Abstract
Spontaneous Ca2+ release occurs in cardiac cells during sarcoplasmic reticulum Ca2+ overload, a process we refer to as store-overload-induced Ca2+ release (SOICR). Unlike cardiac cells, skeletal muscle cells exhibit little SOICR activity. The molecular basis of this difference is not well defined. In this study, we investigated the SOICR properties of HEK293 cells expressing RyR1 or RyR2. We found that HEK293 cells expressing RyR2 exhibited robust SOICR activity, whereas no SOICR activity was observed in HEK293 cells expressing RyR1. However, in the presence of low concentrations of caffeine, SOICR could be triggered in these RyR1-expressing cells. At the single-channel level, we showed that RyR2 is much more sensitive to luminal Ca2+ than RyR1. To identify the molecular determinants responsible for these differences, we constructed two chimeras between RyR1 and RyR2, N-RyR1(1–4006)/C-RyR2(3962–4968) and N-RyR2(1–3961)/C-RyR1(4007–5037). We found that replacing the C-terminal region of RyR1 with the corresponding region of RyR2 (N-RyR1/C-RyR2) dramatically enhanced the propensity for SOICR and the response to luminal Ca2+, whereas replacing the C-terminal region of RyR2 with the corresponding region of RyR1 (N-RyR2/C-RyR1) reduced the propensity for SOICR and the luminal Ca2+ response. These observations indicate that the C-terminal region of RyR is a critical determinant of both SOICR and the response to luminal Ca2+. These chimeric studies also reveal that the N-terminal region of RyR plays an important role in regulating SOICR and luminal Ca2+ response. Taken together, our results demonstrate that RyR1 differs markedly from RyR2 with respect to their responses to Ca2+ overload and luminal Ca2+, and suggest that the lack of spontaneous Ca2+ release in skeletal muscle cells is, in part, attributable to the unique intrinsic properties of RyR1.
INTRODUCTION
Muscle contraction is initiated by the release of Ca2+ from the sarcoplasmic reticulum (SR) through the Ca2+-release channel (ryanodine receptor, RyR). The mechanism by which SR Ca2+ release is triggered by membrane depolarization differs in cardiac and skeletal muscle (1). In the heart, membrane depolarization activates the cardiac L-type Ca2+ channel, the dihydropyridine receptor (DHPR), resulting in a small influx of Ca2+. This Ca2+ entry triggers a large Ca2+ release from the SR by opening the cardiac RyR (RyR2) via a mechanism known as Ca2+-induced Ca2+ release (2). On the other hand, Ca2+ entry is not required for excitation-contraction (EC) coupling in skeletal muscle. Depolarization-induced conformational changes in the DHPR are believed to activate the skeletal RyR (RyR1) via a direct physical interaction between DHPR and RyR1 (1–3).
Besides their differences in EC coupling, cardiac and skeletal muscles also differ in their propensities for depolarization-independent SR Ca2+ release (spontaneous Ca2+ release). It is well known that cardiac cells exhibit spontaneous Ca2+ waves or oscillations in the absence of membrane depolarization during SR Ca2+ overload (4–8). Considering its dependence on the SR Ca2+ store, we have referred to this depolarization-independent, Ca2+ overload-induced SR Ca2+ release as store-overload-induced Ca2+ release (SOICR) (9). It has long been recognized that SOICR in cardiac cells can activate inward currents such as the Na+/Ca2+ exchanger current. These inward currents can alter the surface membrane potential of cardiac cells and generate delayed afterdepolarizations, which can lead to triggered arrhythmia (10). Despite its important role in arrhythmogenesis, the molecular basis and regulatory mechanism of SOICR are not well understood.
In contrast to cardiac cells, skeletal muscle cells show little spontaneous Ca2+ release (11–16). The reason for this lack of spontaneous Ca2+ release is unclear. Under certain conditions, however, spontaneous Ca2+ release can occur in skeletal muscle. For example, treatments that disrupt the sarcolemmal membrane or cause membrane deformations, such as saponin permeabilization, mechanical skinning, or osmotic shock, can induce spontaneous Ca2+ release in skeletal muscle fibers (16–20). Altering the metabolic and redox states of mitochondria can also trigger spontaneous Ca2+ release (21,22). These observations indicate that skeletal muscle is susceptible to spontaneous Ca2+ release. In line with this view, spontaneous Ca2+ release can also occur in SR vesicles isolated from skeletal muscle (23–25). These findings have led to the suggestion that spontaneous Ca2+ release in intact skeletal muscle is actively suppressed (21,26), though the exact molecular mechanism of this suppression is unknown. It has been proposed that the DHPR is an important suppressor of spontaneous Ca2+ release in skeletal muscle (27).
Skeletal and cardiac muscles express different subtypes of DHPRs and RyRs with unique properties. These unique properties are thought to be the major determinants of the type of EC coupling operating in cardiac and skeletal muscles (28). The expression of RyR1 in dyspedic skeletal muscle cells lacking endogenous RyR1 restores skeletal-type EC coupling, whereas the expression of RyR2 in dyspedic skeletal muscle cells does not restore skeletal type EC coupling, but does support cardiac-type EC coupling (11,12). Interestingly, spontaneous Ca2+ waves and oscillations were observed in dyspedic skeletal muscle cells expressing RyR2, but not in cells expressing RyR1(11,12). These observations suggest that the RyR isoform not only confers the type of EC coupling, but also influences the occurrence of spontaneous Ca2+ release. However, it remains to be determined whether differences in the intrinsic channel properties of RyR1 and RyR2 contribute to the different propensities for spontaneous Ca2+ release observed in skeletal and cardiac muscles.
We recently showed that the sensitivity of the RyR2 channel to activation by luminal Ca2+ is a critical determinant of the threshold for SOICR (9). Disease-causing RyR2 mutations enhance the sensitivity of the channel to luminal Ca2+ activation and reduce the SOICR threshold (29). Considering the role of luminal Ca2+ in SOICR, we propose that the lack of SOICR activity in skeletal muscle may be ascribed to the lesser response of RyR1 to luminal Ca2+. To test this hypothesis, we expressed RyR1 and RyR2 in an equivalent cellular environment, HEK293 cells, which lack a number of muscle-specific Ca2+-handling proteins, and compared their SOICR activity without the influence of DHPR. We further determined the responses of RyR1 and RyR2 to luminal Ca2+ using single-channel recordings in planar lipid bilayers. We found that SOICR occurred readily in HEK293 cells expressing RyR2, but not in cells expressing RyR1, and that RyR2 is much more sensitive to luminal Ca2+ than RyR1. Moreover, we demonstrated that the C-terminal domain of RyR is a critical determinant of both SOICR and the response to luminal Ca2+, and that the N-terminal region of RyR also plays an important regulatory role in these processes. Our results suggest that the different responses of RyR1 and RyR2 to luminal Ca2+ contributes, in part, to the different propensities for spontaneous Ca2+ release observed in cardiac and skeletal muscles.
MATERIALS AND METHODS
Construction of RyR1, RyR2, and RyR1/RyR2 chimeras
The cDNA encoding the rabbit RyR1 was kindly provided by Dr. David H. MacLennan (University of Toronto). The full-length mouse RyR2 cDNA was cloned and constructed as described previously (30). An XhoI restriction site was introduced into the mouse RyR2 at amino acid position 3961 by site-directed mutagenesis (31). The XhoI-EcoRV cDNA fragment of RyR1 was removed and used to replace the corresponding fragment of RyR2 to form the N-RyR2/C-RyR1 chimera. To produce the N-RyR1/C-RyR2 chimera, the XhoI-NotI fragment of RyR2 was removed and used to replace the corresponding fragment of RyR1. All chimeric constructs were confirmed by DNA sequencing. The full-length RyR1, RyR2, and RyR1/RyR2 chimeras were subcloned into the mammalian expression vector pcDNA5 and used to generate stable, inducible HEK293 cell lines.
Generation of stable, inducible HEK293 cell lines
Stable, inducible HEK293 cell lines expressing RyR1, RyR2, and the chimeras were generated using the Flp-In T-REx Core Kit from Invitrogen (9) (Carlsbad, CA). Briefly, the full-length cDNA encoding RyR1, RyR2, or the chimeras was subcloned into the inducible expression vector, pcDNA5/FRT/TO. Flp-In T-REx-293 cells were then cotransfected with the inducible expression vector, pcDNA5/FRT/TO, containing the RyR1, RyR2, or RyR1/RyR2 chimeric cDNAs and the pOG44 vector encoding the Flp recombinase in 1:5 ratios using the Ca2+ phosphate precipitation method. The transfected cells were washed with phosphate-buffered saline (PBS) (137 mM NaCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCl) 1 day after transfection and allowed to grow for one more day in fresh medium. The cells were then washed again with PBS, harvested, and plated onto new dishes. After the cells had attached (∼4 h), the growth medium was replaced with a selective medium containing 200 μg/ml hygromycin (Invitrogen). The selective medium was changed every 3–4 days until the desired number of cells had grown. These hygromycin-resistant cells were pooled, aliquoted, and stored at −80°C. These positive cells are believed to be isogenic, because the integration of RyR cDNA is mediated by the Flp recombinase at a single FRT site. Each HEK293 cell line was tested for RyR expression using Western blotting and immunocytofluorescence staining. The RyR protein was detected in all cells examined.
