Abstract
Using carbon fiber amperometry, we exploited the natural variation in quantal size (Q) among individual granules in rat chromaffin cells to examine the influence of Q on quantal release kinetics. Although it is generally accepted that granules with larger Q have slower kinetics of release, we found that this trend was applicable only to granules with Q1/3 < 0.6 pC1/3. Granules with larger Q adapted specific mechanisms to maintain a rapid kinetic of release. The semistable fusion pores in the large-Q granules persisted for a longer duration and could reach a bigger size before the onset of very rapid dilation to allow a longer and larger foot signal. Most importantly, a large proportion of large-Q granules maintained a relatively short half-width in the main spike. This suggests that the most rapid phase of fusion pore dilation in many large-Q granules may be faster than that in small-Q granules. Moreover, cAMP selectively advanced the onset of the rapid dilation of the fusion pore in the large- but not the small-Q granules. Thus, our finding raises the possibility that fusion pore and/or granule matrix in small- and large-Q granules may have different molecular structures.
INTRODUCTION
The amount of chemical messenger that is released via the exocytosis of individual granules (i.e., quantal size, Q) can be regulated by multiple factors, including the synthesis and packaging of neurotransmitters (1), the kinetics of fusion pore opening/closure (2), and granule matrix dissolution/expansion (3). The value of Q, in turn, regulates the vesicular size (4) and has been postulated to play an important role in determining the kinetics of quantal release (5–7). A comparison of amperometric events between mouse mast cells (wild-type and beige) and bovine chromaffin cells (5) had reported that granules with larger vesicular radius were associated with slower kinetics of the main spike (which reflects the rapid release during and after the rapid dilation of the fusion pore) and longer foot signals (which reflect the leakage of neurotransmitter via a semistable fusion pore). In leech Retzius cells, the large dense-core granules also exhibited slower kinetics of release in the main spike when compared with the small synaptic vesicles from the same cells (6). The amperometric signal from the small synaptic vesicles did not have any detectable foot signal, and it was suggested that the small synaptic vesicles could undergo rapid release even when their fusion pore did not undergo significant dilation (6) or merely flickered (8). Recent studies suggest that Q can also affect the proportion of events with a foot signal; however, in bovine chromaffin cells (9) and PC 12 cells (7), an increase in the value of Q was reported to have the opposite effect on this aspect.
The chromaffin cell is a popular model for the study of quantal catecholamine release from dense-core granules. We have previously shown that rat chromaffin cells release granules with a large range of Q (10). Here, we exploited the natural variations in Q among individual rat chromaffin granules to examine in detail the correlation between Q and the kinetics of release via the foot signal and the main spike. An increase in cellular cAMP level has been reported to increase Q and cause a dramatic slowing in the main spike in bovine chromaffin cells (11). Recently, we suggested that cAMP elevation in rat chromaffin cells might cause a uniform increase in the Q of individual granules (10). This raises the issue whether cAMP affects the kinetics of quantal release directly or indirectly via an increase in Q. Here, we addressed this issue by analyzing the effect of cAMP on the kinetics of release in rat chromaffin granules at matched values of Q.
MATERIALS AND METHODS
Cell preparation
Single rat chromaffin cells were prepared as described previously (12,13). Briefly, male Sprague-Dawley rats (200–250 g) were killed in accordance to the standards of the Canadian Council on Animal Care. The adrenal medullas were dissociated enzymatically, and single chromaffin cells were plated on uncoated plastic culture dishes (Corning, Corning, NY) and maintained in a defined medium (MEM supplemented with 1% (v/v) insulin-transferrin-selenium-A; Gibco, Grand Island, NY) that was supplemented with 50 U ml−1 penicillin G and 50 μg ml−1 streptomycin (Gibco). Recordings were performed on cells maintained in culture for 3 days.
Measurement of quantal catecholamine release
Carbon-fiber (tip diameter 7 μm) amperometry was employed to monitor quantal catecholamine release as described in our previous studies (12,13). Briefly, +680 mV was applied to the carbon fiber electrode, using a modified Axopatch-1B amplifier (Axon Instruments, Foster City, CA). An individual chromaffin cell was stimulated by bath application of a high-[K+] (50 mM) extracellular solution which typically raised intracellular [Ca2+]i to 0.5–1 μM for at least 5 min (12). In all experiments, we collected data for 5 min while extracellular [K+] was raised. To further minimize the slight variation in sensitivity and noise of individual carbon-fiber electrodes, each electrode was discarded after being used twice (in randomized order) for one control cell and one cell that received the experimental manipulation. All experiments were conducted at room temperature (20–23°C). Amperometric currents were recorded at 10 kHz (filtered at 1 kHz) with the Fetchex function of pCLAMP version 6.03 (Axon Instruments) and then analyzed with the Mini Analysis Program version 5.24 (Synaptosoft, Decatar, GA). Q was calculated from the time integral of individual amperometric events.
Criteria for selection of amperometric signals
Details on the criteria employed to select the amperometric events for analysis were as described in our previous study (10). Because events detected from release sites beyond the rim of the exposed carbon fiber can cause significant distortion to the amperometric signals and such events are expected to have artificially long rise times (14,15), we minimized the contribution of these distorted signals by restricting our analysis to signals with short rise times (16). We found that all the trends shown in the current study were unaffected when the 50–90% rise time was restricted to <0.5 ms, <1.0 ms, <1.5 ms, or <5 ms. Therefore, we adopted the criterion of <5 ms for 50–90% rise time here.
