SYNOPSIS
Objective
It is well documented that injection drug users (IDUs) have a high prevalence of antibodies to hepatitis C virus (HCV). Sexual transmission of HCV can occur, but studies have shown that men who have sex with men (MSM) without a history of injection drug use are not at increased risk for infection. Still, some health-care providers believe that all MSM should be routinely tested for HCV infection. To better understand the potential role of MSM in risk for HCV infection, we compared the prevalence of antibody to HCV (anti-HCV) in non-IDU MSM with that among other non-IDU n at sexually transmitted disease (STD) clinics and human immunodeficiency virus (HIV) counseling and testing sites in three cities.
Methods
During 1999–2003, public health STD clinics or HIV testing programs in Seattle, San Diego, and New York City offered counseling and testing for anti-HCV for varying periods to all clients. Sera were tested using enzyme immunoassays, and final results reported using either the signal-to-cutoff ratio or recombinant immunoblot assay results. Age, sex, and risk information were collected. Prevalence ratios and 95% confidence intervals were calculated.
Results
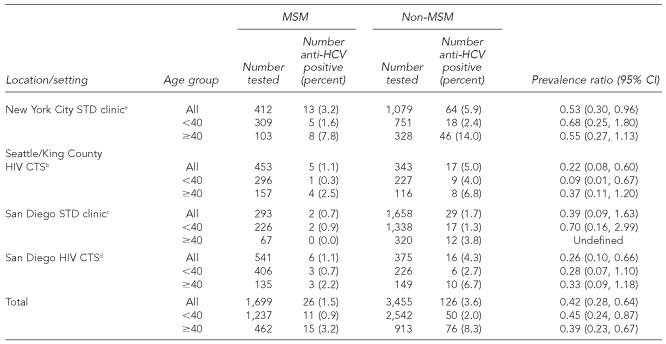
Anti-HCV prevalence among IDUs (men and women) was between 47% and 57% at each site, with an overall prevalence of 51% (451/887). Of 1,699 non-IDU MSM, 26 (1.5%) tested anti-HCV positive, compared with 126 (3.6%) of 3,455 other non-IDU men (prevalence ratio 0.42, 95% confidence interval 0.28, 0.64).
Conclusion
The low prevalence of anti-HCV among non-IDU MSM in urban public health clinics does not support routine HCV testing of all MSM.
Hepatitis C virus (HCV) is the most common chronic blood-borne virus infection in the United States, with an estimated 3.2 to 4 million people chronically infected.1,2 Large or repeated percutaneous exposures to blood such as through transfusion from unscreened donors or injection drug use have been the primary sources of infection. Sexual transmission occurs, but appears to be inefficient compared with other sexually transmitted viruses.3 Multiple studies published in the 1990s have shown that men who have sex with men (MSM) without a history of injection drug use who are seen in sexually transmitted disease (STD) clinics or human immunodeficiency virus (HIV) counseling and testing sites (CTS) have a prevalence of antibody to HCV (anti-HCV) that is no higher than other men who deny injection drug use in these settings, or adult men in the general population.4–7 More recently, similar findings were reported among non-injection drug user (non-IDU) MSM seen in an STD clinic in San Diego8 and among a large cohort of MSM recruited for an HIV transmission study in Canada.9 The Centers for Disease Control and Prevention (CDC) recommends that people at increased risk for HCV infection be identified and offered counseling and testing.5 Such people generally include those with a high prevalence of infection, such as injection drug users (IDUs). Because non-IDU MSM without other known risk factors for HCV infection are not at increased risk, HCV testing is not recommended routinely for this population. Recent reports of increased HCV infection among HIV-positive non-IDU MSM have again raised concerns of sexual transmission of HCV. Consequently, some health-care providers and MSM advocates believe that all MSM should be tested routinely for HCV infection.10–13
To further examine this issue, we compared anti-HCV prevalence between non-IDU MSM clients and other non-IDU male clients in selected STD clinics and HIV CTS in three large cities.