Preparation of cell lysate, GST-FKBP12.6 pull-down, and immunoblotting analysis
Stable, inducible HEK293 cells grown for various durations after induction by 1 μg/ml tetracycline were washed with PBS plus 2.5 mM EDTA and harvested in the same solution by centrifugation for 8 min at 700 × g in an IEC Centra-CL2 centrifuge (International Equipment, Needham Heights, MA). The cells were then washed with PBS without EDTA and centrifuged again at 700 × g for another 8 min. The PBS-washed cells were solubilized in a lysis buffer containing 25 mM Tris/50 mM Hepes (pH 7.4), 137 mM NaCl, 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 0.5% soybean phosphatidylcholine, 2.5 mM DTT, and a protease inhibitor mix (1 mM benzamidine, 2 μg/ml leupeptin, 2 μg /ml pepstatin A, 2 μg /ml aprotinin, and 0.5 mM PMSF). This mixture was incubated on ice for 1 h. Cell lysate was obtained by centrifuging twice at 16,000 × g in a microcentrifuge at 4°C for 30 min to remove the unsolubilized materials. The cell lysates were then incubated with glutathione-Sepharose (15 μl) that had been prebound with 60 μg GST-FKBP12.6 at 4°C for 17–19 h. GST-FKBP12.6 was produced using the pGEX-4T-1 GST Gene fusion System (Pharmacia, Milan, Italy). The glutathione-precipitates were washed with PBS three times, each time for 10 min. The proteins bound to the Sepharose beads were then solubilized by the addition of 20 μl of 2× Laemmli's sample buffer plus 5% β-mercaptoethanol and boiled for 5 min. An equal portion of the solubilized proteins from different samples was then separated by 6% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (32). The SDS-polyacrylamide-gel-electrophoresis-resolved proteins were transferred to nitrocellulose membranes at 45 V for 18–20 h at 4°C in the presence of 0.01% SDS according to the method of Towbin et al. (33). The nitrocellulose membranes containing the transferred proteins were blocked for 30 min with PBS containing 0.5% Tween-20 and 5% skim milk powder. The blocked membrane was then incubated with the anti-RyR(34c), anti-RyR1, or anti-RyR2 antibodies (34), and washed for 15 min three times, with PBS containing 0.5% Tween-20. The membrane was then incubated with the secondary antimouse IgG (H&L) antibodies conjugated with horseradish peroxidase (1:20,000) for 30 min. After washing for 15 min three times, the bound antibodies were detected using an enhanced chemiluminescence kit from Pierce (Rockford, IL) (34).
Immunofluorescent staining
Stable, inducible HEK293 cells were grown on glass coverslips placed in a 100-mm tissue culture dish. Twenty-four hours after induction by tetracycline, the coverslips were washed three times with PBS, fixed with 4% formaldehyde in PBS for 15 min, and washed again, once with PBS and three times with PBS containing 0.1% saponin, for 5 min each time. The coverslips were blocked with buffer A (2% skim milk powder, 0.1% saponin in PBS) for 30 min before washing and incubating with the anti-RyR antibody (34c). The coverslips were then washed with buffer A and incubated with rhodamine-conjugated antimouse IgG in buffer A for 1 h. The coverslips were then washed, mounted in 95% glycerol, and analyzed with a Leica DMRB fluorescent microscope (Leica Microsystems, Wetzlar, Germany) using a 40× objective.
Single-cell Ca2+ imaging
Intracellular Ca2+ transients in HEK293 cells expressing RyR1, RyR2, or RyR1/RyR2 chimeras were measured using single-cell Ca2+ imaging and the fluorescent Ca2+ indicator dye fura-2 acetoxymethyl ester (fura-2 AM). Briefly, cells grown on glass coverslips for various durations after induction by 1 μg/ml tetracycline (Sigma, St. Louis, MO) were loaded with 5 μM fura-2 AM in Krebs-Ringer-Hepes (KRH) buffer (125 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 6 mM glucose, 1.2 mM MgCl2, 25 mM Hepes, pH 7.4) plus 0.02% pluronic F-127 (Molecular Probes, Eugene, OR) and 0.1 mg/ml BSA for 20 min at room temperature. The coverslips were then mounted in a perfusion chamber (Warner Instruments, Hamden, CT) on a Zeiss Axiovert 135 microscope (Carl Zeiss, Oberkochen, Germany) and continuously perfused with KRH buffer containing various concentrations of CaCl2 (0.1–10 mM) at room temperature. Fura-2 fluorescence was captured every 4 s through a Fluor 20× objective and a Chroma filter set using the ImageMaster System and a DeltaRAM rapid-wavelength-switching illuminator (Photon Technology International, Lawrenceville, NJ). For measuring the store Ca2+ content, HEK293 cells expressing various RyR constructs were perfused with KRH buffer containing different concentrations of Ca2+ (0–10mM). The cells were then challenged with 5 mM caffeine in the same KRH perfusion buffer to assess the store Ca2+ content at each [Ca2+]o concentration. It should be noted that the stable, inducible HEK293 Flp-In cells that express RyR1, RyR2, or the RyR1/RyR2 chimeras are all sensitive to caffeine, whereas the parental HEK293 Flp-In cells that do not express RyRs are caffeine-insensitive. We used caffeine to estimate the store Ca2+ content only in those cells that express RyRs.
Single-channel recordings
Recombinant RyR1, RyR2, and the RyR1/RyR2 chimeric proteins were partially purified from cell lysate by sucrose density gradient centrifugation. Heart phosphatidylethanolamine and brain phosphatidylserine (Avanti Polar Lipids, Alabaster, AL), dissolved in chloroform, were combined in a 1:1 ratio (w/w), dried under nitrogen gas, and suspended in 30 μl of n-decane at a concentration of 12 mg lipid/ml. Bilayers were formed across a 250-μm hole in a Delrin partition separating two chambers. The trans chamber (800 μl) was connected to the head-stage input of an Axopatch 200A amplifier (Axon Instruments, Austin, TX). The cis chamber (1.2 ml) was held at virtual ground. A symmetrical solution containing 250 mM KCl and 25 mM Hepes (pH 7.4) was used for all recordings, unless indicated otherwise. A 4-μl aliquot (≈ 1 μg of protein) of the sucrose density gradient-purified recombinant proteins was added to the cis chamber. Spontaneous channel activity was always tested for sensitivity to EGTA and Ca2+. The chamber to which the addition of EGTA inhibited the activity of the incorporated channel presumably corresponds to the cytoplasmic side of the Ca2+-release channel. The direction of single-channel currents was always measured from the luminal to the cytoplasmic side of the channel, unless specified otherwise. Recordings were filtered at 2,500 Hz. Data analyses were carried out using the pclamp 8.1 software package (Axon Instruments) (29). Free Ca2+ concentrations were calculated using the computer program of Fabiato and Fabiato (35).
RESULTS
Generation of stable, inducible HEK293 cell lines expressing RyR1 and RyR2
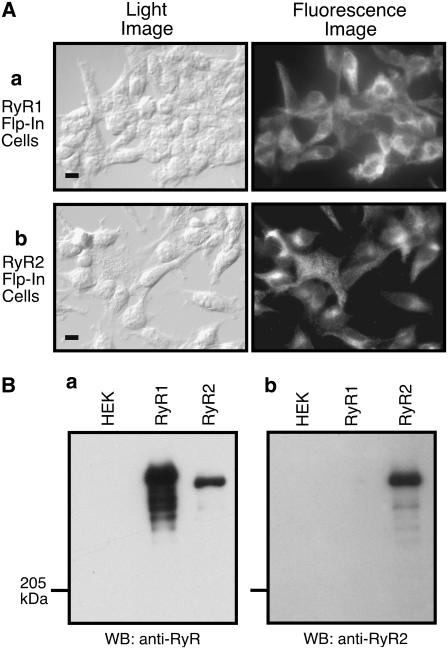
It is possible that the different SOICR activities observed in skeletal and cardiac muscle cells result from their different cellular environments. Alternatively, since SR Ca2+ release is mediated by RyRs, it is also possible that this difference is due to the different intrinsic properties of the RyR isoforms expressed in skeletal muscle (RyR1) and cardiac muscle (RyR2). To address these possibilities, we generated stable, inducible HEK293 cell lines expressing RyR1 or RyR2 and compared their SOICR properties in this equivalent nonmuscle cellular environment. The expression of RyR proteins in these cell lines was confirmed by immunofluorescent staining using an anti-RyR antibody (34c) that recognizes both RyR1 and RyR2 (Fig. 1 A), and was further verified by Western blotting using the anti-RyR antibody (34c) and an RyR2-specific antibody. As shown in Fig. 1 B, the anti-RyR antibody (34c) detected a high-molecular-weight band in the HEK293 cell lines expressing RyR1 or RyR2, but not in parental HEK293 cells (Fig. 1 B a). The major immunoreactive band detected in cells expressing RyR1 migrated slightly more slowly than that from cells expressing RyR2 (Fig. 1 B a). Bands with lower molecular weights were also detected in lysate from cells expressing RyR1, which presumably represent degradation products of RyR1. On the other hand, the RyR2-specific antibody detected immunoreactive signals only in cells expressing RyR2, but not in cells expressing RyR1 or in parental HEK293 cells (Fig. 1 B b).