Criteria for detection of foot signal
In this study, the existence of a foot for each amperometric event was identified with the criteria of the Mini Analysis Program, which is based on the existence of a point of “inflection” during the “rising phase”. We empirically determined that the Mini Analysis Program defined the end of the foot signal (depicted as the square symbol in Fig. 1) as the first point of “inflection” (i.e., when the second derivative of the signal either reached zero or changed sign) to the left of the detected peak. We also empirically determined that the Mini Analysis Program defined the “rising phase” as the interval between the detected peak and the first data point at which the signal exceeded the value of the calculated baseline (to the left of the peak signal; as depicted by the circle symbol in Fig. 1). However, it is possible that a signal classified as without an “inflection” may have a foot that is sufficiently small in amplitude to be masked by the noise of the recordings. To address this possibility, we recorded some amperometric signals at 25 kHz and then low-pass filtered the signals at 0.1, 0.3, 1, 3, or 10 kHz. When the signals were filtered at 3 or 10 kHz, the percentage of events detected as having a foot signal by the Mini Analysis program was ∼30% less in comparison to the results obtained with 1-kHz filtering. Thus, the higher level of noise in the signals with the 3- or 10-kHz filtering masked the point of “inflection” for some events with a foot. When the filtering was set to 0.3 or 0.1 kHz, the percentage of events detected as having a foot was only ∼5% more in comparison to the results obtained with 1-kHz filtering. However, some of the foot signals detected with the 0.1 or 0.3 kHz filtering were indistinguishable from baseline noise (particularly 60 Hz), and the overall kinetic of individual spikes was clearly distorted. Therefore, all sets of data described here were low-pass filtered at 1 kHz (which is the best compromise between maximizing the detection of foot signals and minimizing the distortion of spike kinetics). With this procedure, a foot signal is detectable if its amplitude is >1 pA and its duration is >1 ms. Note that even for the smallest events (e.g., Q1/3 of ∼0.3 pC1/3), the mean duration of the foot signal was ∼3.3 ms (see Fig. 3, A and E) and mean foot amplitude (i.e., foot area divided by foot duration) was ∼1.7 pA (see Fig. 3, D and H). Thus, our procedure is adequate to resolve foot signals from the small-Q events.
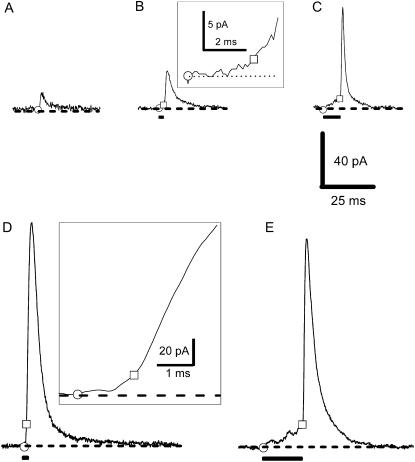
FIGURE 1.
Wide variety of amperometric events could be detected in rat chromaffin cells. The circle and square symbols depict the threshold for detecting an amperometric event and the end of the foot signal, respectively (see Materials and Methods). The bars in B–E depict the duration of foot signals for the events. The values of Q (in pC) in A–E was 0.054, 0.181, 0.258, 1.556, and 1.534, respectively; the corresponding values of Q1/3 (in pC1/3) were 0.378, 0.566, 0.636, 1.159, and 1.153, respectively. To illustrate the short duration of the foot signals for the events in B and D, we plotted the transition between the foot and the spike portion of the events at an expanded timescale in the insets.
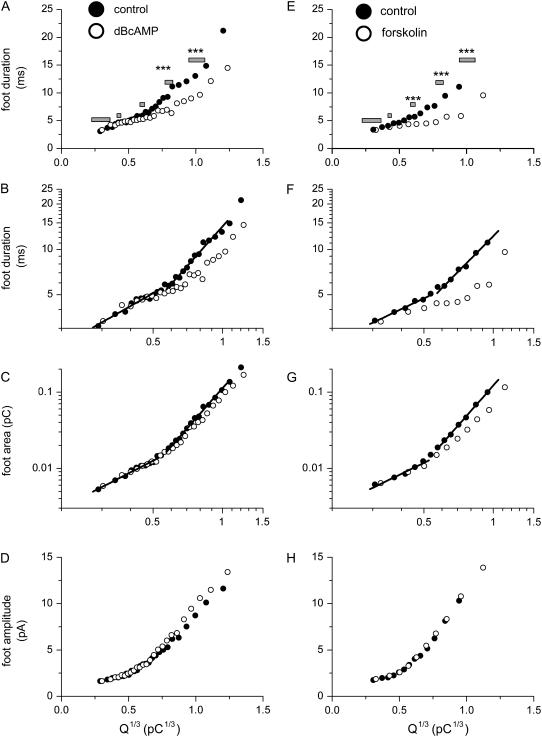
FIGURE 3.
Influence of quantal size and cAMP on the mean duration, area, and amplitude of the foot signals. (A–D) Data from dBcAMP-treated cells and their time-matched controls. (E–H) Data from forskolin-treated cells and their corresponding controls. Plots of the Q1/3 distribution of the mean foot duration in linear (A and E) and log-log scale (B and F). (C and G) Plots of the Q1/3 distribution of the mean foot area in log-log scale. (D and H) Plots of the Q1/3 distribution of the mean foot amplitude (foot area divided by duration) in linear scale. Each data point shown here was the average from 300 amperometric events. (A and E) The bars denote the five ranges of Q1/3 used for the distributions shown in Fig. 4.
Statistical analysis
Functions in Origin version 6.0 (OriginLab Corporation, Northampton, MA) or the Mini Analysis Program were employed for statistical analysis and curve fitting. Because the distribution of every parameter at any range of Q deviated from a single Gaussian, we employed the Kolmogorov-Smirnov (K-S) test for comparing pairs of distribution (17).
Chemicals
All chemicals were purchased from Sigma (Oakville, Ontario, Canada). The standard bath solutions contained (in mM): 150 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 8 glucose, and 10 Na-Hepes (pH 7.4). During stimulation of chromaffin cells, [K+] in the standard solution was raised to 50 mM (equal molar replacement of NaCl by KCl).
RESULTS
Influence of quantal size and cAMP on the foot signals
When rat chromaffin cells were stimulated by KCl, nonoverlapping amperometric events of diverse quantal size and kinetics could be observed (Fig. 1). Note that all the events shown in Fig. 1 (except for the insets) were plotted in the same scale. In some events, the main spike was preceded by a foot (e.g., Fig. 1 B–E; the duration of the foot signal for each event was marked by a bar). Note that although the events shown in Fig. 1, D and E, have similar amplitudes and Q values, the foot signal for the event shown in Fig. 1 E was much longer in duration. For some events (e.g., Fig. 1 D), the foot was very short such that the transition to the main spike was much less conspicuous (but see the expanded timescale of the same trace in the inset). In bovine chromaffin cells, an event was classified as having a foot when the duration of the foot signal was >1ms (9). Using the same criterion, we plotted the percentage of events with a foot from 60 rat chromaffin cells (control) in Fig. 2 A. In this plot, all the amperometric events (with or without a foot) were first sorted with an ascending value of Q1/3 and then separated into groups each containing ∼300 amperometric events. For each group, we calculated the percentage of events with a foot and the corresponding mean value of Q1/3 and plotted the data (as a single data point) in Fig. 2 A. Similar to that reported in bovine chromaffin cells (2), we found that in rat chromaffin cells, the percentage of events with a foot increased with the value of Q1/3. There was no major change in this trend when we altered the criterion for minimum duration of foot signal to 0 or 3 ms (data not shown). Therefore, the criterion of foot duration >1 ms for event with a foot was adopted for all subsequent analysis. As reported previously (12), the frequency of the “stand-alone foot” signals in rat chromaffin cells was very low (<1%) and thus was not analyzed in this study.
