes (Mackman et al., 1985; Oropeza-Wekerle et al., 1989; Balakrishnan et al., 2001). Guthmiller et al. (Guthmiller et al., 1995) reported that mutations in A. actinomycetemcomitans ltxB or ltxD resulted in a significant decrease in the level of LtxA protein and concluded that LtxA is mislocalized in these mutants. We found that ltxB and ltxD mutants of A. actinomycetemcomitans fail to secrete LtxA, indicating that these gene products are involved in the active transport of the toxin out of the cell (M. P. Palacio, M. S. Duncan, and S. C. Kachlany, unpublished).
In E. coli, secretion of α -hemolysin requires a third protein, TolC (Wandersman and Delepelaire, 1990; Thanabalu et al., 1998). TolC is an outer membrane protein (OMP) that forms a trimeric export channel in the outer membrane of bacteria (Koronakis, 2003; Koronakis et al., 2004). The model for α -hemolysin secretion suggests that HlyD (in the periplasm) contacts both HlyB (at the inner membrane) and TolC (at the outer membrane) to form a channel through which the toxin is transported (Schlor et al., 1997; Balakrishnan et al., 2001; Gentschev et al., 2002).
In addition to its role in protein export, TolC also participates in the efflux of diverse small molecules including toxic substances and antibiotics, as part of a multidrug resistance (MDR) mechanism (Koronakis, 2003; Koronakis et al., 2004). The machinery required for drug efflux is similar to that involved in the secretion of protein toxins. TolC is also an important virulence factor as exemplified by the fact that TolC has been shown to be required for several bacteria to cause disease including Salmonella enterica serovar Enteritidis in mice (Stone and Miller, 1995; Nishino et al., 2006), S. enterica serovar Typhimurium in chicks (Baucheron et al., 2005), Vibrio cholerae in mice (Bina and Mekalanos, 2001), and Erwinia chrysanthemi in plants (Barabote et al., 2003)
To date, there is no published report or communication regarding the existence of a functional TolC-like protein in A. actinomycetemcomitans. Because LtxA is secreted from A. actinomycetemcomitans (Kachlany et al., 2000) and LtxB and LtxD are both required for this process (Guthmiller et al., 1995); M. P. Palacio, M. S. Duncan, and S. C. Kachlany, unpublished), we asked whether a TolC-like protein exists in A. actinomycetemcomitans. We report here the identification of a gene encoding a TolC-like protein that is required for LtxA secretion and involved in drug efflux in A. actinomycetemcomitans. This is the first report of a protein in A. actinomycetemcomitans that is involved in the efflux of antimicrobial compounds.
2. MATERIALS AND METHODS
2.1. Bacterial strains and culture conditions
A. actinomycetemcomitans strain IDH781 is an adherent, rough isolate and minimally leukotoxic strain (Haubek et al., 1995) that can be genetically transformed. Bacteria were grown in A. actinomycetemcomitans growth medium (AAGM; TSB + 0.6% yeast extract + 0.8% glucose + 0.4% NaHCO3) as previously described (Fine et al., 1999) or on Columbia agar with 5% sheep blood (PML Microbiologicals, Wilsonville, OR). After streaking bacteria on solid media, plates were incubated at 37° C in 10% CO2 for 3–4 days. Colonies were inoculated in AAGM broth and incubated for 24 hours at 37° C unless otherwise noted. The E. coli strain used for routine recombinant DNA manipulations was TOP10F’ (Invitrogen Corporation, Carlsbad, California). E. coli strains were grown on Luria-Bertani (LB) agar or in LB broth supplemented with Ampicillin or carbenicillin at 50 μ g/ml or kanamycin at 50 μ g/ml (Sambrook et al., 1989).
2.2. Generation of gene disruption mutants
Gene disruption mutants were generated using a vector (pMB78) (M. K. Bhattacharjee, B. A. Perez, S. C. Kachlany, and D. H. Figurski, unpublished) that contains A. actinomycetemcomitans uptake sequences (AAAGTGCGGTC) (Thomson et al., 1999). Briefly, genes to be disrupted (ltxA, tdeA, and macB) were first amplified using the EXPAND PCR kit (Roche Molecular Systems Inc, Branchburg, NJ) and cloned into pCR2.1 TOPO in E. coli (Invitrogen Corporation, Carlsbald, CA). The ORFs were then subcloned into pMB78. Plasmid DNA was purified from E. coli and in vitro transposon mutagenesis (EZ-Tn<KAN-2>; Epicentre Biotechnologies, Madison, WI) was then used to obtain kanamycin resistance gene disruptions. Gene disruption mutants were isolated on LB containing 50 μ g/ml kanamycin. The fragments carrying the kanamycin transposable element interrupting the desired genes plus the uptake sequence were obtained and used to transform IDH781 based on a procedure described by Wang et al. (Wang et al., 2002). Briefly, adherent IDH781 cells from a 25 ml overnight culture (AAGM) were removed from the wall of the tube, centrifuged, and resuspended in 5 ml of fresh culture medium. The mixture was vortex mixed for about 1 minute and allowed to stand for 2 minutes in order to allow large clumps and debris to settle. The OD600 of the resulting suspension was adjusted to a value of 0.1 by adding fresh AAGM. A 10 ml aliquot of this suspension was supplemented with cyclic AMP to 2 mM (Sigma, St. Louis, MO) and incubated at 37° C for one hour. Cells were then collected by brief centrifugation, resuspended in 200 μ l fresh liquid medium with cAMP, and mixed with DNA in a 1.5 ml microcentrifuge tube. After incubation at 37° C for two hours, the cells were plated on selective medium (AAGM with 40 μ g/ml kanamycin). Antibiotic resistant colonies appeared between 3 and 6 days later. Correct recombination of the gene disrupted by the kan transposable element was confirmed by PCR.
2.3. Reverse transcription-PCR
Total bacterial RNA was isolated using TRIzol (Invitrogen Corporation, Carlsbad, CA) and further purified by DNase I treatment followed by passage through an RNeasy spin column (Qiagen, Valencia, CA). The RT-PCR reaction was subsequently carried out using the OneStep RT-PCR Kit (Qiagen, Valencia, CA). Primers used are shown in Table 1. Reactions were carried out in duplicate at least twice.