FIGURE 1.
Immunofluorescent staining and Western blot analysis of stable, inducible HEK293 cell lines expressing RyR1 or RyR2. (A) Stable, inducible HEK293 cells were fixed and permeabilized 24 h after induction by tetracycline. RyR1 or RyR2 proteins were detected using anti-RyR antibody and secondary rhodamine-conjugated antimouse IgG antibody. Light (left) and fluorescent (right) images are shown (scale bar, 10 μm). (B) Cell lysates were prepared from parental HEK293 cells (HEK), RyR1-expressing HEK293 cells (RyR1), and RyR2-expressing HEK293 cells (RyR2). RyR proteins were pulled down by GST-FKBP12.6 from the same amount of cell lysate. The GST-FKBP12.6 precipitates were immunoblotted with anti-RyR antibody (a) or anti-RyR2 antibody (b).
HEK293 cells expressing RyR1 and RyR2 differ markedly in SOICR
Having established the expression of RyR1 and RyR2 in these stable, inducible HEK293 cell lines, we moved on to assess their SOICR properties. Cells were loaded with fura-2 AM and perfused with elevating [Ca2+]o. Single-cell Ca2+ imaging analysis revealed that elevating [Ca2+]o as high as 10 mM did not induce SOICR in HEK293 cells expressing RyR1 (Fig. 2, A and B). On the other hand, under the same conditions SOICR was readily observed in HEK293 cells expressing RyR2 (Fig. 2, A–C). As seen in HEK293 cells expressing RyR2 (9), elevating [Ca2+]o also increases the store Ca2+ content in HEK293 cells expressing RyR1 (Fig. 2 E). Furthermore, as reported with skeletal muscle cells, SOICR could be triggered in HEK293 cells expressing RyR1 in the presence of low concentrations of caffeine (0.5–0.7 mM) (Fig. 2 D) (11,15,36). These observations indicate that, as in muscle cells, RyR2 is much more sensitive to Ca2+ overload than RyR1 in an equivalent nonmuscle cellular environment, suggesting that the intrinsic properties of the RyR channels are critical determinants for the occurrence of SOICR.
FIGURE 2.
SOICR occurs in HEK293 cells expressing RyR2, but not in cells expressing RyR1, at elevated [Ca2+]o. Stable, inducible HEK293 RyR1 or RyR2 cells were induced with tetracycline for 24 h and loaded with 5 μM fura-2 AM in KRH buffer at room temperature for 20 min. Cells were perfused continuously with KRH buffer containing 0.1–10 mM Ca2+ or 10mM Ca2+ plus 5 mM caffeine. (A) Fluorescent Ca2+ images of single RyR1 cells (upper) and RyR2 cells (lower) at various [Ca2+]o. (B) Fura-2 ratios of representative RyR1 (red) and RyR2 (blue) cells at elevated [Ca2+]o. (C) The fraction (%, mean ± SE) of RyR1 (solid circle) and RyR2 (open circle) cells that displayed Ca2+ oscillations at various [Ca2+]o. The total numbers of cells analyzed for Ca2+ oscillations were 389 for RyR1 and 501 for RyR2 from four to six separate experiments. (D) The fraction (%, mean ± SE) of RyR1 cells that displayed Ca2+ oscillations in the presence of 0.5 mM (open circle) or 0.7 mM (solid circle) caffeine. The total numbers of cells analyzed for Ca2+ oscillations were 401 for cells treated with 0.5 mM caffeine and 454 with 0.7mM caffeine from four separate experiments. (E) Store Ca2+ content in HEK293 cells expressing RyR1 at various [Ca2+]o was estimated by measuring the amplitude of caffeine (5 mM)-induced Ca2+ release and normalized to the maximum level obtained at 10 mM [Ca2+]o. Data shown are mean ± SE from three to six separate experiments.
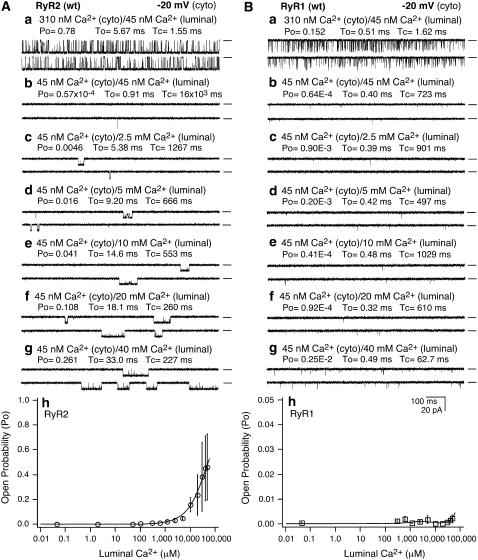
Single RyR1 and RyR2 channels exhibit different responses to luminal Ca2+
Considering that SOICR is triggered by SR Ca2+ overload, and that elevated SR luminal Ca2+ activates RyR2, it is likely that the different SOICR activities observed in RyR1- and RyR2-expressing cells may be due to their different responses to luminal Ca2+. To test this hypothesis, we examined the response of single RyR1 and RyR2 channels to a wide range of luminal Ca2+ concentrations. As shown in Fig. 3, a single RyR2 channel was activated by ∼300 nM cytosolic Ca2+ (Fig. 3 A a), and was inhibited by the addition of EGTA (cytosolic) (Fig. 3 A b), which reduced cytosolic Ca2+ to ∼45 nM. The luminal Ca2+ was then increased stepwise from 45 nM to 50 mM. At luminal Ca2+ concentrations between 45 nM and ∼1 mM, the activity of the channel did not change much. Long opening events started to appear at ∼2.5 mM luminal Ca2+ (Fig. 3 A c). Further increases in luminal Ca2+ led to a greater activation of the channel. At 50 mM luminal Ca2+, the channel was considerably activated with an average open probability (Po) of ∼0.5 (Fig. 3 A h). In contrast, under the same conditions, single RyR1 channels hardly responded to increasing luminal Ca2+ concentrations (Fig. 3 B). These data indicate that single RyR1 channels are much less sensitive to luminal Ca2+ than single RyR2 channels. Given the link between SOICR and the luminal Ca2+ activation of RyR, this lack of response of RyR1 to luminal Ca2+ likely contributes to the absence of SOICR in cells expressing RyR1.
FIGURE 3.
Single RyR1 and RyR2 channels differ in their responses to luminal Ca2+. Single-channel activities of RyR2 (A) and RyR1 (B) were recorded in a symmetrical recording solution containing 250 mM KCl and 25 mM Hepes (pH 7.4) at a holding potential of −20 mV. EGTA was added to either the cis or trans chamber to determine the orientation of the incorporated channel. The side of the channel to which an addition of EGTA inhibited the activity of the incorporated channel presumably corresponds to the cytoplasmic face. The incorporated channel was first activated by 310 nM cytoplasmic Ca2+ (a). The Ca2+ concentration on both the cytoplasmic and luminal side of the channel was then adjusted to ∼45 nM (b). The luminal Ca2+ concentration was then increased to various levels by the addition of aliquots of CaCl2 solution. Single-channel current traces at 2.5 mM (c), 5 mM (d), 10 mM (e), 20 mM (f), and 40 mM (g) luminal Ca2+ are shown. The relationships between Po and luminal Ca2+ concentrations are shown in h. Openings are downward. Open probability (Po), arithmetic mean open time (To), and arithmetic mean closed time (Tc) are indicated on the top of traces. A short line to the right of each current trace indicates the baseline. Data points shown are mean ± SE from 10 single RyR2 and seven single RyR1 channels.
Generation of stable, inducible HEK293 cell lines expressing RyR1/RyR2 chimeras
In an attempt to understand the molecular determinants underlying the different responses of RyR1 and RyR2 to SOICR and luminal Ca2+, we made two chimeric constructs. One construct, N-RyR1/C-RyR2, encompassed the N-terminal ∼4000 amino acid residues of RyR1 and the C-terminal ∼1000 residues of RyR2, and the other, N-RyR2/C-RyR1, was composed of the N-terminal region of RyR2 and the C-terminal region of RyR1 (Fig. 4, A and B). The rationale for the design of these chimeras was based on the finding by Bhat et al. that the C-terminal ∼1000 residues of RyR1 form a functional Ca2+-release channel when expressed in CHO cells (37). These chimeric constructs were used to generate stable, inducible HEK293 cell lines expressing N-RyR1/C-RyR2 and N-RyR2/C-RyR1. The expression of these chimeras in HEK293 cells was confirmed by immunoblotting. As expected, an RyR1-specific antibody, anti-RyR1(13C2) raised against a peptide sequence (aa 4478–4512) in the C- terminal region of RyR1(38), reacted with RyR1 and the N-RyR2/C-RyR1 chimera, but not with RyR2 or the N-RyR1/C-RyR2 chimera (Fig. 4 C a), since the latter constructs did not contain the epitope for anti-RyR1(13C2). On the other hand, an RyR2-specific antibody, anti-RyR2, raised against a peptide sequence (aa 4674–4697) in the C-terminal region of RyR2, recognized RyR2 and the N-RyR1/C-RyR2 chimera, but not RyR1 or the N-RyR2/C-RyR1 chimera (Fig. 4 C b), as they lacked the epitope for the anti-RyR2 antibody.