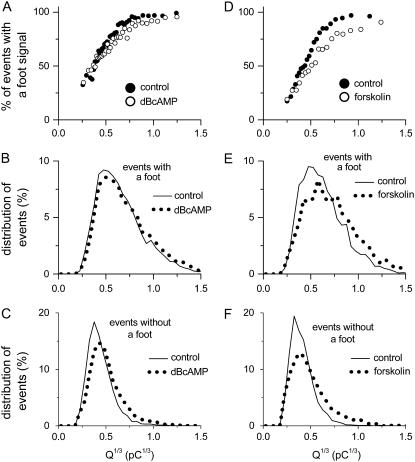
FIGURE 2.
Influence of quantal size and cAMP on the frequency of amperometric events with a foot signal. (A–C) Data from dBcAMP-treated cells and their time-matched controls. (D–F) Data from forskolin-treated cells and their corresponding controls. A and D show the percentage of events with a foot at different values of Q1/3. All the events (with or without a foot) in each data set were first sorted with an ascending value of Q1/3. Each data point is the percentage of events with a foot (in 300 amperometric events) and its corresponding mean value of Q1/3. (B and E) Frequency of events with a foot at different values of Q1/3 (bin size, 0.05 pC1/3). (C and F) Frequency of events without a foot at different values of Q1/3 (bin size, 0.05 pC1/3).
To examine whether there was any difference between the Q1/3 distributions for events with or without a foot, we separated all the events obtained from the 60 control cells (Fig. 2 A) into two groups. One group contained all the events with a foot (n = 8278), and another group contained all the events that did not have a foot (n = 2947). The two data sets were sorted with an ascending value of Q1/3 and then separated into multiple bins (size = 0.05 pC1/3). The percentage of events at different Q1/3 values was then plotted separately for the data set containing the events with a foot (Fig. 2 B) and the data set containing the events without a foot (Fig. 2 C). Note that the distribution of Q1/3 for the events with a foot (Fig. 2 B) spread over a much larger range of Q1/3 when compared with the distribution of events without a foot (Fig. 2 C). The mean Q value for events with a foot (n = 8278) was 3.8 times that of events without a foot (n = 2794). Thus, it is unlikely that the events with a foot in rat chromaffin cells arise mainly from the prefusion between two smaller-Q granules as proposed previously in bovine chromaffin cells (9). In rat chromaffin cells, we previously reported that there were at least three distinct Gaussian populations of granules with small (∼0.4 pC1/3), medium (∼0.6 pC1/3), and large (∼0.8 pC1/3) modal Q1/3 values (10). Interestingly, the Q1/3 distribution for events without a foot (Fig. 2 C) has a modal Q1/3 value of ∼0.4 pC1/3, similar to the population of small modal Q granules (10). However, the distributions for events without a foot (Fig. 2 C) are far too right-skewed to be described by a single Gaussian distribution (P ≪ 0.0001 in K-S test). Moreover, the Q1/3 distribution for events with a foot (Fig. 2 B) had far too large a proportion of events with small Q to have arisen solely from the medium-Q and large-Q granule populations (10). Therefore, we conclude that in rat chromaffin cells, multiple populations of granules contribute to events both with and without a foot.
To further examine the correlation between Q and the occurrence of a foot, we tested whether the frequency of amperometric events with a foot could be altered when the Q values were increased by elevation of cellular cAMP level. Our previous study has shown that treatment of rat chromaffin cells with dibutyryl cAMP (dBcAMP; 1 mM) for 3 days increased the mean cellular Q by ∼35% when compared with time-matched controls (10). Therefore, in this study, we elevated the cAMP level in rat chromaffin cells by incubating the cells with dBcAMP (1 mM) or with forskolin (10 μM) for 3 days (which increased the mean cellular Q by 35% and 59%, respectively). The comparisons between dBcAMP-treated cells and their time-matched controls are shown in Fig. 2, A–C. The comparisons between the forskolin-treated cells and their corresponding time-matched controls are shown in Fig. 2, D–F. Fig. 2, A and D, show that treatment with dBcAMP or forskolin did not affect the general trend of an increase in the frequency of events with a foot with Q1/3. In 60 cells treated with dBcAMP, the percentage of events with a foot (72% of 10,777 events) was only slightly smaller than their time-matched controls (74%). For the 30 cells treated with forskolin, a foot signal was detected in 58% of the 5466 events collected, also slightly smaller than that of the controls (63% of 6642 events). The decrease in the frequency of events with foot by forskolin was prominent for large-Q events (Fig. 2 D). As shown later, it is at the same range of Q values that forskolin increased the proportion of events with short foot duration (see Figs. 3 E and 4, H–J). Thus, it is possible that forskolin increased the number of events with ultrashort foot signals (e.g., <1 ms), and these signals were classified as being without a foot. Note that both dBcAMP and forskolin shifted the distributions of the events with (Fig. 2, B and E) or without a foot (Fig. 2, C and F) toward larger values of Q1/3. This result is consistent with our previous finding that elevation of cellular cAMP typically increased the Q value of individual granules in rat chromaffin cells (10).
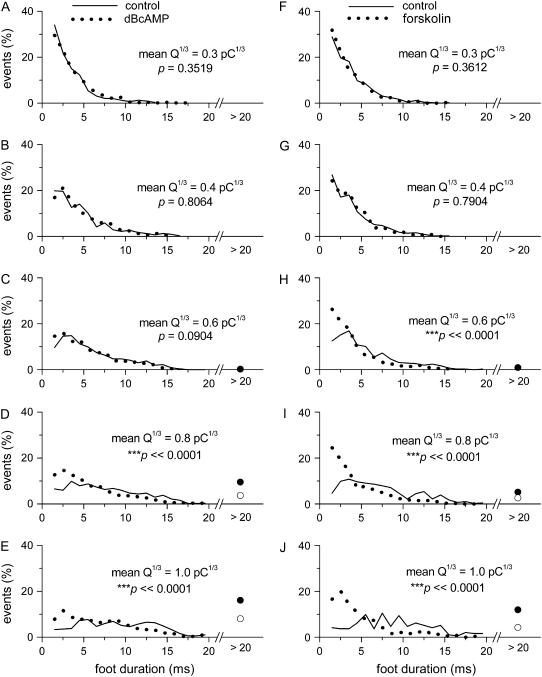
FIGURE 4.