Table 1.
Primer pairs used for RT-PCR
| PCR product | Primer Pairs | Sequence (5’ → 3’) |
|---|---|---|
| A | MacA_F1L | CACCGCCGAAACCAACGTGGGC |
| MacB_R2 | GGCTGCGTAAATCGGAAAGCTG | |
| B | MacA_3'F | GAAACCTTCGGCGATCCTGATGCC |
| TdeA_R2 | GATAAGAATCGCCAATATTGG | |
| C | MacB_3'F | CCGATTACAGCGTTGGCGCAAG |
| TdeA_R1 | CGCCTAAGGTAATTTCCGGG | |
| G | MacA_3'F | GAAACCTTCGGCGATCCTGATGCC |
| Kan2_R | GTGCAATGTAACATCAGAGATTTTGAGAC | |
| H | MacB_3'F | CCGATTACAGCGTTGGCGCAAG |
| TdeA_R1 | CGCCTAAGGTAATTTCCGGG | |
| K | MacB_3'F | CCGATTACAGCGTTGGCGCAAG |
| Kan2_R | GTGCAATGTAACATCAGAGATTTTGAGAC | |
| L | MacB_F2 | GCTTTCCGATTTACGCAGCC |
| MacB_R1 | ATCGTCATTGTGTTTGTGCCGATACCG |
2.4. Sequence analysis
DNA was sequenced by the New Jersey Medical School Molecular Resource Facility. DNA and predicted protein sequences were analyzed using Internet-based bioinformatics tools. The initial batch-BLAST search was done using the BLAST++ server at the National University of Singapore (http://xena1.ddns.comp.nus.edu.sg/~genesis/blast++) (Wang et al., 2003). Protein sequence analysis was done using the resources available from the Max Planck Institute for Developmental Biology (http://protevo.eb.tuebingen.mpg.de/toolkit/).
2.5. SDS-PAGE and western blot analysis
Secreted proteins were concentrated from culture supernatants using trichloroacetic acid, washed with cold acetone and dissolved in Laemmli sample buffer (Sambrook et al., 1989). Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose membranes as described previously (Sambrook et al., 1989). Immunodetection was carried out using rabbit polyclonal anti-LtxA antibody (Diaz et al., 2006) and alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (Bio-Rad Laboratories, Hercules, CA). Bound antibody was detected using BCIP (5-bromo-4-chloro-3-indolylphosphate) and nitroblue tetrazolium (Bio-Rad Laboratories, Hercules, CA).
2.6. Antimicrobial susceptibility testing
The minimal inhibitory concentrations (MICs) of 14 antimicrobial agents (Sigma, St. Louis, MO) were determined by a modified version of the standard agar dilution susceptibility testing method (Reynolds et al., 2003). The chosen compounds have different chemical characteristics that have previously been used to test for the function of outer membrane efflux proteins (Sulavik et al., 2001). Briefly, stock solutions of the antimicrobial agents at concentrations of 6.4 mg/ml were prepared and serial 1:2 dilutions were made and one hundred microliters of each solution was mixed with 5 ml of agar to give the required concentrations between 128 and 0.015 μ g/ml. Drug-supplemented AAGM agar was prepared in 6-well tissue culture plates and cell suspensions containing ~1x103 CFUs of the strains being tested were plated. The assays were done at least twice in duplicate. After incubation at 37°C in 10% CO2 for 72 h, the plates were evaluated and the lowest drug concentration that did not allow any growth was designated as the MIC.
2.7. Measurement of drug accumulation and efflux
We followed the procedure described by Stermitz et al. (Stermitz et al., 2000). Briefly, cells were cultured as described above, washed in PBS and resuspended in PBS at an OD600 of 0.3. Berberine uptake was measured by mixing an aliquot of cells with an equal volume of a 60 μ g/ml berberine sulfate (Sigma, St. Louis, MO) solution in PBS supplemented with 0.4% glucose. To measure efflux, cells were resuspended in PBS containing 0.2 μ g/ml carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and 30 μ g/ml berberine followed by incubation at 37°C for 30 min. The cells were centrifuged, washed twice, and resuspended in PBS + 0.4% glucose at an OD600 of 0.15. Fluorescence was measured using a Synergy HT microplate reader (Bio-Tek Instruments, Inc., Winooski, VT), with a 360/40-nm excitation filter and a 530/25-nm emission filter.
2.8. DNA accession numbers
The DNA sequences reported here have been submitted to EMBL-GenBank. Accession number DQ378165 has been assigned to macA/macB and DQ378166 to tdeA.
3. RESULTS
3.1. Identification of a tolC-like gene in A. actinomycetemcomitans
To identify tolC-like candidate genes in the genome of A. actinomycetemcomitans strain HK1651 (http://www.genome.ou.edu/act.html), we first selected all of the translated open reading frames (ORFs) that encode putative proteins larger than 400 amino acid residues. From this criterion, we obtained 478 ORFs. We then carried out a batch-BLAST search (Wang et al., 2003) against the SWISSPROT database. This search revealed an ORF with significant similarity to bacterial proteins involved in drug efflux. This ORF corresponds to AA02077 in the annotated ORALGEN genome database (http://www.oralgen.lanl.gov/) and will be referred to as TdeA. We identified two potential start codons for tdeA 36 nucleotides apart. AA02077 initiates at the second start codon; however, based on the requirement of signal sequences to achieve proper localization in other outer membrane proteins, we believe that tdeA initiates at the first codon to include a potential signal sequence. Thus, tdeA encodes a putative 457 aminoacid polypeptide (51.1 kDa).