FIGURE 4.
Generation and characterization of RyR1/RyR2 chimeras. (A) The linear sequences of RyR1 (solid box) and RyR2 (open box), and the N-RyR1/C-RyR2 and N-RyR2/C-RyR1 chimeras are shown. An XhoI restriction site was introduced into the RyR2 sequence at amino acid position 3961, which corresponds exactly to the endogenous XhoI site in the RyR1 sequence at position 4006. (B) Amino acid sequences around residues 4006 and 3961 in RyR1 and RyR2, respectively. (C) Western blot analysis of stable inducible HEK293 cell lines expressing RyR1, RyR2, and the chimeras. RyR proteins from the same amount of cell lysate were immunoblotted with anti-RyR1 antibody (a) and anti-RyR2 antibody (b).
Role of the C-terminal region of RyR in SOICR
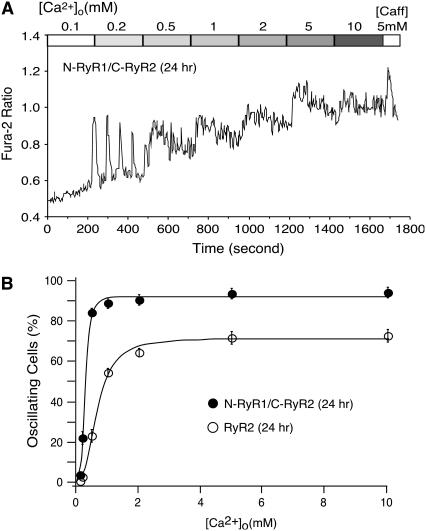
The C-terminal region of RyR, which is believed to contain the channel conduction pore, likely plays an important role in sensing luminal Ca2+ and mediating SOICR. To test this hypothesis, we compared the SOICR activity of HEK293 cells expressing RyR1 with that of cells expressing the N-RyR1/C-RyR2 chimera. The N-RyR1/C-RyR2 chimera differs from RyR1 in the C-terminal region (Fig. 4 A). To ensure a similar level of expression of RyR1 and N-RyR1/C-RyR2, we determined the time course of expression of the N-RyR1/C-RyR2 chimera by immunoblotting using the anti-RyR antibody that recognizes both RyR1 and the chimera. As shown in Fig. 5 A, the level of expression of N-RyR1/C-RyR2 after 6–9 h induction was similar to that of RyR1 after 24 h induction. Therefore, we compared SOICR between RyR1-expressing cells induced for 24 h and N-RyR1/C-RyR2-expressing cells induced for 6 or 9 h. As seen in Fig. 5, B and C, SOICR was readily observed in HEK293 cells expressing the N-RyR1/C-RyR2 chimera after 6 or 9 h induction, whereas no SOICR was detected in cells expressing RyR1 after 24 h induction (Fig. 2 C). Thus, replacing the C-terminal region of RyR1 with the corresponding region of RyR2 confers SOICR activity.
FIGURE 5.
RyR expression and SOICR in HEK293 cells expressing the N-RyR1/C-RyR2 chimera. (A) Time course of N-RyR1/C-RyR2 protein expression in HEK293 cells. N-RyR1/C-RyR2 HEK293 cells were induced with tetracycline for different lengths of time (0–24 h). RyR1 cells were induced for 24 h. RyR proteins from the same amount of cell lysate were immunoblotted with anti-RyR antibody. (B and C) Fura-2 ratios of single N-RyR1/C-RyR2 cells induced for 6 h and 9 h at elevated [Ca2+]o. (D) The fraction (%, mean ± SE) of cells that display Ca2+ oscillations at various [Ca2+]o, N-RyR1/C-RyR2 induced for 6 h (open circle), N-RyR1/C-RyR2 induced for 9 h (solid circle), and RyR1 induced for 24 h (open square). The total numbers of cells analyzed for Ca2+ oscillations were 496 (N-RyR1/C-RyR2, 6 h), 520 (N-RyR1/C-RyR2, 9 h) and 389 (RyR1, 24 h) from four to five separate experiments.
To assess the impact of the C-terminal region of RyR1 on SOICR, we compared the SOICR activity of HEK293 cells expressing RyR2 with that of cells expressing the N-RyR2/C-RyR1 chimera, which differs from RyR2 in the C-terminal region (Fig. 4 A). Similarly, to ensure a comparable level of expression of RyR2 and the N-RyR2/C-RyR1 chimera for comparative studies, we determined the time course of expression of RyR2. The expression level of RyR2 after 12 h induction was found to be similar to that of N-RyR2/C-RyR1 after 24 h induction (Fig. 6 A). SOICR was then compared between cells expressing RyR2 after 12 h induction and cells expressing the chimera after 24 h induction. As shown in Fig. 6, SOICR occurred in HEK293 cells expressing RyR2 at lower [Ca2+]o than in cells expressing the N-RyR2/C-RyR1 chimera (Fig. 6, B and C). Furthermore, HEK293 cells expressing the N-RyR2/C-RyR1 chimera exhibited a reduced propensity for SOICR compared to cells expressing RyR2 (Fig. 6 D). The fraction of oscillating cells in HEK293 cells expressing the N-RyR2/C-RyR1 chimera was significantly lower than that in cells expressing RyR2 at [Ca2+]o ≥ 0.2 mM (p < 0.05) (Fig. 6 D). Therefore, replacing the C-terminal region of RyR2 with the corresponding region of RyR1 reduces the propensity for SOICR. Collectively, these data indicate that the C-terminal region of RyR is a critical determinant of SOICR. As observed in HEK293 cells expressing RyR1 or RyR2, elevating [Ca2+]o also increased the store Ca2+ content in HEK293 cells expressing the N-RyR1/C-RyR2 or the N-RyR2/C-RyR1 chimeras (Fig. 6 E).
FIGURE 6.
RyR expression and SOICR in HEK293 cells expressing RyR2 and the N-RyR2/C-RyR1 chimera. (A) Western blot analysis of RyR proteins in HEK293 cells expressing RyR2 or the N-RyR2/C-RyR1 chimera. HEK293 cells containing the RyR2 gene were induced by tetracycline for different lengths of time, whereas cells containing the chimera gene were induced for 24 h. RyR proteins from the same amount of cell lysate were immunoblotted with anti-RyR antibody. (B and C) Fura-2 ratio of single RyR2 cells induced for 12 h (B) and of single N-RyR2/C-RyR1 cells induced for 24 h (C) at elevated [Ca2+]o. (D) The fraction (%, mean ± SE) of RyR2 cells (12 h induction, open circle) and N-RyR2/C-RyR1 (24 h induction, solid circle) that display Ca2+ oscillations at various [Ca2+]o. The total numbers of cells analyzed for Ca2+ oscillations were 436 (RyR2, 12 h) and 876 (N-RyR2/C-RyR1, 24 h) from five to eight separate experiments. (E) Store Ca2+ content in HEK293 cells expressing N-RyR1/C-RyR2 induced for 6 h or in HEK293 cells expressing N-RyR2/C-RyR1 induced for 24 h at various [Ca2+]o was estimated by measuring the amplitude of caffeine (5 mM)-induced Ca2+ release and normalized to the maximum level obtained at 10 mM [Ca2+]o. Data shown represent the mean ± SE from four separate experiments.