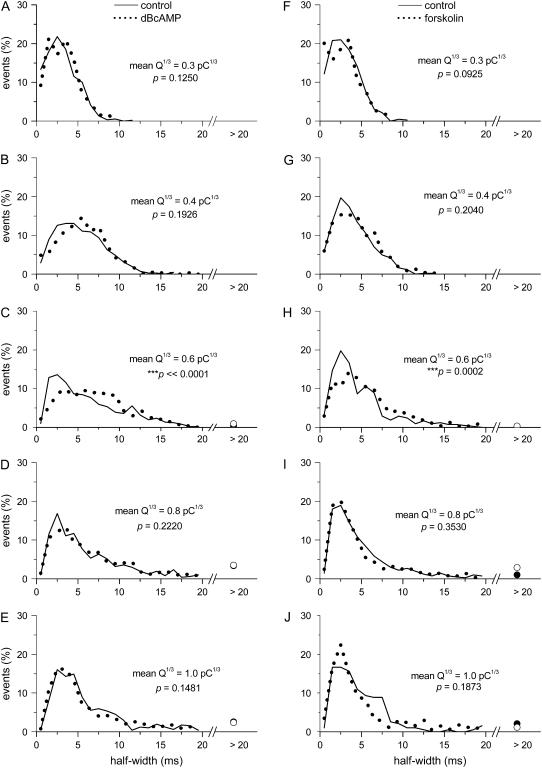
Changes in the distribution of foot duration with quantal size and cAMP. (A–E) data from dBcAMP-treated cells and their time-matched controls. (F–J) Data from forskolin-treated cells and their corresponding controls. Each plot was obtained by selecting ∼250–600 events from a matched range of Q1/3 (shown in Fig. 3, A and E). The percentage of events with foot duration >20 ms was represented as a circle symbol: solid circle for the control cells and open circle for the cAMP-elevated cells. The p-values shown in each panel (as well as in Figs. 7 and 8) were obtained from K-S tests (performed on the cumulative versions of the corresponding pair of distributions).
Previous amperometric studies (5,7) showed that an increase in Q was associated with an increase in foot duration. We found that this trend was robust in control rat chromaffin cells over the entire range of Q1/3 values (Fig. 3, A and E). However, for values of Q1/3 > 0.6 pC1/3, the foot duration increased more steeply with Q1/3. For Q1/3 values between ∼0.3 and ∼0.5 pC1/3, the slope of the linear fit to the log-log plot of the two groups of control cells (Fig. 3, B and F) was 0.87 and 0.73, respectively. For values of Q1/3 > 0.6 pC1/3, the slope of the linear fit to the log-log plot for the two groups of control cells essentially doubled (to 1.71 and 1.54, respectively; Fig. 3, B and F). Note that the log-log plots of mean foot area against Q1/3 for the two groups of control cells (Fig. 3, C and G) also had very different slopes at Q1/3 values below or above ∼0.6 pC1/3. At Q1/3 < 0.6 pC1/3, the slopes of the linear fit for Fig. 3, C and G, were 1.55 and 1.48, respectively; at Q1/3 > 0.6 pC1/3, the slopes essentially doubled (to 3.43 for both sets of control cells). Fig. 3, D and H, show that the mean foot amplitude (i.e., the foot area divided by the foot duration) in control cells also increased with Q.
Interestingly, in cells treated with dBcAMP or forskolin, when Q1/3 > ∼0.6 pC1/3, the foot duration increased less steeply than the controls (Fig. 3, B and F). Note that the plots in Fig. 3 did not have any standard error bars. This is because of the non-Gaussian distributions of foot duration at any particular range of Q1/3 (see examples in Fig. 4), and thus the mean foot duration between the cAMP-elevated cells and their time-matched control could not be compared with simple parametric statistics. We selected five different ranges of Q1/3 (denoted by the bars in Fig. 3, A and E) and plotted the distribution of the foot duration from these five groups of events (each plot was generated from 250–600 events) in Fig. 4. For events with smaller mean Q1/3 values (∼0.3 or ∼0.4 pC1/3), the distribution of foot duration was not affected by dBcAMP (Fig. 4, A and B) or forskolin (Fig. 4, F and G). For events with larger mean Q1/3 values (∼0.6, ∼0.8, or ∼1.0 pC1/3), forskolin (Fig. 4, H–J) increased the proportion of events with very short foot duration (<4 ms). This effect was also robust among cells treated with dBcAMP for events with mean Q1/3 of ∼0.8 and ∼1.0 pC1/3 (Fig. 4, D and E). For a statistical analysis of the effects of dBcAMP and forskolin, we applied the K-S test to compare the cumulative distributions of foot duration (at each of the five selective ranges of Q1/3) between the cAMP-elevated cells and their Q-matched controls (cumulative distributions not shown here). The results of the K-S test are summarized in Fig. 4 as well as Fig. 3, A and E. Note that the shift in the distribution of foot duration toward smaller values by forskolin was indeed significant for events with larger mean Q1/3 (∼0.6, ∼0.8, and ∼1.0 pC1/3; Fig. 3 E). For cells treated with dBcAMP, the effect was smaller; nevertheless, a significant shift in the distribution of the foot duration was also observed for events with larger mean Q1/3 (∼0.8 and ∼1.0 pC1/3; Fig. 3 A). Fig. 3, C and G, shows that the reduction in the mean foot duration for events with larger mean Q1/3 by cAMP also decreased the foot area. Note, however, that neither dBcAMP nor forskolin caused any appreciable change in the mean foot amplitude (Fig. 3, D and H). The lack of effect of cAMP elevation on foot amplitude was further confirmed by comparing the cumulative distributions of foot amplitude at narrowly matched ranges of Q1/3 and foot duration with the K-S test (data not shown). Therefore, the major effect of cAMP on the foot signal is a reduction in foot duration in events with Q1/3 > 0.6 pC1/3.
