Abstract
During sequence learning individuals exhibit motor skill acquisition and an ability to verbally describe items within the sequence. We disrupted this latter declarative component by having participants learn a word list immediately after sequence learning. This induced off-line skill improvements. We conclude that off-line memory processing relies not only upon the engagement of neuroplastic mechanisms but also upon the disengagement of an interaction between declarative and procedural memory systems.
An important and fertile concept in neuroscience is the distinction between a declarative memory – a memory for a fact or event – and a procedural memory – a memory supporting improved skill 1. These different memories are usually examined separately, yet memories are seldom acquired in isolation 2, 3. For example, sequence learning tasks such as the serial reaction time task (SRTT) have a blend of declarative and procedural components 4. We are able to verbally describe, or declare the sequence; and with practice we also acquire skill at producing the sequence. These latter skill improvements can also develop “off-line” during consolidation. When the declarative component of the SRTT is present – shown by participants being able to verbally describe some, or all, of the sequence - off-line improvements only develop over a night of sleep, not over wake 5–7. One possible explanation is that the declarative component inhibits the off-line enhancement of motor skill memories over wake (see Supplementary Introduction). We sought to test this possibility by disrupting the declarative component: participants learnt a word list immediately after being trained and tested (8am, skill1) on the SRTT (Figure 1; informed written consent was obtained; for details see Supplementary Methods). Twelve hours later, participants were re-tested (8pm, skill2) on the SRTT and a free recall test of the sequence was administered. The difference between SRTT performance at testing and retesting (skill2 – skill1) provided a measure of off-line learning over wake. These performance changes were compared against those that developed when participants – rather than learning a word list - stated the number of vowels within nonsense letter strings. This engaged participants in a demanding task without requiring them, or providing them with an opportunity to encode declarative information. Contrasting these experimental groups allowed an assessment of how declarative learning, immediately after the initial SRTT, influenced later skilled performance.
Figure 1. Experimental Design.
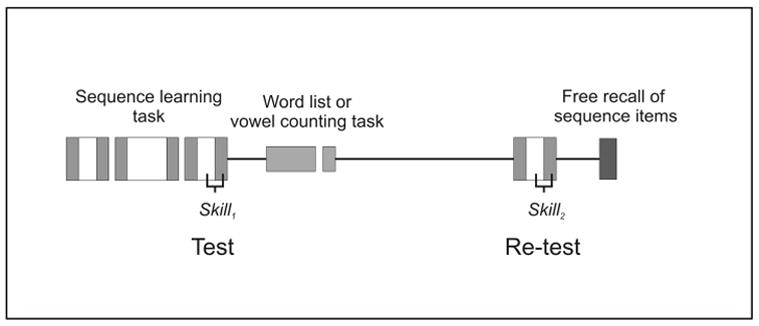
Participants were trained, tested (skill1) on the SRTT, and then either learnt a list of words or were asked to state the number of vowels within nonsense letter strings. Following a 12-hr interval participants were retested (skill2) on the SRTT, and finally asked to declaratively recall items from the sequence. Skill was measured by comparing the response times of the sequential trials (white blocks) against those of the random trials (gray blocks). The interval between testing (skill1) and word list learning was extended to 4-hr in one control group while in another the interval between testing and retesting was reduced to 30 minutes.
At initial testing, prior to the word list task, there was no significant difference in skill (skill1) between the experimental groups (both groups, n = 12; ANOVA, F(1,22) = 0.004, P = 0.95, Figure 2). However, the off-line improvement detected 12-hr later differed significantly between the two groups (ANOVA, F(1,22) = 6.32, P<0.05). Improvements only developed when the SRTT was immediately followed by learning a word list (30±11ms, mean±sem, paired t-test, t(11) = 2.8, P<0.05, Figure 2); whereas, when participants stated the number of vowels within nonsense letter strings immediately after SRTT, there was no significant offline improvement (−7±10ms, paired t-test, t(11) = 0.73, P = 0.48, Figure 2). This latter group is consistent with previous studies showing that offline improvements, in this and similar tasks, do not develop over wake 5, 8, 9. Instead, it is only over sleep that improvements normally develop with a magnitude comparable to the improvements induced by word list learning (see Supplementary Results). In both groups participants performed a cognitively demanding task immediately after the SRTT; however, in only one group; the group requiring participants to encode a word list was off-line learning observed. Therefore, by encoding a word list immediately after learning the SRTT, it became possible to induce offline motor skill improvements.