Role of the N-terminal region of RyR in SOICR
Although the N-terminal region of RyR, which is thought to form the cytoplasmic domain of the channel, is unlikely to directly participate in sensing luminal Ca2+ and mediating SOICR, it may play a regulatory role in luminal Ca2+ activation and SOICR. To test this possibility, we compared the SOICR activity between HEK293 cells expressing RyR2 and cells expressing the N-RyR1/C-RyR2 chimera, and between cells expressing RyR1 and cells expressing N-RyR2/C-RyR1. Each of these pairs differs in the N-terminal region (Fig. 4 A). Under the same induction conditions (24 h), the expression level of the N-RyR1/C-RyR2 chimera was comparable to that of RyR2 (Fig. 4 C b), whereas the expression level of the N-RyR2/C-RyR1 chimera was slightly lower than that of RyR1 (Fig. 4 C a). SOICR activities were compared 24 h after induction. As shown in Fig. 7, SOICR started to occur in HEK293 cells expressing the N-RyR1/C-RyR2 chimera at 0.1–0.2 mM [Ca2+]o (Fig. 7 A). Interestingly, elevated [Ca2+]o resulted in a marked increase in basal intracellular Ca2+ level and erratic Ca2+ transients, which were not observed in cells expressing RyR2. The fraction of oscillating cells in HEK293 cells expressing the N-RyR1/C-RyR2 chimera was significantly higher than that in cells expressing RyR2 at 0.2 mM external Ca2+ or greater (p < 0.05) (Fig. 7 B). Hence, replacing the N-terminal region of RyR2 with the corresponding region of RyR1 enhances the propensity for SOICR. Similarly, compared to HEK293 cells expressing RyR1 (Fig. 2 C), cells expressing the N-RyR2/C-RyR1 chimera displayed enhanced SOICR activity (Fig. 6 D). In other words, replacing the N-terminal region of RyR1 with the corresponding region of RyR2 also increases the propensity for SOICR, even though the expression level of N-RyR2/C-RyR1 is slightly lower than that of RyR1 (Fig. 5 C a). These observations indicate that regardless of the isoform (either RyR1 or RyR2), changing the N-terminal region of RyR leads to increased SOICR activity, suggesting that the N-terminal region of RyR may normally play an inhibitory role in SOICR in an isoform-specific manner.
FIGURE 7.
SOICR properties of HEK293 cells expressing the N-RyR1/C-RyR2 chimera. (A) Fura-2 ratio of single N-RyR1/C-RyR2 cells induced for 24 h at elevated [Ca2+]o. (B) Comparison of the fraction (%, mean ± SE) of RyR2 cells (open circle) and N-RyR1/C-RyR2 cells (solid circle) that display SOICR activity at various [Ca2+]o. The total numbers of cells analyzed for Ca2+ oscillations were 501 for RyR2 and 622 for N-RyR1/C-RyR2 from five to six separate experiments.
Responses of the RyR1/RyR2 chimeras to luminal Ca2+
To determine whether the different SOICR activities observed in HEK293 cells expressing different RyR1/RyR2 chimeras are linked to differences in their responses to luminal Ca2+, we examined the response of single RyR1/RyR2 chimeric channels to a wide range of luminal Ca2+ concentrations (45 nM to 50 mM). As shown in Fig. 8 A, a single N-RyR1/C-RyR2 chimeric channel was activated by ∼300 nM cytosolic Ca2+ and was inhibited when the cytosolic Ca2+ concentration was reduced to 45 nM. The channel was then reactivated by increasing the luminal Ca2+ concentration. Comparing their responses to luminal Ca2+ revealed that single N-RyR1/C-RyR2 channels are much more sensitive to luminal Ca2+ than single RyR2 channels. For example, at 300 μM luminal Ca2+, single N-RyR1/C-RyR2 channels exhibited an average Po of ∼0.3, whereas very little activity was observed with single RyR2 channels at this luminal Ca2+ concentration (Fig. 8 A h). On the other hand, under the same conditions, single N-RyR2/C-RyR1 channels responded only weakly to elevated luminal Ca2+ compared to single RyR2 channels (Fig. 8 B). However, compared to single RyR1 channels, single N-RyR2/C-RyR1 channels were more sensitive to luminal Ca2+ (Fig. 8 B h). The results of these single-channel analyses are consistent with those from studies of SOICR. RyR1, which has the lowest response to luminal Ca2+, also displays the lowest propensity for SOICR, whereas the N-RyR1/C-RyR2 chimera, which has the highest response to luminal Ca2+, also exhibits the highest propensity for SOICR. Overall, the responsiveness of RyR1, RyR2, and the chimeras to luminal Ca2+ follow the same order as that of their propensities for SOICR (i.e., RyR1 < N-RyR2/C-RyR1 < RyR2 < N-RyR1/C-RyR2). This close correlation between the luminal Ca2+ response of the RyR isoform and the propensity for SOICR of the cell line expressing it suggests that the latter is a consequence of the former.
FIGURE 8.
Single chimeric N-RyR1/C-RyR2 and N-RyR2/C-RyR1 channels exhibit different responses to luminal Ca2+. Single-channel activities of N-RyR1/C-RyR2 (A) and N-RyR2/C-RyR1 (B) were recorded in a symmetrical recording solution containing 250 mM KCl and 25 mM Hepes (pH 7.4), as described in the legend to Fig. 3. (A) The control current traces for N-RyR1/C-RyR2 at 310 nM cytoplasmic Ca2+ are shown in a and single-channel current traces at 45 nM (b), 2 μM (c), 100 μM (d), 600 μM (e), 2.5 mM (f), and 5 mM (g) luminal Ca2+ are also depicted. (B) The control current traces for N-RyR2/C-RyR1 are shown in a and single-channel current traces at 45 nM (b), 2.5 mM (c), 5 mM (d), 10 mM (e), 20 mM (f), and 40 mM (g) luminal Ca2+ are also shown. The holding potential was −20 mV. Openings are downward. Open probability (Po), arithmetic mean open time (To), and arithmetic mean closed time (Tc) are indicated on the top of traces. A short line to the right of each current trace indicates the baseline. The relationships between Po and luminal Ca2+ concentrations of single N-RyR1/C-RyR2 (A h) and N-RyR2/C-RyR1 (B h) channels are shown in comparison with those of single RyR2 and RyR1 channels, respectively. Data points shown are mean ± SE from six single N-RyR1/C-RyR2 and five single N-RyR2/C-RyR1 channels.
Fig. 8 also shows that the gating properties of the N-RyR1/C-RyR2 chimera differ from those of the N-RyR2/C-RyR1 chimera. Single N-RyR1/C-RyR2 channels activated by ∼300 nM cytosolic Ca2+ displayed a mean open time of ∼20 ms, which is much longer than that of single N-RyR2/C-RyR1 channels (∼0.5 ms). The mean open time of single RyR2 channels activated by ∼300 nM cytosolic Ca2+ (∼5 ms) was also much longer than that of single RyR1 channels (∼0.5 ms) (Fig. 3). Since N-RyR1/C-RyR2 and RyR2, and N-RyR2/C-RyR1 and RyR1 share the same C-terminal regions, respectively, these data indicate that the C-terminal region of RyR contains not only the determinants for SOICR and luminal Ca2+ response, but also the determinants for channel gating.
Effect of rapamycin on RyR1-expressing HEK293 cells
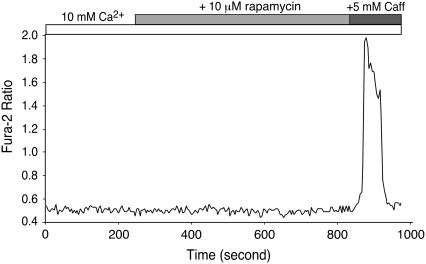
HEK293 cells express a considerable amount of FKBP12.0, which interacts specifically with RyR1, but not with RyR2, and an undetectable level of FKBP12.6, which can bind to both RyR1 and RyR2 (39,40). It is possible that the observed differences in SOICR in HEK293 cells expressing RyR1 and RyR2 could result from the specific interaction of FKBP12.0 with RyR1. To test this possibility, we examined SOICR in cells expressing RyR1 that were treated with 10 μM rapamycin, which dissociates FKBP12.0 from RyR1. As shown in Fig. 9, HEK293 cells expressing RyR1 showed no SOICR activity in either the absence or presence of 10 μM rapamycin. These results suggest that the lack of SOICR in HEK293 cells expressing RyR1 is unlikely to be due to the association of FKBP12.0 with RyR1.
FIGURE 9.
Effect of rapamycin on the response of HEK293 cells expressing RyR1 to elevating [Ca2+]o. HEK293 cells expressing RyR1 grown on glass coverslips were induced with tetracycline for ∼24 h and loaded with 5 μM fura-2 AM in KRH buffer for 20 min at room temperature. Cells were continuously perfused with KRH buffer containing 10 mM Ca2+ with or without 10 μM rapamycin. Caffeine (5 mM) was applied at the end of the experiment to trigger Ca2+ release from the intracellular Ca2+ store. The trace shown is fura-2 ratios of a representative cell out of 178 cells from three separate experiments.
DISCUSSION
In contrast to cardiac cells, skeletal muscle cells show little SOICR (11–16). The reason for this difference is not completely understood. We have recently shown that RyR2 is a key component governing SOICR, and that abnormal RyR2 function can lead to altered SOICR (29). Based on these observations, we propose that the lack of SOICR in skeletal muscle cells may be attributable to the unique properties of RyR1. In this study, we compared SOICR in HEK293 (nonmuscle) cells expressing RyR1 or RyR2. Our results show that SOICR occurs readily in HEK293 cells expressing RyR2, whereas HEK293 cells expressing RyR1 display no SOICR activity, although SOICR could be triggered in these cells in the presence of caffeine. We also compared the responses of single RyR1 and RyR2 channels to luminal Ca2+, and demonstrated that single RyR2 channels are much more sensitive to luminal Ca2+ than single RyR1 channels. These observations demonstrate that RyR1 and RyR2 differ in their responses to Ca2+ overload and luminal Ca2+, and that these differences may underlie, in part, the different propensities for SOICR in cardiac and skeletal muscle cells.