Analysis of TdeA reveals features that are typical for TolC-like proteins (Figure 1A). At the N-terminus a peptide of 22 amino acids (MFTIKKLTLTIVVATTLTGCAN) is predicted (Bendtsen et al., 2004). This sequence would be essential for proper localization of TdeA (Nilsson et al., 1993). Secondary structure prediction for TdeA yields a structure that includes two β -sheet-loop-β -sheet segments flanked by α-helical domains. The β -sheets would participate in the formation of the outer membrane channel as has been described for other bacterial OMPs, while the α -helical segments will form the ‘tunnel domain’ within the periplasmic space (Koronakis, 2003). In addition, weak internal similarity (~20% amino acid identity) is found between the two halves of TdeA, a property that is characteristic of the outer membrane drug efflux family of proteins (Andersen et al., 2000; Sharff et al., 2001). Thus, our analysis of TdeA indicates that this putative protein conforms to known properties of other outer membrane channel-forming proteins.
Figure 1.

A, Structural properties of A. actinomycetemcomitans TdeA that are shared among TolC family members. TolC (from E. coli), VceC (from Vibrio cholerae), and TdeA all have a similar number and arrangement of α -helical and β -sheet regions. TdeA has a predicted cleavable signal peptide, similar to other TolC family members. B, Arrangement of macA, macB, and tdeA genes in the A. actinomycetemcomitans genome. The strain number and arrowheads denote the locations of kan cassette insertions within the macB and tdeA mutants.
A BLAST search revealed that TdeA is similar to other putative outer membrane proteins including those from H. influenzae (NP_439611.1; 74% amino acid identity), Pasteurella multocida (NP_245464.1; 63% amino acid identity), and Actinobacillus pleuropneumoniae (gi46143366; 55% amino acid identity). The closest related proteins for which biological activity has been demonstrated and crystal structures are available are VceC from Vibiro cholerae (21% amino acid identity) (Federici et al., 2005) and E. coli TolC (21% amino acid identity) (Koronakis et al., 2000; Koronakis, 2003).
3.2. TdeA is required for LtxA secretion
Because of the TolC-like properties displayed by TdeA, we tested whether the protein was involved in secretion of LtxA from bacteria. The gene was disrupted with a kanamycin resistance transposon and transformed into A. actinomycetemcomitans as described in Materials and methods. Recombinants that contained an inactivated chromosomal copy of tdeA were confirmed by PCR analysis and the PCR products were sequenced to determine the precise site of insertion (data not shown). Figure 1B shows the relative location of the transposon within the tdeA gene (strain Aa323).
We next determined whether the tdeA mutant was able to secrete LtxA. LtxA secretion was assayed using two different approaches. First, we took advantage of the knowledge that LtxA is required for β -hemolysis on blood agar (Balashova et al., 2006). Like the ltxA mutant (Balashova et al., 2006), we found that the tdeA mutant showed no β -hemolysis on Columbia agar with 5% sheep blood (Figure 2A), indicating inability to secrete LtxA. We also examined secreted LtxA directly using western blot analysis (Figure 2B). In contrast to wild type strain IDH781 (Figure 2B, lane 1), the tdeA mutant did not secrete LtxA into culture supernatants (Figure 2B, lane 2). These results indicate that TdeA is required for LtxA secretion.
Figure 2.

Phenotypes of the A. actinomycetemcomitans mutants. A, β -hemolysis assay on Columbia agar with 5% sheep blood. The wild type (W.T.) strain and various mutants were streaked onto blood agar and then incubated for several days as described in Materials and methods. A β -hemolytic reaction was seen as a clearing around the bacteria. B, western blot analysis of secreted protein. Secreted protein subjected to western blot analysis probing with anti-LtxA antibody as described in Materials and methods. Lane 1, wild type strain IDH781; lane 2, tdeA::kan; lane 3, ltxA::kan; lane 4, macB::kan. The arrowhead notes the location of LtxA.
3.3. TdeA is involved in drug efflux
Because other bacterial TolC-like proteins are involved in drug efflux, we tested the sensitivity of the tdeA mutant to 14 drugs and toxic compounds that belong to several different chemical classes. Figure 3A shows that the wild type strain is significantly more resistant than the tdeA mutant to at least 8 out of the 14 drugs tested here. The greatest sensitivity of the mutant was seen towards clotrimazole (CLO), erythromycin (ERY), and ethidium bromide followed by cationic detergents, bile-acids, anionic detergents, and chloramphenicol (Figure 3A). The overall reduction in antimicrobial resistance of the tdeA mutant compared to the wild type strain is consistent with the phenotype of tolC mutants of other gram-negative bacteria (Koronakis et al., 2004; Nishino et al., 2006). We therefore conclude that TdeA is also part of a general efflux system involved in the export of antimicrobial agents.
Figure 3.

The role of TdeA in drug resistance and efflux. A, Decreased resistance of the tdeA mutant to antimicrobial agents. Bacteria were plated on media that contained the various agents noted in the graph, and the MIC was determined to be the lowest concentration at which bacteria did not grow. The graph shows the ratio of the MIC values for the wild type versus the tdeA mutant strain. The absolute MIC values for the wild type (w.t.) and the tdeA mutant strains are noted below the graph. DOC, deoxycholate; HTAB, hexadecyl trimethyl ammonium bromide; SDS, sodium dodecyl sulfate; EtBr, ethidium bromide; CLO, clotrimazole; ERY, erythromycin; PLU, plumbagin; ACR, acriflavine hydrochloride; CAM, chloramphenicol; NAL, nalidixic acid; TET, tetracycline; CCCP, Carbonyl cyanide 3-chlorophenylhydrazone; IRG, irgasan; NOR, norfloxacin. B, Kinetics of accumulation (left) and efflux (right) of berberine in wild-type and tdeA::kan and macB::kan mutants of A. actinomycetemcomitans. Cells were resuspended in PBS and treated with berberine as described in Materials and methods. Accumulation is measured immediately after adding the dye. Fluorescence is proportional to the cell-associated dye. For efflux, the results are shown as percent of the maximum (first time point).