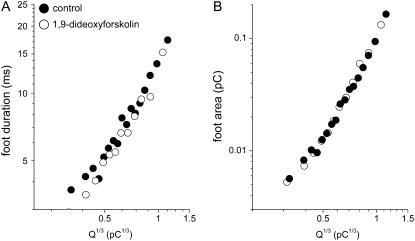
To confirm that the effect of dBcAMP and forskolin treatment on foot signal is indeed related to elevation of cellular cAMP, we treated rat chromaffin cells with the inactive analog of forskolin, 1,9-dideoxyforskolin (10 μM), for 3 days. Fig. 5 shows the relation of the foot duration (Fig. 5 A) and foot area (Fig. 5 B) with Q1/3 for the 1,9-dideoxyforskolin-treated cells (n = 16; 1213 events) and the corresponding controls (n = 17; 1732 events). Note that neither the foot duration nor the foot area was affected by the inactive analog of forskolin.
FIGURE 5.
Foot signals were not affected by the inactive analog of forskolin. (A) Plots of the Q1/3 distribution of the mean foot duration for cells treated with 1,9-dideoxyforskolin and their corresponding controls. (B) Plots of the Q1/3 distribution of the mean foot area for cells treated with the inactive forskolin analog and their corresponding controls. Each point shown here was the average of 100 events.
Influence of quantal size and cAMP on the main amperometric spikes
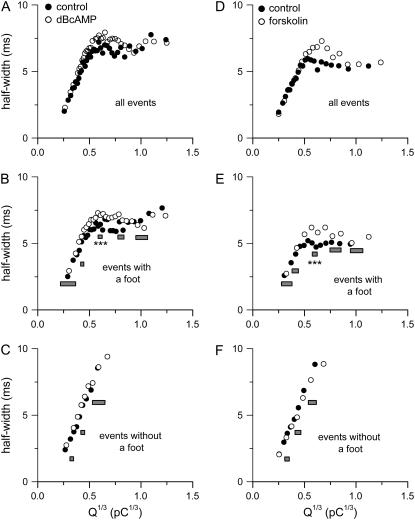
To examine the influence of Q on the kinetics of the main spike, we plotted the mean half-width duration of all events (both with and without a foot) as a function of Q1/3 for both groups of control cells (Fig. 6, A and D). When the value of Q1/3 was <0.6 pC1/3, the mean half-width of main spike increased almost linearly with Q1/3. At larger Q1/3 values, the mean half-width of main spikes stayed near a plateau mean value of ∼6–7 ms. A similar trend was observed when the analysis was restricted to events with a foot (Fig. 6, B and E). Interestingly, when the analysis included only events without a foot, the half-width of main spikes increased linearly over the entire range of Q1/3 (up to ∼0.7 pC1/3; Fig. 6, C and F). At Q1/3 values of 0.6–0.74 pC1/3 (where both events with or without feet were present), the mean value of half-width for events without a foot was 9.30 ± 0.02 ms (n = 186 events) in control cells and 9.28 ± 0.01 ms (n = 390 events) in dBcAMP treated cells (Fig. 6 C). In contrast, the mean value of the half-width for events with a foot at the same range of Q1/3 was 7.02 ± 0.01 ms (n = 1497 events) in control cells and 6.31 ± 0.01 ms (n = 1698 events) in dBcAMP-treated cells (Fig. 6 B). Thus, at Q1/3 > 0.6 pC1/3, the events without a foot indeed have longer half-widths than the events with a foot. This result raises the possibility that the kinetics of the main spike in events with a foot may be different from those without a foot.
FIGURE 6.
Influence of quantal size and cAMP on the mean half-width of the main spike. (A–C) Data from dBcAMP-treated cells and their time-matched controls. (D–F) Data from forskolin-treated cells and their time-matched controls. Each point shown here was the average of 300 events.
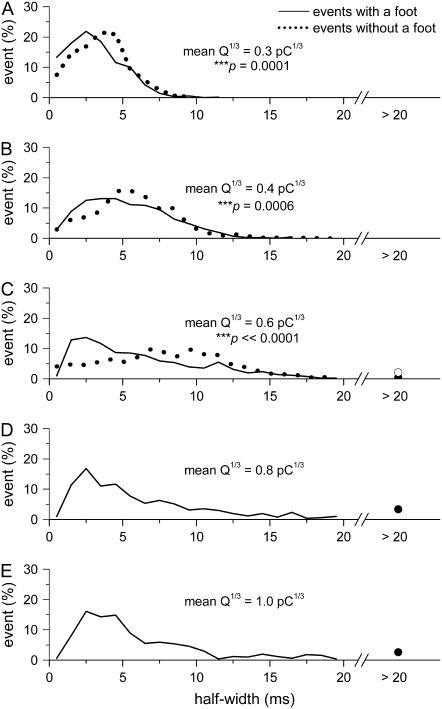
To further examine this, we compared the distributions of half-width values for events with or without a foot at narrowly matching ranges of Q1/3 of 0.3, 0.4, and ∼0.6 pC1/3 (Fig. 7, A–C). Note that the pattern of change in the distribution of half-width with increasing values of Q1/3 was very different for the two types of events. For events without a foot, the modal value as well as the variance in half-width increased as Q1/3 got larger. At any range of Q1/3, the events without a foot always had a lower proportion of signals with very short half-width (<3 ms). For events with a foot, there was little shift in the modal value, but the distribution of half-widths skewed toward longer duration with increasing values of Q1/3 (Fig. 7, A–C). Note that the overall distributions in Fig. 7, C–E, are quite similar. This led to the trend that the mean half-width for events with a foot remained near a plateau value when Q1/3 > 0.6 pC1/3 (Fig. 6, B and E). When we applied the K-S statistics to compare the cumulative distributions of half-width from events with a foot and events without a foot at Q1/3 values of 0.3, 0.4, and 0.6 pC1/3 (cumulative distributions not shown), very significant differences (0.0001 < P ≤ 0.0006) were found. This suggests that the kinetics of the main spike is fundamentally different between events with and without a foot.
FIGURE 7.
Comparisons between the distributions of half-width for events with or without a foot signal. The range of Q1/3 used in each of the distributions was shown in Fig. 6, B and C. The percentage of events with half-width >20 ms was represented as a circle symbol: solid circle for events with a foot signal and open circle for events without a foot signal.