METHODS
HCV counseling and testing was offered in selected STD clinics and HIV CTS in San Diego, New York City (NYC), and Seattle/King County (SKC), Washington, as part of efforts to integrate viral hepatitis prevention services into public health clinics serving people at high risk for infection.14,15 Hepatitis services, including testing and vaccination, were offered to all clients initially as part of routine clinic services, and data were collected on all clients as part of routine STD or HIV clinic protocol. During the CDC Institutional Review Board and human subjects review process, these services and the data collected for this study were determined to be part of program implementation and evaluation, and specific informed consent was not required by clients. From 1999–2003, all people seeking services in these settings were offered HCV counseling and testing for varying time periods. Risk behavior information, collected through interviews and self-administered questionnaires, included sexual and IDU history, as well as other known risk factors for HCV infection (e.g., blood transfusion before 1992). Although African American race has been shown to be significantly associated with a higher prevalence of anti-HCV,1,4 race/ethnicity data were not systematically collected across sites for people receiving anti-HCV testing. However, sites were able to provide estimates of the proportion of African American clients tested for anti-HCV, based on review of clinic testing data.
Testing of sera for anti-HCV was performed using an enzyme immunoassay (EIA 2.0, Abbott Laboratories, Abbott Park, IL, or ELISA 3.0, Ortho-Clinical Diagnostics, Raritan, NJ). In San Diego and NYC, all repeatedly reactive sera were tested by recombinant immunoblot assay (RIBA 3.0, Chiron Corp., Emeryville, CA) and in SKC, RIBA testing was performed only on repeat reactive specimens with a mean signal-to-cutoff ratio of <3.8.16 A positive test was defined as EIA/ELISA repeat reactive with either a signal-to-cutoff ratio of ≥3.8 or RIBA-positive.
Among men not reporting a history of injection drug use, anti-HCV prevalence was calculated among MSM and among men reporting no history of sex with other men (non-MSM). Prevalence ratios (PRs) and 95% confidence intervals (CIs) were calculated using Epi-Info version 6.0 software.17
RESULTS
From 1999 to 2003, approximately 7,000 STD and HIV CTS clients received anti-HCV testing at these sites, including 887 IDUs (both men and women) and 5,154 non-IDU men. Clinic estimates of the proportion of people tested for anti-HCV who were African American were 32% at the NYC clinic, 20% in San Diego, and 7% in SKC.
Anti-HCV prevalence among IDUs was 51% (451/887) overall: 47% (105/224) in the San Diego STD clinic, 50% (190/382) in the SKC HIV CTS, 53% (53/100) in the San Diego HIV CTS, and 57% (103/181) in the NYC STD clinic. Among 5,154 non-IDU men, anti-HCV prevalence was 1.5% (26/1,699) among MSM and 3.6% (126/3,455) among non-MSM (PR 0.42, 95% CI 0.28, 0.64) (Table). Among all non-IDU men <40 years of age, overall anti-HCV prevalence was 1.6% (61/3,756) compared with 6.6% (91/1,369) among those ≥40 years of age (PR 0.24, 95% CI 0.18, 0.34). However, anti-HCV prevalence was lower among non-IDU MSM than among other non-IDU men in both age strata at all sites (Table).
Table.
Prevalence of antibodies to hepatitis C virus among non-injection drug user men, by report of having sex with men and age younger and older than 40, in STD clinics and HIV counseling and testing sites in three U.S. cities, 1999–2003
Tested May 2000–March 2003.
Counseling and testing programs took place in multiple venues including an STD clinic, a needle exchange program, and selected community-based organizations and bathhouses from May 2000–September 2003.
Tested October 1999–April 2000.
Tested October 2000–May 2001.