Mechanisms regulating spontaneous SR Ca2+ release in skeletal muscle cells
A unique feature of skeletal muscle cells distinguishing them from cardiac cells is their lack of spontaneous Ca2+-release activity. This feature is perhaps related to the ability of skeletal muscle to stay in a relaxed state for a long period of time. To do so, skeletal muscle cells must be able to tightly control Ca2+ release from the SR via the RyR1 channel. This tight control of SR Ca2+ release is believed to be achieved by the skeletal muscle DHPR, which physically interacts with the RyR1 channel (1,28,41). It has been shown that the skeletal muscle DHPR plays an important role not only in the activation, but also in the termination of SR Ca2+ release (42). Furthermore, the suppression of spontaneous SR Ca2+ release in skeletal muscle cells can be alleviated by removing the transverse tubular membrane or by osmotic shock, which is thought to cause membrane deformation and disrupt the RyR1-DHPR interaction (16,17,19,20). In addition, dysgenic muscle cells, which lack DHPR, do not exhibit significant repression of spontaneous SR Ca2+ release (27). Clearly, the DHPR is a major factor in the inhibition of RyR1-mediated spontaneous Ca2+ release in skeletal muscle cells.
Spontaneous SR Ca2+ release was also detected in mechanically skinned skeletal muscle fibers, a process that is thought to leave the DHPR-RyR1 interaction intact (18). This observation suggests that the presence of DHPR alone is not sufficient to completely suppress spontaneous Ca2+ release, and that other factors are also involved in the suppression (27). Moreover, suppression of RyR1 activity was observed in SR vesicles that were devoid of the components of the transversal tubular membrane including DHPR, further indicating the existence of a DHPR-independent mechanism of inhibiting spontaneous Ca2+ release (43,44). Interestingly, spontaneous SR Ca2+ release was enhanced in skeletal muscle fibers after saponin permeabilization, inhibition of mitochondrial function, or an elevation of cytosolic Ca2+ concentrations (17,21,22,45). Each of these manipulations can alter cytosolic Ca2+ homeostasis and may affect SR Ca2+ loading. In line with this view, the impact of elevated cytosolic Ca2+ concentrations on spontaneous Ca2+ release manifested with a time delay, which is believed to be due to the time required to load the SR (26). Taken together, these observations suggest that the SR Ca2+ load is another important factor that influences the propensity for spontaneous Ca2+ release in skeletal muscle cells.
Since RyR1 mediates SR Ca2+ release in skeletal muscle, it is logical to assume that the activity of RyR1 would have a considerable impact on the propensity for spontaneous Ca2+ release in skeletal muscle. Indeed, spontaneous Ca2+ release in skeletal muscle fibers can be induced in the presence of caffeine, an activator of RyR1 (11,15,36). It is known that in cardiac cells, caffeine reduces the threshold SR Ca2+ content at which spontaneous Ca2+ release occurs by increasing the activity of the RyR2 channel (46). It is likely that by activating RyR1, caffeine likewise reduces the threshold for SOICR in skeletal muscle cells. The lack of spontaneous Ca2+ release in skeletal muscle cells in the absence of caffeine may imply that they have a higher SOICR threshold than cardiac cells. As a result, a considerable overloading of SR Ca2+ and/or a reduction in the threshold (e.g., by caffeine) would be required to trigger spontaneous Ca2+ release in skeletal muscle. In line with this view, we observed SOICR in HEK293 cells expressing RyR1 in the presence of low concentrations of caffeine (Fig. 2). Taken together, the mechanisms underlying the different SOICR behaviors of skeletal and cardiac muscle cells are likely complex and involve multiple factors. Nevertheless, differences in the intrinsic properties of the RyR1 and RyR2 channels are likely an important factor.
RyR1 and RyR2 exhibit different responses to luminal Ca2+
Why HEK293 cells expressing RyR1 and RyR2 differ in their propensities for SOICR is not altogether clear. We have shown that single RyR2 channels are much more sensitive to luminal Ca2+ than single RyR1 channels (Fig. 3). This difference in their responses to luminal Ca2+ is likely to contribute to the different SOICR propensities observed in cells expressing RyR1 and RyR2.
It should be noted that to specifically study and compare the intrinsic properties of different RyR channels with respect to luminal Ca2+ regulation, in this study, we determined the responses of single RyR1, RyR2, and RyR1/RyR2 chimeric channels incorporated into planar lipid bilayers to a wide range of luminal Ca2+ concentrations under identical conditions, without the presence of channel agonists, such as ATP, caffeine, or sulmazole. Most of the previous studies on the impact of luminal Ca2+ on RyR channels incorporated into lipid bilayers were carried out in the presence of channel agonists to enhance luminal Ca2+ activation (47–51). In the absence of these channel agonists, single RyR channels display little response to physiologically relevant concentrations (1–2 mM) of luminal Ca2+. Consistent with these observations, we found that with Ca2+ as the sole channel activator, single RyR2 channels were considerably activated only when the luminal Ca2+ concentration was increased to 10 mM or greater. However, under the same conditions, single RyR1 channels were hardly activated by luminal Ca2+ even at 40 mM. Had we included channel agonists in our protocol, the luminal Ca2+ load required to activate the channel would have been more physiologically relevant, but the results would have been confounded by the impossibility of discerning whether differences between isoforms were attributable to different sensitivities to luminal Ca2+ or to different sensitivities to the agonists.
Although it is clear that the RyR1 and RyR2 channels respond differently to luminal Ca2+, precisely how luminal Ca2+ activates RyR1 and RyR2 to different extents is not. It has been suggested that luminal Ca2+ activates RyRs by passing through the open channel and acting on the cytosolic Ca2+ activation site (a “feed-through” regulation hypothesis) (47,49). Thus, it is possible that the different responses of RyR1 and RyR2 to luminal Ca2+ could result from their different sensitivities to cytosolic Ca2+. However, the role of the luminal-to-cytosolic Ca2+ fluxes in the activation of RyR by luminal Ca2+ is controversial. Gyorke et al. and Ching et al. found that luminal-to-cytosolic Ca2+ fluxes are not necessary for the activation of RyR by luminal Ca2+ (50,51). Furthermore, the application of trypsin to the luminal side of the RyR channels diminishes luminal Ca2+ activation, but not Ca2+ fluxes, arguing against the “feed-through” mechanism and suggesting the existence of a luminal Ca2+ regulation site distinct from the cytosolic Ca2+ activation site (51). Hence, the different responses of RyR1 and RyR2 to luminal Ca2+ could result from their different sensitivities to luminal Ca2+. Alternatively, luminal Ca2+ may bind to the putative luminal Ca2+ sensor and sensitize the channel to activation by cytosolic Ca2+ in an allosteric manner. This allosteric regulation of the channel by luminal Ca2+ could be further modulated by other ligands, such as Mg2+, which is known to inhibit the RyR1 channel more potently than the RyR2 channel. Therefore, the different responses of RyR1 and RyR2 to luminal Ca2+ and Ca2+ overload could result from differences in their allosteric regulation by luminal Ca2+. Clearly, further systematic and detailed studies are required to define the exact mechanism underlying the luminal Ca2+ activation of RyRs.
The activation of RyR2 by luminal Ca2+ is likely modulated by a number of factors. It has been proposed that calsequestrin, a low-affinity, high-capacity SR Ca2+-binding protein, acts as a luminal Ca2+ sensor and is responsible for the activation of RyR2 by luminal Ca2+ (52). However, this view is apparently inconsistent with the observation that purified native RyRs remain sensitive to luminal Ca2+ activation (48,49). Moreover, recent studies have shown that SR Ca2+ release in cardiac myoctyes isolated from calsequestrin knock-out mice remains steeply nonlinear with increasing SR Ca2+ content, indicating that the RyR2 channel can sense luminal Ca2+ in the absence of calsequestrin. These observations have led to the conclusion that calsequestrin, although it modulates SR Ca2+ release, is not required for luminal Ca2+ sensing (53). Consistent with this finding, we found that recombinant RyR2 expressed in HEK293 cells, which lack calsequestrin, is activated by luminal Ca2+.