Consistent with the previous report in bovine chromaffin cells that elevation of cellular cAMP increases the half-width of the main spike (11), we found that treatment with dBcAMP and forskolin increased the mean half-width of all events by 14% and 21%, respectively. However, a different picture emerged when we analyzed the effect of cellular cAMP elevation on the half-width of main spikes at matching values of Q1/3 (Fig. 6). For events without a foot, cAMP elevation had no appreciable effect on their half-width (Fig. 6, C and F). For events with a foot, the cAMP-elevated cells appeared to have a slightly longer half-width for events with a mean Q1/3 of ∼0.6 pC1/3 (Fig. 6, B and E). In Fig. 8, we compared the distributions of half-width (at five selected ranges of Q1/3) from events with a foot between the cAMP-elevated cells and their corresponding controls. Note that both dBcAMP and forskolin caused a significant effect only at the distribution with mean Q1/3 of ∼0.6 pC1/3 (Fig. 8, C and H). At this range of Q1/3, cAMP elevation clearly reduced the contribution of events with a very short half-width (< 4 ms) and slightly increased the contribution of events with an intermediate half-width (e.g., 5–10 ms). Thus, the major effect of cAMP elevation on the main spike of events with a foot was a small increase in the half-width, but this was significant only for events with mean Q1/3 of ∼0.6 pC1/3 (summarized in Fig. 6, B and E). In contrast, elevation of cAMP did not affect the distribution of half-width for the events without a foot at Q1/3 of 0.3, 0.4, or 0.6 pC1/3(data not shown).
FIGURE 8.
Influence of cAMP on the distribution of half-widths from events with a foot signal. (A–E) Data from dBcAMP-treated cells and their time-matched controls. (F–J) Data from forskolin-treated cells and their time-matched controls. The range of Q1/3 used in each of the distributions was shown in Fig. 6, B and E. The percentage of events with half-width >20 ms was represented as a circle symbol: solid circle for the control cells and open circle for the cAMP-elevated cells.
DISCUSSION
Influence of Q on the kinetics of the foot and the main spike
Among the small-Q rat chromaffin granules (Q1/3 < 0.6 pC1/3), we found that increasing vesicular size (and Q) resulted in an increase in the foot duration (Fig. 3). This result is consistent with a previous comparison of granules with distinct vesicular diameters from multiple cell types (5). However, the increase in foot duration with Q was steeper for the large-Q rat chromaffin granules (Q1/3 > 0.6 pC1/3). The slopes of the log-log plot for foot duration versus Q1/3 for large-Q granules (1.7 and 1.5; Fig. 3, B and F) are almost double those of the slopes for small-Q granules (0.7 and 0.9; Fig. 3, B and F). For events without a foot, the mean half-width of the main spike increased monotonically with Q1/3 (Fig. 6, C and F). A similar trend was also observed for small-Q events with a foot. Note, however, that for large-Q events with a foot, the mean half-width of the main spike was maintained at a plateau value (Fig. 6, B and E), and there was little change in the overall distribution of the half-width with Q1/3 between 0.6 and 1.0 pC1/3 (Fig. 7, C–E). A recent study on mouse chromaffin cells (18) also reported that at Q1/3 > 0.5 pC1/3, there was clearly no further increase in either the half-width (or rise time) of the main spike. Overall, our findings suggest that the kinetics of the foot and the main spike in the large-Q rat chromaffin granules are regulated differently from the small-Q granules. Most important, despite their large quantal size, the large granules were able to maintain rapid kinetics of release.
Our previous study has shown that random prefusion between two dense-core granules before exocytosis could account for neither the skewed distribution of Q1/3 in the control rat chromaffin cells nor the increase in Q induced by elevation of cAMP (10). Granule prefusion has been proposed to increase the duration of the foot signal (9). Our observation that the mean foot duration increased at a faster rate for events with Q1/3 > 0.6 pC1/3 in the control cells (Fig. 3, A–C) raises the possibility that events with large Q arise from granule prefusion. Granule prefusion may lead to the presence of more than one matrix in some granules before exocytosis. The parallel dissolution of multiple matrices during subsequent exocytosis of such prefused granules can, in theory, cause the mean half-width of the main spike to stay at a plateau value. A major problem with this scenario is that ∼30% of the rat chromaffin granules have Q1/3 > 0.6 pC1/3. Thus, prefusion must occur in ∼30% of the granules undergoing exocytosis; this magnitude of granule prefusion in the context of compound exocytosis was not detected in chromaffin cells (19,20). Moreover, if cAMP increased Q via an increase in the frequency or magnitude of granule prefusion, the foot signal of the prefused large-Q events is expected to be longer. However, we found that cAMP reduced the foot duration of the large-Q events (Fig. 3, A and E). Therefore, we must consider that mechanisms other than granule prefusion contribute to the difference in kinetics between the small- and large-Q events in rat chromaffin cells.
Differences in the kinetics of the fusion pore may account for the rapid main spike in large-Q granules
Some recent models of quantal release (3,21) suggest that the end of the foot signal (i.e., the onset of the rapid dilation of the fusion pore) occurs when the energy associated with the constrained dissolution/expansion of the granule matrix suddenly overcomes the edge energy of the initially semistable pore. Such models also predict that the rapid fusion pore dilation should be prominent only among larger-Q granules. Also, according to these models, granules that do not give rise to any significant foot signal may have a fusion pore that dilated with minimal delay, albeit at a slower maximal rate, because such granules have either little matrix or a matrix that does not undergo significant dissolution/expansion. In this context, the deviations of the kinetics of the large-Q rat chromaffin granules from that predicted by their quantal size may arise from the following scenarios. First, the semistable fusion pore of larger-Q granules can reach a larger size but needs more energy to reach the state of rapid dilation. This will account for the steeper rate of increase in foot duration and foot area with Q observed in the large-Q granules (Fig. 3). Second, in at least some large-Q granules with a significant matrix, the most rapid dilation of the fusion pore (which coincides with the most rapid dissolution and expansion of the granule matrix) may proceed at a much faster rate relative to that of the small-Q granules. This will allow some large-Q granules to discharge their catecholamine during the main spike with kinetics comparable to the smaller-Q granules. Note that it is theoretically possible for the main spike of large- to very large-Q granules to have similar half-widths, even if they have identical fusion pore kinetics. For this to happen, the granules must have similar diameters, but the ones with a larger Q must contain a higher concentration of releasable catecholamine. However, a 27-fold increase in catecholamine concentration would be required to maintain the half-width of the main spike at the same value when Q1/3 increased from ∼0.5 to ∼1.5 pC1/3. This scenario is unlikely because a recent study has shown that the maximum variation in total catecholamine concentration among individual bovine chromaffin granules from different cells was only ∼4-fold (4).