To assess the contribution of TdeA to drug efflux in A. actinomycetemcomitans, we examined the kinetics of accumulation and efflux of berberine, an alkaloid whose fluorescence is enhanced when bound to DNA (Yamagishi, 1962). Berberine resembles ethidium bromide, accumulates inside cells driven by the membrane potential, (Mikes and Dadak, 1983) and is extruded by multidrug resistance pumps (MDRs) found in bacteria (Stermitz et al., 2000; Barabote et al., 2003). Figure 3B shows that while the alkaloid is incorporated by the wild-type and tdeA mutant strains, the tdeA mutant accumulates it at a higher rate (left panel). In addition, dye efflux in the tdeA mutant proceeds slowly while the wild-type strain is able to rapidly eliminate the dye (right panel). Taken together, these results indicate that the tdeA mutation does not adversely affect the ability to maintain the integrity and function of the cell membrane, but does impair a general efflux system. Because of the role that TdeA plays in both LtxA secretion and drug efflux, the designation of tdeA was chosen (for toxin and drug export).
3.4. The tdeA gene locus
In many bacteria, tolC-like genes encoding outer membrane efflux proteins (OEPs) are part of an operon encoding other drug efflux proteins (Gotoh et al., 1995; Hagman et al., 1995; Zgurskaya and Nikaido, 2000; Grkovic et al., 2002). We therefore examined the region surrounding tdeA and found two partially overlapping open reading frames upstream of tdeA (Figure 1B). BLASTX searches of these two ORFs produced significant matches to the Neisseria gonorrhoeae macrolide-specific membrane fusion protein, MacA (AA02080; 50% amino acid identity), and the ABC transporter, MacB (AA02081; 65% amino acid identity). In E. coli and N. gonorrhoeae, MacA and MacB are part of drug efflux systems involved in macrolide recognition and export (Kobayashi et al., 2001; Rouquette-Loughlin et al., 2005). The putative A. actinomycetemcomitans MacA protein is 394 residues long (42.3 Kda) and a predicted single transmembrane helix between residues 5 and 23 would serve to anchor the polypeptide to the inner membrane. The putative MacB protein is 657 residues long (71.4 Kda) and its amino terminus contains the Walker A (GxxGxGKST) and B motifs (IILADE), as well as the linker peptide (LSGGQQQRVS) and the D (GALD) and Q loops (FIFQ) that are part the nucleotide binding domain (NBD) characteristic of transport ATPases (Davidson and Chen, 2004). Between four and six transmembrane domains are predicted at the C-terminal half of the protein and these domains may play roles in both inner membrane anchoring and formation of the translocation channel (Davidson and Chen, 2004).
We wished to determine if the putative macAB genes in A. actinomycetemcomitans were expressed. We therefore carried out reverse transcription-PCR (RT-PCR) reactions using primer pairs that amplified the regions noted in Figure 4A. We found that all transcripts were produced indicating that all three genes are transcribed together (Figure 4B, lanes A, B, C). Upon RNase treatment prior to the RT-PCR, no products were obtained, confirming that we were not amplifying DNA (Figure 4B, lanes D, E, and F). Thus, macA, macB, and tdeA are expressed as a single transcript.
Figure 4.
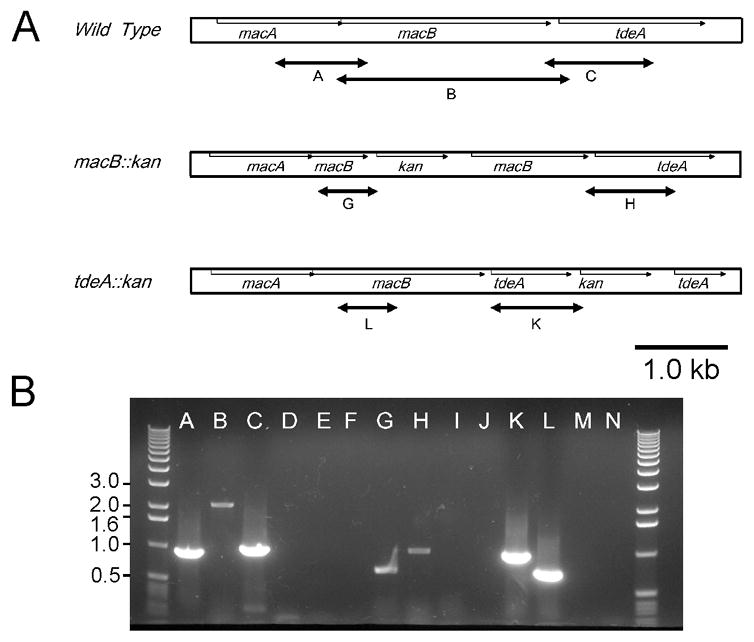
Analysis of macA, macB, and tdeA transcripts by RT-PCR. RNA was prepared from the wild type, macB, and tdeA mutant strains as described in Methods. A, The regions amplified are noted by the lettered-arrows and are to scale. The kan gene designation refers to the transposon insertion. B, Results from RT-PCR to amplify the regions noted in A. The letters of each lane correspond to the regions noted in A. Lanes D, E, F, I, J, M, and N are RNase-treated controls of A, B, C, G, H, K, and L, respectively. The numbers on the side refer to size in kb.
To confirm that the tdeA mutation did not affect the expression of other genes within the locus, we performed RT-PCR with the A. actinomycetemcomitans tdeA mutant using primers to amplify an internal fragment of macB (Figure 4A, fragment L) and the fragment representing the fusion between tdeA and the kan insertion (Figure 5A, fragement K). As shown in Figure 4B, both the macB and tdeA-kan fusion fragments were produced (lanes K and L). Pretreatment of the samples with RNase eliminated the products (Figure 4B, lanes M and N).
3.5. MacB has no detectable role in drug export
Because of the significant similarity of A. actinomycetemcomitans MacB to proteins that may participate in drug efflux, we tested whether it was involved in drug efflux. We reasoned that if MacB is the ATPase component of an efflux system, its inactivation should abolish the function of the pump assembly (van Veen et al., 2000; Fernandez-Recio et al., 2004). A transposon insertion mutation was generated in macB (see Materials and methods). The site of insertion was confirmed by PCR and sequence analysis (data not shown). The insertion occurs just after the predicted Walker A box (Figure 1B; strain Aa324). We tested the macB mutant strain for drug sensitivity and found that the sensitivity profile of this mutant was indistinguishable from that of the wild type strain (data not shown). Furthermore, the behavior of the macB mutant is identical to that of the wild type strain in the dye accumulation and efflux assay (Figure 3B). This indicates that the putative A. actinomycetemcomitans MacB-like protein (AA02081) does not participate in efflux of the drugs we tested. We also wished to determine if the macB mutant was affected in LtxA secretion. Figure 2 shows that the macB mutant was still β -hemolytic (Figure 2A) and the strain secreted LtxA into culture supernatants (Figure 2B, lane 4). Thus, MacB does not play a role in LtxA secretion or drug efflux in A. actinomycetemcomitans.