Figure 2. Off-line learning and sequence recall.
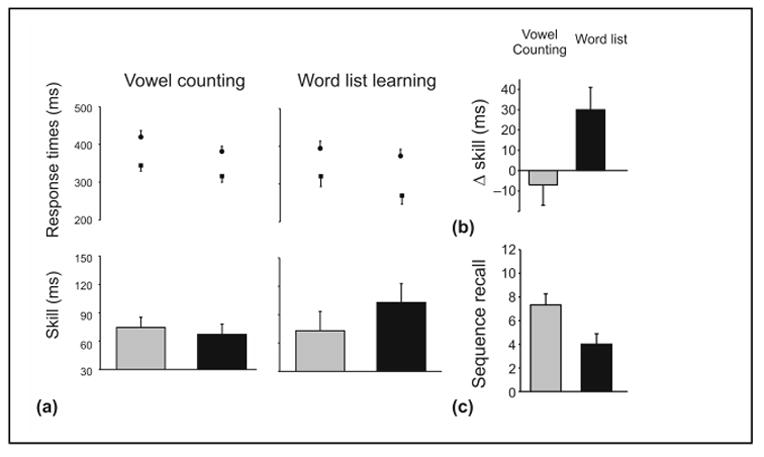
(a) Participants initial (skill1, gray boxes) and later performance on the SRTT (skill2, black boxes), with associated sequential (square±sem) and random (circle±sem) response times. Following word list learning there was only a fall in sequential response times (paired t-test, t(11) = 6.15, P<0.0001); with no significant change in the random response times (circle±sem, paired t-test, t(11) = 1.79, P = 0.1). Following vowel counting, there was a similar non-specific fall in both sequential (paired t-test, t(11) = 2.54, P<0.05) and random (paired t-test, t(11) = 2.36, P<0.05) response times. (b) These response time changes translated into differential off-line improvements: off-line improvements were induced following word list learning but not following vowel counting (unpaired t-test, t(22) = 2.5, P<0.05). (c) The declarative component of the SRTT was disrupted by learning a word list immediately after the SRTT: those learning a word list recalled on average 4.0±0.8 items (mean±sem) of the 12-item sequence; whereas, those stating the number of vowels within nonsense letter strings were able to recall on average 7.3±0.9 sequence items (unpaired t-test, t(22) = 2.8, P<0.05).
Learning a word list also significantly decreased participants’ ability to describe, in a free recall task, the SRTT sequence: participants learning the word list recalled 4.0±0.8 items (mean±sem) of the sequence; whereas those stating the number of vowels within nonsense letter strings recalled 7.3±0.9items (unpaired t-test, t(22) = 2.8, P<0.05, Figure 2). This shows that word list learning, whilst inducing motor skill improvements, also disrupted the declarative component of the SRTT.
Word list learning could exert its influence upon the SRTT by acting retroactively upon information acquired during initial SRTT training. Alternatively, it could function proactively, affecting the subsequent expression of performance at later retesting 10. Increasing the time interval between initial testing and word list learning should, according to the retroactive mechanism; decrease the influence word list learning has upon the SRTT. We compared the induced improvements when word list learning occurred immediately to those of a control group learning the word list 4-hr after initial SRTT testing (n = 12). Greater improvements were induced when word list learning occurring immediately, rather than 4-hr after initial SRTT testing (unpaired t-test, t(22) = 2.7, P<0.05). There were no significant off-line improvements when a 4-hr interval was inserted between SRTT testing and word list learning (−12±12ms, paired t-test, t(11) = 1.0, P = 0.31). Participants’ sequence recall was significantly greater when word list learning took place 4-hr rather than immediately after SRTT testing (7.0±0.8 vs. 4.0±0.8 items, unpaired t-test, t(22) = 2.47, P<0.05). Thus, the influence of word list learning upon the SRTT decreased when the interval between the tasks was increased; implying that word list learning induces skill improvements by modulating information acquired during initial SRTT training.