cAMP selectively advances the onset of rapid fusion pore dilation in large-Q granules
Elevation of cellular cAMP was reported to stimulate tyrosine hydroxylase (22), the rate-limiting enzyme in catecholamine synthesis. In rat chromaffin cells, cAMP has been reported to cause recruitment of T-type Ca2+ channels (23) as well as a potentiation of L-type Ca2+ channels (24). An increase in voltage-gated Ca2+ entry has also been reported to elevate quantal release in calf chromaffin cells (25). Thus, it is possible that cAMP acts via an enhancement of voltage-gated Ca2+ entry as well as an increase in catecholamine synthesis to cause an increase in quantal size of individual granules. However, it is difficult to explain why only the foot signals from the large-Q granules are affected by cAMP. A preferential colocalization of T-type Ca2+ channels with the large-Q granules is unlikely to account for a selective action of cAMP on the large-Q granules because large-Q granules are also released from control cells that do not express T-type Ca2+ channels (23).
In contrast, the selective action of cAMP on the large-Q granules is consistent with the notion that the fusion pore in large-Q granules may have distinct properties. The phenomenon that elevation of cAMP shortened the foot duration superficially resembles an effect reported for overexpression of synaptotagmin IV in PC12 cells (26). However, the Q1/3 of the PC12 granules was <0.5 pC1/3 (26), and at this range of Q1/3, cAMP did not affect the foot signal in rat chromaffin cells. Interestingly, overexpression of synaptotagmin IV was reported to have no measurable effect on the kinetics of transmitter release from the small synaptic vesicles of hippocampal neurons (27).
As described above, models of exocytosis of dense-core granules have postulated that the onset of the rapid fusion pore dilation occurs when the energy associated with the constrained dissolution/expansion of the granule matrix overcomes the edge energy of the pore (3,21). Thus, it is possible that cAMP accelerates this transition by destabilizing the initial fusion pore (i.e., by lowering the critical energy for rapid pore dilation). In theory, the observed decrease in the foot duration can also be caused by a cAMP-mediated acceleration in the rate of granule matrix dissolution/expansion. However, such a mechanism is also expected to accelerate the kinetics of the main spike. Our results show that for events with or without a foot, cAMP never reduced the half-width of the main spike at any value of Q1/3 (Fig. 6, B, C, E, and F). Therefore, it is likely that the action of cAMP involves the molecular machinery of the fusion pore instead of the granule matrix per se. We found that cAMP selectively increased the half-width of the main spike for events with a foot and a mean Q1/3 of ∼0.6 pC1/3 (Fig. 6, B and E). This observation is consistent with the following scenario. A reduction of the critical energy for rapid fusion pore dilation by cAMP would result in less dissociation/expansion of the granule matrix at the moment of fusion pore transition. Thus, the remaining granule matrix may take a longer time to undergo subsequent dissolution during the main spike. This effect may be less prominent for granules with a very large Q because the much accelerated rate of fusion pore dilation in such granules will obscure the relatively small effect of a slightly less dissociated matrix at the moment of fusion pore transition.
Do fusion pores in small- and large-Q rat chromaffin granules have different molecular structures?
The above discussion raises the possibility that the molecular size of the fusion pores (both before and after rapid dilation) may be different between large- and small-Q granules. This may arise from fundamental differences in the molecular machinery (e.g., proteins and/or lipids) of the fusion pore. For example, large- and small-Q granules may have different isoforms of certain proteins. Recent models of fusion pore have also suggested that multiple “fusion machines” in each fusion pore (28) may be arranged in a heteromeric complex that is initially stabilized by a ring of proteins interacting with each other (29). Alternatively, even if the molecular components of individual “fusion machines” are identical between the large- and small-Q granules, it is conceivable that at least some large-Q granules can have a larger number of “fusion machines” per complex and thus formation of an initially larger (undilated) fusion pore that is also more resistant to dilation. It has been proposed that one way for a chromaffin granule to increase Q may involve the fusion of multiple small synaptic microvesicles with a large dense-core granule (4). If this form of fusion is involved in the formation of some of the large-Q rat chromaffin granules, the molecular machinery mediating this type of membrane fusion is likely to remain on the surface of the large-Q granules and may increase the total number of “fusion machines” when such granules undergo exocytosis. Once the ring of proteins surrounding a complex with more “fusion machines” is significantly separated at the end of the foot signal, there are more lipid-protein interfaces to allow the influx of more lipids into the rim of the fusion pore, thus resulting in more rapid fusion pore dilation. Because cAMP selectively acts on large-Q granules by accelerating the onset of rapid fusion pore dilation, it is probable that only large-Q granules have a molecular machinery that can be modulated by cAMP. In light of the diverse downstream mechanisms that can be regulated by cAMP (PKA and/or Epac; (30,31)), the challenge of future research in this area will be to pinpoint the specific molecules involved and ascertain how they participate in the opening and dilation of the fusion pore.
Acknowledgments
We thank Dr. Andy Lee for discussion.
This work was supported by the Canadian Institute of Health Research and the Alberta Heritage Foundation for Medical Research.
Kim San Tang's present address is Division of Neurodegenerative Disorders, St. Boniface General Hospital Research Center, Winnipeg, Manitoba, Canada.