To confirm that the mutation in macB did not affect expression of genes within the locus, we examined RNA transcript in the macB mutant strain using RT-PCR (Figure 4). Fragment G represents the fusion between macB and the kan insertion; fragment H represents the transcript spanning from the 3’ end of macB through the 5’ half of tdeA (Figure 4A). Consistent with the antimicrobial susceptibility, dye accumulation and β -hemolysis results noted above, we found that the macB mutant still expressed the ORF encoding TdeA (Figure 4B, lane H).
4. DISCUSSION
We have identified a gene in A. actinomycetemcomitans whose product is required for secretion of LtxA and resistance to antimicrobial compounds. Based on similarities to other efflux pumps, TdeA is likely an essential component of a drug efflux system in A. actinomycetemcomitans. Because of the phenotypes observed here, we have named the gene tdeA (toxin and drug export). Based on the sequence similarity and predicted structural properties of TdeA, we propose that TdeA belongs to the bacterial outer membrane efflux protein (OEP) family, which includes TolC as the prototypical member (Sharff et al., 2001; Koronakis, 2003; Holland et al., 2005). In contrast to the Oralgen database annotation, we have identified a 22 amino acid signal sequence that would allow the protein to be transported across the inner membrane, an essential step for the assembly of a functional outer membrane channel.
A characteristic among the TolC-family of proteins is lack of significant amino acid similarity (Andersen et al., 2000). Indeed, Andersen et al. (Andersen et al., 2000) noted that TolC-like proteins share remarkable structural similarities with each other, but often lack significant sequence similarities. Structural predictions for TdeA correspond closely with known structures of other bacterial TolC proteins including the presence of a leader peptide, the number and locations of β -sheets (which form the outer membrane pore) and α -helical domains, and internal similarity between the two halves of the protein (Koronakis et al., 2000; Koronakis et al., 2004; Federici et al., 2005).
The TolC family of outer membrane proteins is ubiquitous among Gram negative bacteria and their role in protein secretion has been well established (Wandersman and Delepelaire, 1990). During secretion of HlyA in E. coli, the toxin interacts with both HlyB and HlyD, which then triggers the recruitment of trimeric TolC to the complex (Holland et al., 2005). HlyA is then transported to the surface of the cell through the transenvelope channel in an unfolded state (Holland et al., 2005). Prior to recruitment to the HlyB/D complex, the TolC pore is closed on the periplasmic side, but open to the extracellular environment. When TolC is recruited by HlyB/D, the pore opens on the periplasmic side via an iris-like mechanism, allowing passage of the toxin directly from the cytosol to the outside (Koronakis, 2003; Koronakis et al., 2004; Holland et al., 2005). In A. actinomycetemcomitans, we propose that LtxB, LtxD, and TdeA associate with each other to form a complex through which LtxA is secreted.
In this work, we show that TdeA is the otherwise missing component of a type I secretion system required for LtxA export. While LtxA can be associated with the outer membrane (Ohta et al., 1991; Berthold et al., 1992; Johansson et al., 2000; Diaz et al., 2006), released within lipid vesicles (Kato et al., 2002; Demuth et al., 2003), and secreted from cells as soluble protein (Brogan et al., 1994; Kachlany et al., 2000), we believe that TdeA plays a role in all of these processes. Because RTX toxins are transported through the bacterial cell envelope without a periplasmic intermediate (Gray et al., 1986; Koronakis et al., 2004), LtxA would have to be exported through TdeA before localizing to the outer membrane or within vesicles. Observations about LtxA localization fit the model proposed by Balsalobre et al. (Balsalobre et al., 2006), who recently reported that E. coli α -hemolysin localization to outer membrane-derived vesicles occurs upon assembly of the complete type I secretion machinery.
Gram-negative bacteria utilize drug efflux pumps to actively resist antimicrobial agents. Efflux pumps are widespread and some bacteria harbor multiple efflux systems. In many cases, the functional pump consists of three components: the inner membrane transporter, the periplasmic lipoprotein, and the outer membrane channel (Zgurskaya and Nikaido, 2000). TolC-like proteins are required for several different drug efflux systems including the AcrAB (Nikaido and Zgurskaya, 2001), EmrAB (Lomovskaya and Lewis, 1992), MacAB (Kobayashi et al., 2001), MdtABC (Perreten et al., 2001; Nagakubo et al., 2002), and AcrEF (Lau and Zgurskaya, 2005) efflux systems. We have shown here that TdeA is required for the resistance of A. actinomycetemcomitans to diverse antimicrobial agents and that its function is directly linked to an active efflux system, as demonstrated by the berberine accumulation/efflux assay. Thus, based on the multi-functionality of TolC-like proteins in other bacteria and data we present here, we believe that TdeA indeed plays a central role in the resistance to toxic compounds by A. actinomycetemcomitans. To our knowledge, this is the first report of a protein that is involved in antimicrobial resistance in A. actinomycetemcomitans.
We identified two genes immediately upstream of tdeA that encode putative proteins with similarity to components of a drug efflux pump. The two ORFs –AA02081 and AA02080– would encode proteins with similarity to MacB and MacA, respectively, of N. gonorrhoeae (Rouquette-Loughlin et al., 2005) and E. coli (Kobayashi et al., 2001).