Molecular determinants of SOICR and luminal Ca2+ response
We took advantage of the marked differences between RyR1 and RyR2 to identify regions in the RyR channel that are important for SOICR and luminal Ca2+ response by constructing chimeras between RyR1 and RyR2. Although the C-terminal region of RyR is likely to be the region responding to SR luminal Ca2+ (48), as it is thought to be the transmembrane, channel pore-forming region that has access to luminal Ca2+, this hypothesis has yet to be tested directly. Our chimeric studies demonstrated that replacing the C-terminal region of RyR1with the corresponding region of RyR2 (N-RyR1/C-RyR2) dramatically enhanced the response of the channel to luminal Ca2+. Significant activation of single N-RyR1/C-RyR2 channels was already detected at 2–100 μM luminal Ca2+ (Fig. 8 A). In accordance with this observation, HEK293 cells expressing the N-RyR1/C-RyR2 chimera displayed SOICR even at 0.1 mM [Ca2+]o. Elevating [Ca2+]o also caused a steady increase in the resting Ca2+ level (Figs. 5 C and 7 A). The exact mechanism for this increase is not clear. We found that decreasing the expression level of N-RyR1/C-RyR2 by reducing the induction time decreased the cytoplasmic Ca2+ level upon elevating [Ca2+]o, comparing Fig. 5 B (with 6 h induction) with Fig. 7 A (with 24 h induction). It should be noted that at a comparable level of expression, RyR2-expressing cells do not display a marked increase in the cytoplasmic Ca2+ level in response to elevating [Ca2+]o. These observations suggest that the increase in the cytoplasmic Ca2+ in N-RyR1/C-RyR2-expressing cells may result from severe spontaneous Ca2+ release from N-RyR1/C-RyR2, which is beyond the capacity of the Ca2+-ATPase, thus leading to an elevated resting Ca2+ level.
In contrast to the N-RyR1/C-RyR2 chimera, the N-RyR2/C-RyR1 chimera, in which the C-terminal region of RyR2 was replaced with the corresponding region of RyR1, exhibited a reduced luminal Ca2+ response and propensity for SOICR (Fig. 6). In other words, the transfer of the C-terminal region of RyR1 into the corresponding region of RyR2 confers a “low-response” phenotype to RyR2, whereas, the transfer of the C-terminal region of RyR2 into the corresponding region of RyR1 confers a “high-response” phenotype to RyR1. On the other hand, replacing the N-terminal region of RyR2 with the corresponding region of RyR1 did not give rise to a channel with a low-response phenotype, as seen in RyR1. Similarly, replacing the N-terminal region of RyR1 with the corresponding region of RyR2 did not result in a channel with a high-response phenotype, as seen in RyR2. These observations suggest that the N-terminal regions of RyR1 and RyR2 are unlikely to be responsible for the low- and high-response phenotypes of RyR1 and RyR2, respectively. Taken together, these results indicate that the C-terminal region of RyR is an essential determinant of SOICR and the response to luminal Ca2+.
The chimeric studies also revealed some interesting new insight into the role of the N-terminal region of RyR in SOICR and luminal Ca2+ response. If the C-terminal region of RyR were the sole determinant, one would expect that the response to luminal Ca2+ and the propensity for SOICR of RyR2 and N-RyR1/C-RyR2 and of RyR1 and N-RyR2/C-RyR1 would be similar, as they share the same C-terminal region. However, this is not the case. Replacing the N-terminal region of RyR2 with the corresponding region of RyR1 led to an augmented luminal Ca2+ response and propensity for SOICR compared to RyR2. Similarly, replacing the N-terminal region of RyR1 with the corresponding region of RyR2 resulted in an increased luminal Ca2+ response and propensity for SOICR compared to RyR1. These observations indicate that the N-terminal region of RyR also plays an important role in SOICR and luminal Ca2+ response.
It has been suggested that the cytoplasmic (N-terminal) domain of RyR interacts with its transmembrane (C-terminal) domain (54). This domain-domain interaction is likely to be isoform-specific, and if so, should be weakened or abolished in the N-RyR1/C-RyR2 and N-RyR2/C-RyR1 chimeras. Since we observed enhanced activities when exchanging the cytoplasmic regions between isoforms, the cytoplasmic region in intact RyR1 and RyR2 is likely involved in the suppression of SOICR and luminal Ca2+ regulation. Based on this reasoning, we propose that the increased SOICR activity and luminal Ca2+ responsiveness observed with the N-RyR1/C-RyR2 and N-RyR2/C-RyR1 chimeras result from the disruption of the interaction between the cytoplasmic and transmembrane domains, thus relieving the suppression normally exerted on RyR by its cytoplasmic region. It is likely that the transmembrane region of RyR mediates SOICR and luminal Ca2+ response. However, it remains to be determined whether the cytoplasmic region is able to modulate SOICR and luminal Ca2+ response by interacting with the transmembrane region in an isoform-specific manner.
Implications of SOICR in muscular diseases
We recently showed that a number of naturally occurring RyR2 mutations linked to cardiac arrhythmia and sudden death enhance the sensitivity of the channel to activation by luminal Ca2+ and reduce the threshold for SOICR (29). Enhanced SOICR has also been observed in isolated cardiac cells and muscles from failing, hypertrophied, and ischemic/reperfused hearts (10). Altered RyR2 function, and particularly luminal Ca2+ activation, is likely a common cause of increased SOICR in many cardiac conditions. Given its potential to induce delayed afterdepolarizations, increased SOICR would enhance the propensity for triggered arrhythmia.
The link between SOICR and various cardiac diseases suggests that enhanced SOICR may also be involved in skeletal muscle diseases. Indeed, enhanced spontaneous Ca2+ release has been shown in skeletal muscle cells from patients susceptible to malignant hyperthermia (MH) and central core disease (CCD) and in skeletal muscle cells expressing MH/CCD mutations (55,56). Furthermore, it has recently been shown that intact skeletal muscle fibers from dystrophic mice display enhanced localized Ca2+ transients (Ca2+ sparks) under hypertonic or hypotonic conditions (20). Thus, enhanced SOICR may also be common to various skeletal muscle conditions. In addition, our results suggest that the N-terminal region also plays an important role in the regulation of RyR by luminal Ca2+. It has been shown that disease-linked RyR2 mutations in the N-terminal, central, and C-terminal regions alter the sensitivity of the channel to luminal Ca2+ activation. How these mutations, and especially those located in the N-terminal and central regions, affect luminal Ca2+ activation is not clear. Some of the disease-linked mutations may affect the mechanism by which the N-terminal region regulates luminal Ca2+ response.
In summary, we have demonstrated that RyR1 and RyR2 have different responses to Ca2+ overload and luminal Ca2+. The C-terminal region of RyR is not only an essential determinant for SOICR and luminal Ca2+ response, but also a determinant for channel gating, whereas the N-terminal region plays an important regulatory role in SOICR and luminal Ca2+ response. The intrinsic properties of the RyR1 and RyR2 channels likely contribute to the different propensities for SOICR observed in skeletal and cardiac muscle cells. As in RyR2-associated cardiac arrhythmia, altered luminal Ca2+ response of RyR1 may be involved in skeletal muscle abnormalities, such as MH, CCD, and muscular dystrophy.
Acknowledgments
The authors thank Dr. Anthony Lai for providing the anti-RyR2 antibody, Dr. Jonathan Lytton for the use of his single-cell Ca2+ imaging facility, Jeff Bolstad for critical reading of the manuscript, and Jodie Lee for excellent technical assistance.
This work was supported by National Institutes of Health grant 1RO1HL75210 to S.R.W.C., and research grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Alberta, Northwest Territory, and Nunavut to S.R.W.C., Y.S., and H.J.D. H.K. is the recipient of the Alberta Heritage Foundation for Medical Research (AHFMR) Studentship Award.