References
- 1.Sulzer, D., and E. N. Pothos. 2000. Regulation of quantal size by presynaptic mechanisms. Rev. Neurosci. 11:159–212. [DOI] [PubMed] [Google Scholar]
- 2.Burgoyne, R. D., and J. W. Barclay. 2002. Splitting the quantum: regulation of quantal release during vesicle fusion. Trends Neurosci. 25:176–178. [DOI] [PubMed] [Google Scholar]
- 3.Amatore, C., Y. Bouret, E. R. Travis, and R. M. Wightman. 2000. Interplay between membrane dynamics, diffusion and swelling pressure governs individual vesicular exocytotic events during release of adrenaline by chromaffin cells. Biochimie. 82:481–496. [DOI] [PubMed] [Google Scholar]
- 4.Gong, L. W., I. Hafez, G. A. de Toledo, and M. Lindau. 2003. Secretory vesicles membrane area is regulated in tandem with quantal size in chromaffin cells. J. Neurosci. 23:7917–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez de Toledo, G., R. Fernandez-Chacon, and J. M. Fernandez. 1993. Release of secretory products during transient vesicle fusion. Nature. 363:554–558. [DOI] [PubMed] [Google Scholar]
- 6.Bruns, D., and R. Jahn. 1995. Real-time measurement of transmitter release from single synaptic vesicles. Nature. 377:62–65. [DOI] [PubMed] [Google Scholar]
- 7.Sombers, L. A., H. J. Hanchar, T. L. Colliver, N. Wittenberg, A. Cans, S. Arbault, C. Amatore, and A. G. Ewing. 2004. The effects of vesicular volume on secretion through the fusion pore in exocytotic release from PC12 cells. J. Neurosci. 24:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staal, R. G. W., E. V. Mosharov, and D. Sulzer. 2004. Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci. 7:341–346. [DOI] [PubMed] [Google Scholar]
- 9.Amatore, C., S. Arbault, I. Bonifas, Y. Bouret, M. Erard, A. G. Ewing, and L. A. Sombers. 2005. Correlation between vesicle quantal size and fusion pore release in chromaffin cell exocytosis. Biophys. J. 88:4411–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang, K. S., A. Tse, and F. W. Tse. 2005. Differential regulation of multiple populations of granules in rat adrenal chromaffin cells by culture duration and cyclic AMP. J. Neurochem. 92:1126–1139. [DOI] [PubMed] [Google Scholar]
- 11.Machado, J. D., A. Morales, J. F. Gomez, and R. Borges. 2001. cAMP modulates exocytotic kinetics and increases quantal size in chromaffin cells. Mol. Pharmacol. 60:514–520. [PubMed] [Google Scholar]
- 12.Xu, J., and F. W. Tse. 1999. Brefeldin A increases the quantal size and alters the kinetics of catecholamine release from rat adrenal chromaffin cells. J. Biol. Chem. 274:19095–19102. [DOI] [PubMed] [Google Scholar]
- 13.Xu, J., Y. M. Xu, G. C. R. Ellis-Davies, G. J. Augustine, and F. W. Tse. 2002. Differential regulation of exocytosis by alpha- and beta-SNAPs. J. Neurosci. 22:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow, R. H., and L. von Ruden. 1995. Electrochemical detection of secretion from single cells. In Single-Channel Recording. B. Sakmann, and E. Neher, editors. Plenum Press, New York. 245-275.
- 15.Hafez, I., K. Kisler, K. Berberian, G. Dernick, V. Valero, M. G. Yong, H. G. Craighead, and M. Lindau. 2005. Electrochemical imaging of fusion pore openings by electrochemical detector arrays. Proc. Natl. Acad. Sci. USA. 102:13879–13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruns, D., D. Riedel, J. Klingauf, and R. Jahn. 2000. Quantal release of serotonin. Neuron. 28:205–220. [DOI] [PubMed] [Google Scholar]
- 17.Van der Kloot, W. 1991. The regulation of quantal size. Prog. Neurobiol. 36:93–130. [DOI] [PubMed] [Google Scholar]
- 18.Grabner, C. P., S. D. Price, A. Lysakowski, and A. P. Fox. 2005. Mouse chromaffin cells have two populations of dense core vesicles. J. Neurophysiol. 94:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angleson, J. K., A. J. Cochilla, G. Kilic, I. Nussinovitch, and W. J. Betz. 1999. Regulation of dense cove release from neuroendocrine cells revealed by imaging single exocytic events. Nat. Neurosci. 2:440–446. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto, T., R. Kimura, T. T. Liu, T. Nemoto, N. Takahashi, and H. Kasai. 2006. Vacuolar sequential exocytosis of large dense-core vesicles in adrenal medulla. EMBO J. 25:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troyer, K. P., and R. M. Wightman. 2002. Temporal separation of vesicle release from vesicle fusion during exocytosis. J. Biol. Chem. 277:29101–29107. [DOI] [PubMed] [Google Scholar]
- 22.Hwang, O., M. L. Kim, and J. D. Lee. 1994. Differential induction of gene expression of catecholamine biosynthetic enzymes and preferential increase in norepinephrine by forskolin. Biochem. Pharmacol. 48:1927–1934. [DOI] [PubMed] [Google Scholar]
- 23.Giancippoli, A., M. Novara, A. de Luca, P. Baldelli, A. Marcantoni, E. Carbone, and V. Carabelli. 2006. Low-threshold exocytosis induced by cAMP-recruited CaV3.2 (alpha1H) channels in rat chromaffin cells. Biophys. J. 90:1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carabelli, V., A. Giancippoli, P. Baldelli, E. Carbone, and A. R. Artalejo. 2003. Distinct potentiation of L-type currents and secretion by cAMP in rat chromaffin cells. Biophys. J. 85:1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elhamdani, A., H. C. Palfrey, and C. R. Artalejo. 2001. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 31:819–830. [DOI] [PubMed] [Google Scholar]
- 26.Wang, C. T., R. Grishanin, C. A. Earles, P. Y. Chang, T. F. J. Martin, E. R. Chapman, and M. B. Jackson. 2001. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 294:1111–1115. [DOI] [PubMed] [Google Scholar]
- 27.Ting, J. T., B. G. Kelley, and J. M. Sullivan. 2006. Synaptotagmin IV does not alter excitatory fast synaptic transmission or fusion pore kinetics in mammalian CNS neurons. J. Neurosci. 26:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogel, S. S., P. S. Blank, and J. Zimmerberg. 1996. Poisson-distributed active fusion complexes underlie the control of the rate and extent of exocytosis by calcium. J. Cell Biol. 134:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokumaru, H., K. Umayahara, L. L. Pellegrini, T. Ishizuka, H. Saisu, H. Betz, G. J. Augustine, and T. Abe. 2001. SNARE complex oligomerization by synaphin/complexin is essential for synaptic vesicle exocytosis. Cell. 104:421–432. [DOI] [PubMed] [Google Scholar]
- 30.Bos, J. L. 2006. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 31:680–686. [DOI] [PubMed] [Google Scholar]
- 31.Holz, G. G., G. Kang, M. Harbeck, M. W. Roe, and O. G. Chepurny. 2006. Cell physiology of cAMP sensor Epac. J. Physiol. 577:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]