MSM = men who have sex with men
CI = confidence interval
HCV = hepatitis C virus
STD = sexually transmitted disease
HIV = human immunodeficiency virus
CTS = counseling and testing site
DISCUSSION
The low prevalence of anti-HCV among non-IDU MSM compared with other non-IDU men in this study is consistent with findings from other studies. Although a study published in the early 1990s suggested that a higher prevalence of HCV infection in MSM (6.9%) compared with heterosexual subjects (1.0%) attending a genitourinary clinic in London was strong evidence of sexual transmission of HCV among MSM, the study did not account for a history of injection drug use.18 A larger, more recent study in genitourinary clinics in the United Kingdom, using similar methods, found a low prevalence of anti-HCV among non-IDU, with the anti-HCV prevalence in MSM not significantly different from non-MSM (0.92% vs. 0.75%, PR 1.23; 95% CI 0.76, 1.98).19 The prevalence of HCV infection among MSM in STD clinics has generally been found to be no higher than among heterosexuals.6–8 Only one study of STD clinic clients found male homosexual activity to be an independent risk factor for HCV infection, but this association became nonsignificant when HIV infection was included in the multivariate model.20
A limitation of the current study is that MSM who seek services in publicly funded STD or HIV CTS may not be representative of MSM in general. However, there is no reason to believe that MSM who seek care in the private sector are more likely to engage in behaviors that may put them at increased risk for HCV infection compared with those who seek services in publicly funded clinics. In addition, the accuracy of self-reported risk factor information in this study was not validated, and because race/ethnicity data were not systematically collected across sites, potential differences in anti-HCV prevalence by race/ethnicity could not be analyzed. The higher overall estimated proportion of African Americans (known to have higher prevalence of anti-HCV than other race/ethnic groups) tested in the NYC clinic could explain in part the higher prevalence found in both MSM and non-MSM compared with other sites, but is unlikely to account for the consistency of findings across age strata in all sites.
Screening for risk factors (particularly injection drug use) and testing people at risk for HCV infection in STD and HIV CTS settings, where large numbers of IDUs may be seen, provides the potential for efficiently identifying HCV-infected people and providing them with referral for medical evaluation to determine their disease status and need for antiviral therapy if appropriate. In addition, identification provides infected people the opportunity to receive other needed services (e.g., hepatitis A or hepatitis B vaccine), and counseling to prevent further liver damage (e.g., avoidance of alcohol) and to keep from transmitting the infection to others.5,21
With decreasing resources to support prevention activities in publicly funded clinics, targeting HCV testing to those most likely to be infected is important. The cost of testing, both in resources and the increasing likelihood of false-positive results when testing lower-risk populations, should be weighed against the expected yield of testing. An evaluation conducted in the San Diego STD clinic showed that using recommended CDC criteria (history of injection drug use or blood transfusion before 1992) would identify 64% of clients with HCV infection while requiring testing of only 8% of clients.8 In addition, a cost study evaluating HCV testing in STD clinics found that testing only IDUs is the most efficient, if funds are limited.22 Although sexual transmission of HCV is possible, it appears to be inefficient, and testing MSM without a risk factor for which routine HCV testing is currently recommended is not supported by data in this report or other studies. HIV-infected people, including MSM, are an exception, and are recommended to be tested for HCV infection regardless of reported risk factors, as co-infection has important implications for progression of and therapy for both diseases.23,24
REFERENCES
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Edlin BR. Five million Americans infected with the hepatitis C virus: a corrected estimate (abstract #44). Hepatology; 56th Annual Meeting of the American Association for the Study of Liver Diseases; 2005 Nov 11–15; Boston, MA. 2005. p. 213A. [Google Scholar]
- 3.Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36(5) Suppl 1:S99–105. doi: 10.1053/jhep.2002.36797. [DOI] [PubMed] [Google Scholar]