We found that a mutation in macB did not produce a noticeable phenotype or alter the drug resistance profile of A. actinomycetemcomitans. Several reasons can explain why the macB mutant had no apparent drug-resistance phenotype. First, it is possible that the activities of MacA and MacB are not at all related to drug efflux, instead being devoted to the secretion of unknown products. Second, it is possible redundant drug efflux systems exist in A. actinomycetemcomitans that are masking the effects of the macB mutation. Sulavik et al. (Sulavik et al., 2001) found that a deletion of the macAB (ybjYZ) genes in E. coli produced no change in drug susceptibility. In their study, the macAB deletion strain was still wild type for all the other transporters, whose activity might have masked the macAB deletion. Third, it is possible that over-expression of MacA and MacB is required to observe the reported drug resistance phenotype. Indeed, the macrolide resistance phenotype was described for an E. coli strain that very likely over-produced the proteins since the genes were carried by a multicopy plasmid (Kobayashi et al., 2001). Alternatively, MacA and MacB may constitute an efflux system that exports compounds that were not tested here. In support of this possibility, other workers have found that nearly identical efflux systems (by sequence similarity) from similar bacteria can confer resistance to different drugs (Lomovskaya and Lewis, 1992; Edgar and Bibi, 1997; Nishino and Yamaguchi, 2001; Baucheron et al., 2005; Nishino et al., 2006). Finally, while the macAB transcript is made, the protein products may be unstable or degraded under laboratory culture conditions. Thus, the function of TdeA does not depend on MacB.
The identification of TdeA in A. actinomycetemcomitans allows us to generate a more complete model of LtxA secretion and resistance to various antimicrobial agents. With the work and tools we present here, it is now possible to further characterize the molecular mechanism of LtxA secretion and antibiotic resistance in A. actinomycetemcomitans.
Acknowledgments
We thank Dr. K. Kannan, Maria Isaza, Nataliya Balashova, Radha Yamarthy, Katrina Chang, and Jigna Patel for suggestions and help throughout the project. We thank Narayanan Ramasubbu for careful review of this manuscript. We acknowledge the A. actinomycetemcomitans Genome Sequencing Project for the availability of their data and the ORALGEN database for annotation data. This work was generously supported by a grant from the National Institute of Dental and Craniofacial Research (R01 DE16133).
Abbreviations
- LtxA
leukotoxin
- LAP
localized aggressive periodontitis, A., Aggregatibacter
- TdeA
toxin and drug export protein A
- ORF
open reading frame
- kan
kanamycin
- MFP
membrane fusion protein
- OEP
outer membrane efflux protein
- OMP
outer membrane protein
- AAGM
A. actinomycetemcomitans growth medium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen C, Hughes C, Koronakis V. Chunnel vision. Export and efflux through bacterial channel-tunnels. EMBO Rep. 2000;1:313–8. doi: 10.1093/embo-reports/kvd075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan L, Hughes C, Koronakis V. Substrate-triggered recruitment of the TolC channel-tunnel during type I export of hemolysin by Escherichia coli. J Mol Biol. 2001;313:501–10. doi: 10.1006/jmbi.2001.5038. [DOI] [PubMed] [Google Scholar]
- Balashova NV, Crosby JA, Al Ghofaily L, Kachlany SC. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect Immun. 2006;74:2015–21. doi: 10.1128/IAI.74.4.2015-2021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre C, Silvan JM, Berglund S, Mizunoe Y, Uhlin BE, Wai SN. Release of the type I secreted alpha-haemolysin via outer membrane vesicles from Escherichia coli. Mol Microbiol. 2006;59:99–112. doi: 10.1111/j.1365-2958.2005.04938.x. [DOI] [PubMed] [Google Scholar]
- Barabote RD, Johnson OL, Zetina E, San Francisco SK, Fralick JA, San Francisco MJ. Erwinia chrysanthemi tolC is involved in resistance to antimicrobial plant chemicals and is essential for phytopathogenesis. J Bacteriol. 2003;185:5772–8. doi: 10.1128/JB.185.19.5772-5778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucheron S, Mouline C, Praud K, Chaslus-Dancla E, Cloeckaert A. TolC but not AcrB is essential for multidrug-resistant Salmonella enterica serotype Typhimurium colonization of chicks. J Antimicrob Chemother. 2005;55:707–12. doi: 10.1093/jac/dki091. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Berthold P, Forti D, Kieba IR, Rosenbloom J, Taichman NS, Lally ET. Electron immunocytochemical localization of Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiol Immunol. 1992;7:24–7. doi: 10.1111/j.1399-302x.1992.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Bina JE, Mekalanos JJ. Vibrio cholerae tolC is required for bile resistance and colonization. Infect Immun. 2001;69:4681–5. doi: 10.1128/IAI.69.7.4681-4685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogan JM, Lally ET, Poulsen K, Kilian M, Demuth DR. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect Immun. 1994;62:501–8. doi: 10.1128/iai.62.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AL, Chen J. ATP-binding cassette transporters in bacteria. Annu Rev Biochem. 2004;73:241–68. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- Demuth DR, James D, Kowashi Y, Kato S. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell Microbiol. 2003;5:111–21. doi: 10.1046/j.1462-5822.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- Diaz R, Ghofaily LA, Patel J, Balashova NV, Freitas AC, Labib I, Kachlany SC. Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microb Pathog. 2006;40:48–55. doi: 10.1016/j.micpath.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Edgar R, Bibi E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J Bacteriol. 1997;179:2274–80. doi: 10.1128/jb.179.7.2274-2280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici L, Du D, Walas F, Matsumura H, Fernandez-Recio J, McKeegan KS, Borges-Walmsley MI, Luisi BF, Walmsley AR. The crystal structure of the outer membrane protein VceC from the bacterial pathogen Vibrio cholerae at 1.8 A resolution. J Biol Chem. 2005;280:15307–14. doi: 10.1074/jbc.M500401200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Recio J, Walas F, Federici L, Venkatesh Pratap J, Bavro VN, Miguel RN, Mizuguchi K, Luisi B. A model of a transmembrane drug-efflux pump from Gram-negative bacteria. FEBS Lett. 2004;578:5–9. doi: 10.1016/j.febslet.2004.10.097. [DOI] [PubMed] [Google Scholar]