References
- 1.Fill, M., and J. A. Copello. 2002. Ryanodine receptor calcium release channels. Physiol. Rev. 82:893–922. [DOI] [PubMed] [Google Scholar]
- 2.Bers, D. M. 2002. Cardiac excitation-contraction coupling. Nature. 415:198–205. [DOI] [PubMed] [Google Scholar]
- 3.Franzini-Armstrong, C., and F. Protasi. 1997. Ryanodine receptors of striated muscles: A complex channel capable of multiple interactions. Physiol. Rev. 77:699–729. [DOI] [PubMed] [Google Scholar]
- 4.Kass, R. S., and R. W. Tsien. 1982. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys. J. 38:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orchard, C., D. Eisner, and D. Allen. 1983. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 304:735–738. [DOI] [PubMed] [Google Scholar]
- 6.Stern, M., A. Kort, G. Bhatnagar, and E. Lakatta. 1983. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca++-dependent cellular mechanical oscillations. J. Gen. Physiol. 82:119–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wier, W., A. Kort, M. Stern, E. Lakatta, and E. Marban. 1983. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc. Natl. Acad. Sci. USA. 80:7367–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marban, E., S. W. Robinson, and W. G. Wier. 1986. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J. Clin. Invest. 78:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, D., B. Xiao, D. Yang, R. Wang, P. Choi, L. Zhang, H. Cheng, and S. R. W. Chen. 2004. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc. Natl. Acad. Sci. USA. 101:13062–13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakatta, E. G. 1992. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc. Res. 26:193–214. [DOI] [PubMed] [Google Scholar]
- 11.Yamazawa, T., H. Takeshima, T. Sakurai, M. Endo, and M. Iino. 1996. Subtype specificity of the ryanodine receptor for Ca2+ signal amplification in excitation-contraction coupling. EMBO J. 15:6172–6177. [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai, J., T. Ogura, F. Protasi, C. Franzini-Armstrong, P. D. Allen, and K. G. Beam. 1997. Functional nonequality of the cardiac and skeletal ryanodine receptors. Proc. Natl. Acad. Sci. USA. 94:1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirokova, N., J. Garcia, and E. Rios. 1998. Local calcium release in mammalian skeletal muscle. J. Physiol. (Lond.). 512:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conklin, M. W., V. Barone, V. Sorrentino, and R. Coronado. 1999. Contribution of ryanodine receptor type 3 to Ca(2+) sparks in embryonic mouse skeletal muscle. Biophys. J. 77:1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fessenden, J. D., Y. Wang, R. A. Moore, S. R. Chen, P. D. Allen, and I. N. Pessah. 2000. Divergent functional properties of ryanodine receptor types 1 and 3 expressed in a myogenic cell line. Biophys. J. 79:2509–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou, J., G. Brum, A. Gonzalez, B. S. Launikonis, M. D. Stern, and E. Rios. 2003. Ca2+ sparks and embers of mammalian muscle. Properties of the sources. J. Gen. Physiol. 122:95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launikonis, B., and D. Stephenson. 1997. Effect of saponin treatment on the sarcoplasmic reticulum of rat, cane toad and crustacean (yabby) skeletal muscle. J. Physiol. (Lond.). 504:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirsch, W. G., D. Uttenweiler, and R. H. A. Fink. 2001. Spark- and ember-like elementary Ca2+ release events in skinned fibres of adult mammalian skeletal muscle. J. Physiol. (Lond.). 537:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla, S., J. N. Skepper, A. R. Hockaday and C. L. Huang. 2001. Calcium waves induced by hypertonic solutions in intact frog skeletal muscle fibres. J. Physiol. (Lond.). 536:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, X., N. Weisleder, C. Collet, J. Zhou, Y. Chu, Y. Hirata, X. Zhao, Z. Pan, M. Brotto, H. Cheng, and J. Ma. 2005. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell Biol. 7:525–530. [DOI] [PubMed] [Google Scholar]
- 21.Isaeva, E. V., and N. Shirokova 2003. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J. Physiol. (Lond.). 547:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaeva, E. V., V.M. Shkryl, and N. Shirokova 2005. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J. Physiol. (Lond.). 565:855–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palade, P., R. Mitchell, and S. Fleischer. 1983. Spontaneous calcium release from sarcoplasmic reticulum. General description and effects of calcium. J. Biol. Chem. 258:8098–8107. [PubMed] [Google Scholar]
- 24.Nelson, T. E. 1983. Abnormality in calcium release from skeletal sarcoplasmic reticulum of pigs susceptible to malignant hyperthermia. J. Clin. Invest. 72:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi, S. T., S. Taylor, and G. A. Gronert. 1983. Calcium-induced Ca2+ release from sarcoplasmic reticulum of pigs susceptible to malignant hyperthermia. The effects of halothane and dantrolene. FEBS Lett. 161:103–117. [DOI] [PubMed] [Google Scholar]
- 26.Zhou, J., B. S. Launikonis, E. Rios, and G. Brum. 2004. Regulation of Ca2+ sparks by Ca2+ and Mg2+ in mammalian and amphibian muscle. An RyR isoform-specific role in excitation-contraction coupling?. J. Gen. Physiol. 124:409–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou, J., J. Yi, L. Royer, B. S. Launikonis, A. Gonzalez, J. Garcia, and E. Rios. 2006. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am. J. Physiol. Cell Physiol. 290:C539–C553. [DOI] [PubMed] [Google Scholar]
- 28.Franzini-Armstrong, C., F. Protasi, and V. Ramesh. 1998. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann. N. Y. Acad. Sci. 853:20–30. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, D., R. Wang, B. Xiao, H. Kong, D. J. Hunt, P. Choi, L. Zhang, and S. R. W. Chen. 2005. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ. Res. 97:1173–1181. [DOI] [PubMed] [Google Scholar]
- 30.Li, P., and S. R. Chen. 2001. Molecular basis of ca(2)+ activation of the mouse cardiac Ca(2)+ release channel (ryanodine receptor). J. Gen. Physiol. 118:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 77:51–59. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- 33.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 76:4350–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao, B., G. Zhong, M. Obayashi, D. Yang, K. Chen, M. Walsh, Y. Shimoni, H. Cheng, H. Ter Keurs, and S. Chen. 2006. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon β-adrenergic stimulation in normal and failing hearts. Biochem. J. 396:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabiato, A., and F. Fabiato. 1979. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris). 75:463–505. [PubMed] [Google Scholar]
- 36.Gonzalez, A., W. G. Kirsch, N. Shirokova, G. Pizarro, M. D. Stern, and E. Rios. 2000. The spark and its ember: separately gated local components of Ca2+ release in skeletal muscle. J. Gen. Physiol. 115:139–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhat, M. B., J. Zhao, H. Takeshima, and J. Ma. 1997. Functional calcium release channel formed by the carboxyl-terminal portion of ryanodine receptor. Biophys. J. 73:1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen, S. R., L. Zhang, and D. H. MacLennan. 1992. Characterization of a Ca2+ binding and regulatory site in the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 267:23318–23326. [PubMed] [Google Scholar]
- 39.Timerman, A. P., H. Onoue, H. B. Xin, S. Barg, J. Copello, G. Wiederrecht, and S. Fleischer. 1996. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J. Biol. Chem. 271:20385–20391. [DOI] [PubMed] [Google Scholar]
- 40.Jeyakumar, L., L. Ballester, D. Cheng, J. McIntyre, P. Chang, H. Olivey, L. Rollins-Smith, J. Barnett, K. Murray, H. Xin, and S. Fleischer. 2001. FKBP binding characteristics of cardiac microsomes from diverse vertebrates. Biochem. Biophys. Res. Commun. 281:979–986. [DOI] [PubMed] [Google Scholar]
- 41.Franzini-Armstrong, C. 1999. The sarcoplasmic reticulum and the control of muscle contraction. FASEB J. 13(Suppl. 2):S266–S270. [DOI] [PubMed] [Google Scholar]
- 42.Suda, N. 1995. Involvement of dihydropyridine receptors in terminating Ca2+ release in rat skeletal myotubes. J. Physiol. (Lond.). 486:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murayama, T., and Y. Ogawa. 2001. Selectively suppressed Ca2+-induced Ca2+ release activity of α-ryanodine receptor (α-RyR) in frog skeletal muscle sarcoplasmic reticulum: potential distinct modes in Ca2+ release between α- and β-RyR. J. Biol. Chem. 276:2953–2960. [DOI] [PubMed] [Google Scholar]
- 44.Murayama, T., and Y. Ogawa. 2004. RyR1 exhibits lower gain of CICR activity than RyR3 in the SR: Evidence for selective stabilization of RyR1 channel. Am. J. Physiol. Cell Physiol. 287:C36–C45. [DOI] [PubMed] [Google Scholar]
- 45.Klein, M., H. Cheng, L. Santana, Y. Jiang, W. Lederer, and M. Schneider. 1996. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 379:455–458. [DOI] [PubMed] [Google Scholar]
- 46.Trafford, A. W., G. C. Sibbring, M. E. Diaz, and D. A. Eisner. 2000. The effects of low concentrations of caffeine on spontaneous ca release in isolated rat ventricular myocytes. Cell Calcium. 28:269–276. [DOI] [PubMed] [Google Scholar]
- 47.Tripathy, A., and G. Meissner. 1996. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys. J. 70:2600–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sitsapesan, R., and A. J. Williams. 1997. Regulation of current flow through ryanodine receptors by luminal Ca2+. J. Membr. Biol. 159:179–185. [DOI] [PubMed] [Google Scholar]
- 49.Xu, L., and G. Meissner. 1998. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+. Biophys. J. 75:2302–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gyorke, I., and S. Gyorke. 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75:2801–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ching, L. L., A. J. Williams, and R. Sitsapesan. 2000. Evidence for Ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ. Res. 87:201–226. [DOI] [PubMed] [Google Scholar]
- 52.Gyorke, I., N. Hester, L. R. Jones, and S. Gyorke. 2004. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86:2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knollmann, B. C., N. Chopra, T. Hlaing, B. Akin, T. Yang, K. Ettensohn, B. E. Knollmann, K. D. Horton, N. J. Weissman, I. Holinstat, W. Zhang, D. M. Roden, L. R. Jones, C. Franzini-Armstrong, and K. Pfeifer. 2006. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 116:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.George, C. H., H. Jundi, N. L. Thomas, M. Scoote, N. Walters, A. J. Williams, and F. A. Lai. 2004. Ryanodine receptor regulation by intramolecular interaction between cytoplasmic and transmembrane domains. Mol. Biol. Cell. 15:2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avila, G., and R. T. Dirksen. 2001. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J. Gen. Physiol. 118:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tilgen, N., F. Zorzato, B. Halliger-Keller, F. Muntoni, C. Sewry, L. M. Palmucci, C. Schneider, E. Hauser, F. Lehmann-Horn, C. R. Muller, and S. Treves. 2001. Identification of four novel mutations in the C-terminal membrane spanning domain of the ryanodine receptor 1: association with central core disease and alteration of calcium homeostasis. Hum. Mol. Genet. 10:2879–2887. [DOI] [PubMed] [Google Scholar]