- 4.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 5.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 6.Thomas DL, Cannon RO, Shapiro CN, Hook EW 3rd, Alter MJ, Quinn TC. Hepatitis C, hepatitis B, and human immunodeficiency virus infections among non-intravenous drug-using patients attending clinics for sexually transmitted diseases. J Infect Dis. 1994;169:990–5. doi: 10.1093/infdis/169.5.990. [DOI] [PubMed] [Google Scholar]
- 7.Weinstock HS, Bolan G, Reingold AL, Polish LB. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA. 1993;269:392–4. [PubMed] [Google Scholar]
- 8.Gunn RA, Murray PJ, Brennan CH, Callahan DB, Alter MJ, Margolis HS. Evaluation of screening criteria to identify persons with hepatitis C virus infection among sexually transmitted disease clinic clients: results from the San Diego Viral Hepatitis Integration Project. Sex Transm Dis. 2003;30:340–4. doi: 10.1097/00007435-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Alary M, Joly JR, Vincelette J, Lavoie R, Turmel B, Remis RS. Lack of evidence of sexual transmission of hepatitis C virus in a prospective cohort study of men who have sex with men. Am J Pub Health. 2005;95:502–5. doi: 10.2105/AJPH.2003.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gambotti L, Batisse D, Colin-de-Verdiere N, Delaroque-Astagneau E, Desenclos JC, Dominguez S, et al. Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001-2004. Euro Surveill. 2005;10:115–7. [PubMed] [Google Scholar]
- 11.Gotz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men—results from contact tracing and public health implications. AIDS. 2005;19:969–74. doi: 10.1097/01.aids.0000171412.61360.f8. [DOI] [PubMed] [Google Scholar]
- 12.Rauch A, Rickenbach M, Weber R, Hirschel B, Tarr PE, Bucher HC, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis. 2005;41:395–402. doi: 10.1086/431486. [DOI] [PubMed] [Google Scholar]
- 13.Ghosn J, Ceveau C, Goujard C, Garrigue I, Saichi N, Galimand J, et al. Increase in HCV incidence in HIV-1 infected patients followed since primary infection. Sex Transm Inf. 2006;82:458–60. doi: 10.1136/sti.2006.021493. Epub 2006 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (US) National hepatitis C prevention strategy. [cited 2006 Dec 1];2001 Summer; Available from: URL: http://www.cdc.gov/ncidod/diseases/hepatitis/c/plan/index.htm.
- 15.Hepatitis B vaccination among high-risk adolescents and adults, San Diego, California, 1998–2001. MMWR Morb Mortal Wkly Rep. 2002;51(28):618–21. [PubMed] [Google Scholar]
- 16.Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and results reporting of antibody to hepatitis C virus. MMWR Recomm Rep. 2003;52(No.RR03):1–16. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (US) Epi-Info Version 6.04. Atlanta: CDC; 1997. [Google Scholar]
- 18.Tedder RS, Gilson RJ, Briggs M, Loveday C, Cameron CH, Garson JA, et al. Hepatitis C virus: evidence for sexual transmission. BMJ. 1991;302:1299–302. doi: 10.1136/bmj.302.6788.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balogun MA, Ramsay ME, Parry JV, Donovan L, Andrews NJ, Newham JA, et al. A national survey of genitourinary medicine clinic attenders provides little evidence of sexual transmission of hepatitis C virus infection. Sex Transm Infect. 2003;79:301–6. doi: 10.1136/sti.79.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas DL, Zenilman JM, Alter HJ, Shih JW, Galai N, Carella AV, et al. Sexual transmission of hepatitis C virus among patients attending sexually transmitted diseases clinics in Baltimore—an analysis of 309 sex partnerships. J Infect Dis. 1995;171:768–75. doi: 10.1093/infdis/171.4.768. [DOI] [PubMed] [Google Scholar]
- 21.Mark KE, Murray PJ, Callahan DB, Gunn RA. Medical care and alcohol use after testing hepatitis C antibody positive at STD clinic and HIV test site screening programs. Public Health Rep. 2007;122:37–43. doi: 10.1177/003335490712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honeycutt AA, Harris JL, Khavjou O, Buffington J, Jones TS, Rein DB. The costs and impacts of testing for hepatitis C virus antibody in public STD clinics. Public Health Rep. 2007;122(Suppl 2):55–62. doi: 10.1177/00333549071220S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institutes of Health (US), and Public Health Service (US) and the Infectious Diseases Society of America. Guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus, 2001. [cited 2006 Dec 1]; Available from: URL: http://www.aidsinfo.nih.gov/guidelines.
- 24.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]