- Fine DH, Furgang D, Schreiner HC, Goncharoff P, Charlesworth J, Ghazwan G, Fitzgerald-Bocarsly P, Figurski DH. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: implications for virulence. Microbiology. 1999;145(Pt 6):1335–47. doi: 10.1099/13500872-145-6-1335. [DOI] [PubMed] [Google Scholar]
- Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;20:136–67. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Gentschev I, Dietrich G, Goebel W. The E. coli alpha-hemolysin secretion system and its use in vaccine development. Trends Microbiol. 2002;10:39–45. doi: 10.1016/s0966-842x(01)02259-4. [DOI] [PubMed] [Google Scholar]
- Gotoh N, Tsujimoto H, Poole K, Yamagishi J, Nishino T. The outer membrane protein OprM of Pseudomonas aeruginosa is encoded by oprK of the mexA-mexB-oprK multidrug resistance operon. Antimicrob Agents Chemother. 1995;39:2567–9. doi: 10.1128/aac.39.11.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L, Mackman N, Nicaud JM, Holland IB. The carboxy-terminal region of haemolysin 2001 is required for secretion of the toxin from Escherichia coli. Mol Gen Genet. 1986;205:127–33. doi: 10.1007/BF02428042. [DOI] [PubMed] [Google Scholar]
- Grkovic S, Brown MH, Skurray RA. Regulation of bacterial drug export systems. Microbiol Mol Biol Rev. 2002;66:671–701. doi: 10.1128/MMBR.66.4.671-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller JM, Kolodrubetz D, Cagle MP, Kraig E. Sequence of the lktB gene from Actinobacillus actinomycetemcomitans. Nucleic Acids Res. 1990a;18:5291. doi: 10.1093/nar/18.17.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller JM, Kolodrubetz D, Kraig E. Mutational analysis of the putative leukotoxin transport genes in Actinobacillus actinomycetemcomitans. Microb Pathog. 1995;18:307–21. doi: 10.1006/mpat.1995.0028. [DOI] [PubMed] [Google Scholar]
- Guthmiller JM, Kraig E, Cagle MP, Kolodrubetz D. Sequence of the lktD gene from Actinobacillus actinomycetemcomitans. Nucleic Acids Res. 1990b;18:5292. doi: 10.1093/nar/18.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995;141(Pt 3):611–22. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- Hardie KR, Issartel JP, Koronakis E, Hughes C, Koronakis V. In vitro activation of Escherichia coli prohaemolysin to the mature membrane-targeted toxin requires HlyC and a low molecular-weight cytosolic polypeptide. Mol Microbiol. 1991;5:1669–79. doi: 10.1111/j.1365-2958.1991.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Haubek D, Poulsen K, Asikainen S, Kilian M. Evidence for absence in northern Europe of especially virulent clonal types of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1995;33:395–401. doi: 10.1128/jcm.33.2.395-401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Nair SP, Ward JM, Wilson M. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol. 2003;57:29–55. doi: 10.1146/annurev.micro.57.030502.090908. [DOI] [PubMed] [Google Scholar]
- Holland IB, Blight MA. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J Mol Biol. 1999;293:381–99. doi: 10.1006/jmbi.1999.2993. [DOI] [PubMed] [Google Scholar]
- Holland IB, Schmitt L, Young J. Type 1 protein secretion in bacteria, the ABC-transporter dependent pathway (review) Mol Membr Biol. 2005;22:29–39. doi: 10.1080/09687860500042013. [DOI] [PubMed] [Google Scholar]
- Johansson A, Sandstrom G, Claesson R, Hanstrom L, Kalfas S. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur J Oral Sci. 2000;108:136–46. doi: 10.1034/j.1600-0722.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- Kachlany SC, Fine DH, Figurski DH. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect Immun. 2000;68:6094–100. doi: 10.1128/iai.68.11.6094-6100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32:1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Nishino K, Yamaguchi A. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J Bacteriol. 2001;183:5639–44. doi: 10.1128/JB.183.19.5639-5644.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodrubetz D, Dailey T, Ebersole J, Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989;57:1465–9. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V. TolC--the bacterial exit duct for proteins and drugs. FEBS Lett. 2003;555:66–71. doi: 10.1016/s0014-5793(03)01125-6. [DOI] [PubMed] [Google Scholar]
- Koronakis V, Eswaran J, Hughes C. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu Rev Biochem. 2004;73:467–89. doi: 10.1146/annurev.biochem.73.011303.074104. [DOI] [PubMed] [Google Scholar]
- Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–9. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- Kraig E, Dailey T, Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990;58:920–9. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally ET, Golub EE, Kieba IR. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J Biol Chem. 1994;269:31289–95. [PubMed] [Google Scholar]
- Lally ET, Golub EE, Kieba IR, Taichman NS, Rosenbloom J, Rosenbloom JC, Gibson CW, Demuth DR. Analysis of the Actinobacillus actinomycetemcomitans leukotoxin gene. Delineation of unique features and comparison to homologous toxins. J Biol Chem. 1989a;264:15451–6. [PubMed] [Google Scholar]
- Lally ET, Kieba IR, Demuth DR, Rosenbloom J, Golub EE, Taichman NS, Gibson CW. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem Biophys Res Commun. 1989b;159:256–62. doi: 10.1016/0006-291x(89)92431-5. [DOI] [PubMed] [Google Scholar]
- Lau SY, Zgurskaya HI. Cell division defects in Escherichia coli deficient in the multidrug efflux transporter AcrEF-TolC. J Bacteriol. 2005;187:7815–25. doi: 10.1128/JB.187.22.7815-7825.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya O, Lewis K. Emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci U S A. 1992;89:8938–42. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N, Nicaud JM, Gray L, Holland IB. Genetical and functional organisation of the Escherichia coli haemolysin determinant. Mol Gen Genet. 2001;201:282–8. doi: 10.1007/BF00425672. [DOI] [PubMed] [Google Scholar]
- Mikes V, Dadak V. Berberine derivatives as cationic fluorescent probes for the investigation of the energized state of mitochondria. Biochim Biophys Acta. 1983;723:231–9. doi: 10.1016/0005-2728(83)90122-6. [DOI] [PubMed] [Google Scholar]