The full expression of skill acquired during learning may have been prevented by the declarative component of the SRTT. Thus, when this component was disrupted – by learning a word list – motor skill improvements could be quickly induced. Alternatively, the declarative component may prevent the off-line processing and slow enhancement of skill. Disrupting the declarative component would allow off-line processing, and in turn permit the slow development of skill improvements over time. The first possibility suggests that induced skill improvements should be observed immediately after word list learning; whereas, the second suggests that induced improvements should require time to develop. To distinguish between these possibilities we compared the induced improvements following a 12-hr interval against those from a 30-min interval between testing and retesting (control group, n = 12). The off-line improvements following the 12-hr interval were significantly greater than those associated with the 30-min interval (unpaired t-test, t(22) = 2.2, P<0.05). The latter showed no significant off-line improvements (−9±15, paired t-test, t(11) = 0.63, P<0.05). Sequence recall was not significantly effected by whether retesting took place 30 mins or 12-hr after word list learning (4.0±1.2 vs. 4.0±0.8 items, unpaired t-test, t(22) = 0.05, P = 0.96). Therefore, these induced improvements require time to develop, being present only after a prolonged time interval, and not immediately, indicating that they are a product of off-line processing.
The expression of off-line improvements at retesting may have been prevented by the declarative component of the SRTT. According, to this view offline improvements are the inevitable consequence of learning any new skill; yet, these improvements can only be expressed when the declarative component has been disrupted by learning a word list. However, it has been demonstrated that off-line improvements can be expressed at retesting even when participants are able to recall a substantial proportion of the sequence (5, 7, 11, see Supplementary Results). Thus, declarative knowledge alone does not appear sufficient to prevent the expression of off-line improvements.
Learning a word list immediately after acquiring the SRTT disrupted the declarative representation of the sequence – reducing participants’ ability to describe the sequence. This induced off-line improvements - normally such improvements are only observed over sleep 5, 6, 8, 9 - suggesting that, over wake, the declarative component of the SRTT may inhibit off-line motor learning. When this declarative component is selectively disrupted by learning a word list, the inhibition is removed, inducing off-line improvements (see Supplementary Discussion). This suggests that memory processing may involve not only the engagement of specific neuroplastic mechanisms, but may also rely upon the disengagement of interacting memory systems 6. Finding such an interaction challenges the concept of segregated and encapsulated memory systems; complements recent work showing that competition between systems may occur during learning and perhaps extends this principle to the off-line processing of memories 12. Together these results deepen our understanding of memory organization and provide new insights into the mechanisms supporting and controlling memory processing.
Supplementary Material
Acknowledgments
The National Institutes of Health (R01 NS051446, EMR) supported this study. We thank Matthew Walker and Chris Miall for their thoughtful and constructive comments, Alvaro Pascual-Leone for his continued guidance, and the reviewers for helping to improve this work.
Footnotes
Author Contributions
RMB conducted the experiments and assisted with the analysis; EMR designed the study, assisted with conducting the experiments, analyzed the data and wrote the manuscript.
References
- 1.Cohen NJ, Squire LR. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 2.Willingham DB. Neuron. 1997;18:5–8. doi: 10.1016/s0896-6273(01)80040-4. [DOI] [PubMed] [Google Scholar]
- 3.Gabrieli JD. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- 4.Willingham DB, Nissen MJ, Bullemer P. J Exp Psychol Learn Mem Cogn. 1989;15:1047–1060. doi: 10.1037//0278-7393.15.6.1047. [DOI] [PubMed] [Google Scholar]
- 5.Robertson EM, Pascual-Leone A, Press DZ. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Walker MP, Stickgold R. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Spencer RM, Sunm M, Ivry RB. Curr Biol. 2006;16:1001–1005. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 8.Fischer S, Hallschmid M, Elsner AL, Born J. Proc Natl Acad Sci USA. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 10.Robertson EM, Pascual-Leone A, Miall RC. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- 11.Fischer S, Drosopoulos S, Tsen J, Born J. J Cogn Neurosci. 2006;18:311–319. [PubMed] [Google Scholar]
- 12.Poldrack RA, Packard MG. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