- Nagakubo S, Nishino K, Hirata T, Yamaguchi A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J Bacteriol. 2002;184:4161–7. doi: 10.1128/JB.184.15.4161-4167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H, Zgurskaya HI. AcrAB and related multidrug efflux pumps of Escherichia coli. J Mol Microbiol Biotechnol. 2001;3:215–8. [PubMed] [Google Scholar]
- Nilsson IM, Gafvelin G, von Heijne G. Different sec-requirements for signal peptide cleavage and protein translocation in a model E. coli protein. FEBS Lett. 1993;318:7–10. doi: 10.1016/0014-5793(93)81316-r. [DOI] [PubMed] [Google Scholar]
- Nishino K, Latifi T, Groisman EA. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2006;59:126–141. doi: 10.1111/j.1365-2958.2005.04940.x. [DOI] [PubMed] [Google Scholar]
- Nishino K, Yamaguchi A. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J Bacteriol. 2001;183:5803–12. doi: 10.1128/JB.183.20.5803-5812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørskov-Lauritsen N, Kilian M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov. and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. Int J Syst Evol Microbiol. 2006 doi: 10.1099/ijs.0.64207-0. in press. [DOI] [PubMed] [Google Scholar]
- Ohta H, Kato K, Kokeguchi S, Hara H, Fukui K, Murayama Y. Nuclease-sensitive binding of an Actinobacillus actinomycetemcomitans leukotoxin to the bacterial cell surface. Infect Immun. 1991;59:4599–605. doi: 10.1128/iai.59.12.4599-4605.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza-Wekerle RL, Muller E, Kern P, Meyermann R, Goebel W. Synthesis, inactivation, and localization of extracellular and intracellular Escherichia coli hemolysins. J Bacteriol. 1989;171:2783–8. doi: 10.1128/jb.171.5.2783-2788.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreten V, Schwarz FV, Teuber M, Levy SB. Mdt(A), a new efflux protein conferring multiple antibiotic resistance in Lactococcus lactis and Escherichia coli. Antimicrob Agents Chemother. 2001;45:1109–14. doi: 10.1128/AAC.45.4.1109-1114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Shackcloth J, Felmingham D, MacGowan A. Comparison of BSAC agar dilution and NCCLS broth microdilution MIC methods for in vitro susceptibility testing of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis: the BSAC Respiratory Resistance Surveillance Programme. J Antimicrob Chemother. 2003;52:925–30. doi: 10.1093/jac/dkg462. [DOI] [PubMed] [Google Scholar]
- Rouquette-Loughlin CE, Balthazar JT, Shafer WM. Characterization of the MacA-MacB efflux system in Neisseria gonorrhoeae. J Antimicrob Chemother. 2005;56:856–60. doi: 10.1093/jac/dki333. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning : a laboratory manual. 2. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1989. [Google Scholar]
- Schlor S, Schmidt A, Maier E, Benz R, Goebel W, Gentschev I. In vivo and in vitro studies on interactions between the components of the hemolysin (HlyA) secretion machinery of Escherichia coli. Mol Gen Genet. 1997;256:306–19. doi: 10.1007/s004380050574. [DOI] [PubMed] [Google Scholar]
- Sharff A, Fanutti C, Shi J, Calladine C, Luisi B. The role of the TolC family in protein transport and multidrug efflux. From stereochemical certainty to mechanistic hypothesis. Eur J Biochem. 2001;268:5011–26. doi: 10.1046/j.0014-2956.2001.02442.x. [DOI] [PubMed] [Google Scholar]
- Stanley P, Koronakis V, Hughes C. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol Mol Biol Rev. 1998;62:309–33. doi: 10.1128/mmbr.62.2.309-333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5'-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci U S A. 2000;97:1433–7. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BJ, Miller VL. Salmonella enteritidis has a homologue of tolC that is required for virulence in BALB/c mice. Mol Microbiol. 1995;17:701–12. doi: 10.1111/j.1365-2958.1995.mmi_17040701.x. [DOI] [PubMed] [Google Scholar]
- Sulavik MC, Houseweart C, Cramer C, Jiwani N, Murgolo N, Greene J, DiDomenico B, Shaw KJ, Miller GH, Hare R, Shimer G. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob Agents Chemother. 2001;45:1126–36. doi: 10.1128/AAC.45.4.1126-1136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman NS, Simpson DL, Sakurada S, Cranfield M, DiRienzo J, Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol Immunol. 1987;2:97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Thanabalu T, Koronakis E, Hughes C, Koronakis V. Substrate-induced assembly of a contiguous channel for protein export from E.coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. Embo J. 1998;17:6487–96. doi: 10.1093/emboj/17.22.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson VJ, Bhattacharjee MK, Fine DH, Derbyshire KM, Figurski DH. Direct selection of IS903 transposon insertions by use of a broad-host-range vector: isolation of catalase-deficient mutants of Actinobacillus actinomycetemcomitans. J Bacteriol. 1999;181:7298–307. doi: 10.1128/jb.181.23.7298-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, McArthur WP, Baehni PC, Hammond BF, Taichman NS. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979;25:427–39. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Shenker BJ, DiRienzo JM, Malamud D, Taichman NS. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984;43:700–5. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen HW, Margolles A, Muller M, Higgins CF, Konings WN. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. Embo J. 2000;19:2503–14. doi: 10.1093/emboj/19.11.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C, Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990;87:4776–80. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ooi BC, Tan KL, Ong TH, Zhou L. BLAST++: BLASTing queries in batches. Bioinformatics. 2003;19:2323–4. doi: 10.1093/bioinformatics/btg310. [DOI] [PubMed] [Google Scholar]
- Wang Y, Goodman SD, Redfield RJ, Chen C. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J Bacteriol. 2002;184:3442–9. doi: 10.1128/JB.184.13.3442-3449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H. Interaction between nucleic acids and berberine sulfate. J Cell Biol. 1962;15:589–92. doi: 10.1083/jcb.15.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon JJ. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- Zgurskaya HI, Nikaido H. Multidrug resistance mechanisms: drug efflux across two membranes. Mol Microbiol. 2000;37:219–25. doi: 10.1046/j.1365-2958.2000.01926.x. [DOI] [PubMed] [Google Scholar]


