SYNOPSIS
Objectives.
In California, injection drug users (IDUs) comprise the second leading risk group for human immunodeficiency virus (HIV) infection and the majority of hepatitis C virus (HCV) cases. Innovative disease screening and prevention activities are needed to improve disease surveillance and to guide appropriate public health responses. This study tested the hypothesis that offering HIV counseling and testing (C&T) concurrently with HCV C&T will increase HIV C&T rates among IDUs.
Methods.
From February through June 2003, HIV and HCV C&T were integrated in five California local health jurisdictions. HIV C&T and disclosure rates among IDUs were monitored when HIV C&T was offered alone during a baseline phase and when offered with HCV C&T during an intervention phase.
Results.
Among IDUs, HIV C&T rates were significantly higher when HIV and HCV C&T were offered together (27.1%, 354/1,305) than when HIV C&T services were offered alone (8.4%, 138/1,645) (p<0.05). HIV disclosure rates increased from 54.3% (75/138) when only HIV test results were disclosed to 71.8% (254/354) when HIV test results were disclosed concurrently with HCV test results (p<0.05). HCV prevalence among IDUs tested ranged from 23% to 75% at the five project sites. Integrating HIV and HCV C&T increased overall C&T time required for staff and clients and increased stress among counselors due to the number of positive test results (HCV) given to clients.
Conclusions.
Study results suggest that integrating HIV and HCV C&T can increase disease screening rates among IDUs. Careful planning of integrated staff activities and schedules is recommended.
In California, injection drug users (IDUs) comprise the second leading risk group for human immunodeficiency virus (HIV) infection and the majority of chronic hepatitis C virus (HCV) cases. State data suggest that more than 1,000 new syringe-mediated HIV infections occur annually.1 As of 2001, an estimated 600,000 Californians were infected with HCV, of whom an estimated 60% acquired HCV through drug injection; during the same time period, at least 5,000 new HCV infections were estimated to occur annually in the state.2,3 These numbers may underestimate the burden of disease among injectors in California because IDUs access HIV counseling and testing (C&T) services less frequently than individuals in other risk groups and, if tested, between 23%4 and 30%5 fail to return for test results.
In recent years, anecdotal evidence in California and other West Coast states has suggested that IDUs are more interested in learning about and being tested for HCV than HIV. A growing body of literature indicates that integrating HIV prevention activities with other viral prevention activities may produce greatly needed synergies that facilitate prevention efforts.6–10 Integration of disease prevention efforts for IDUs and other high-risk groups may have both public health and budgetary benefits. Because of the well-established infrastructure for HIV C&T in public health programs, expanding these services to include prevention for HCV (and hepatitis B virus) infection should be feasible.6
In 2002, the California Department of Health Services, Office of AIDS (CDHS/OA), decided to evaluate integration of HIV and HCV C&T services, targeting IDUs in five local health jurisdictions (LHJs), and to monitor the use of HCV C&T as an incentive to attract more IDUs into HIV C&T services.
METHODS
LHJs were chosen based on their ability to reach relatively large numbers of IDUs, sufficient staffing to administer the project, their ability to provide clients with targeted educational and harm-reduction materials and appropriate referrals, and the presence of little or no ongoing HCV screening or related research. The LHJs selected were Fresno, Humboldt, Riverside, and Solano counties and the city of Berkeley. Site visits and trainings were conducted prior to project initiation to explain project details.
Baseline phase
The project's baseline assessment phase began in February 2003. During the two-month baseline phase, staff members at the five participating sites conducted outreach in traditional locations on the streets; in local parks; adjacent to syringe exchange programs; and at public health vans, clinics, and drug and alcohol treatment centers. Outreach conducted during the baseline phase was identical to outreach typically conducted among IDUs in LHJs.11
All outreach contacts with IDUs were documented. An IDU contact was defined as “a conversation with an IDU in which an HIV test was offered.” An IDU was defined as an individual who reported injecting illicit substances during the past two years. IDUs interested in receiving an HIV test were referred to HIV counselors or, if recruited by a counselor, were invited into the testing venue or scheduled for a later date. HIV C&T took place in private rooms on public health vans, in health clinics, and at other fixed sites, described elsewhere.4,11 Site staff members documented the number of contacts made while recruiting IDU clients for HIV C&T and the number of IDUs who decided to be tested for HIV during the two months. Clients who indicated that they knew they were HIV-positive or who were recently tested for HIV were excluded. During the counseling session preceding the HIV test, counselors used a Client Information Form to guide the counseling session and to gather basic demographic and risk behavior data.
Clients were then tested using an oral testing device (Orasure®) and were asked to return two weeks later to receive their HIV test results. HIV test rates were calculated by dividing the number of HIV tests performed by the number of IDUs contacted, and HIV test results disclosure rates were calculated by dividing the number of HIV test results disclosure sessions by the number of HIV tests conducted.
Intervention phase
In May 2003, one month after the baseline phase, the two-month intervention phase began. During this phase, IDUs were recruited in the same manner and at the same locales used during the baseline phase, but both HCV and HIV C&T were offered. Site staff members actively promoted HCV C&T during this phase, and HIV C&T was offered as an “add-on.”
Site staff members collected data on the number of contacts made with IDUs and the number of IDUs who ultimately tested for HIV or both HIV and HCV during the two-month period. Clients who indicated they already knew they were HCV-positive were still offered an HIV test, whereas HIV-positive clients and those who were recently tested for HIV were not offered a test. HIV-positive clients who did not know their HCV status were given the opportunity to test for HCV but were excluded from the HIV C&T dataset. The HIV Client Information Form and an HCV Testing Form were used to guide counseling sessions and to document client demographics; HIV and HCV risk behaviors, respectively; and referrals provided, for all IDUs who chose to be tested.
Clients were then tested using an oral testing device (Orasure®) for HIV and a finger-stick test device (Hepatitis C Check, Home Access®) for HCV (HCV Version 3.0 ELISA, Ortho®). If the initial HCV ELISA was not reactive (i.e., negative), no further testing was conducted. If the initial ELISA was reactive (i.e., preliminary positive), then a second ELISA was performed in duplicate. If the second ELISA was negative, supplemental testing was performed, using the RIBA® HCV 3.0 to clarify conflicting results from the two ELISAs and to confirm whether the individual was actually HCV positive or negative. If the second ELISA was positive, supplemental testing was also performed using the RIBA® HCV 3.0 to confirm previous positive results. A positive result indicated that the sample was found repeatedly reactive with the Ortho® HCV Version 3.0 ELISA and reported as an HCV antibody positive result.
All testing IDUs were asked to return two weeks later to receive their HIV and HCV test results, and staff members documented the number of IDUs who returned.
Data analysis
Project data captured within quantitative HIV C&T and project-specific HCV databases and through qualitative evaluation methods were analyzed. Quantitative data were analyzed using tests for differences in proportions for independent samples to compare baseline and intervention testing and test results disclosure rates for statistically significant differences at the p<0.05 level. Logistic regression analyses were conducted to compare rates of return for HIV test results while controlling for gender, race/ethnicity (white vs. non-white), reported needle sharing, and use of a syringe exchange program. Qualitative data collection methods, including periodic semiformal, semi-structured telephone interviews, site visits, and e-mailed semi-structured, open-ended questions on project experiences, were used to obtain feedback from program managers and frontline staff members on how well the integration worked, areas for improvement, lessons learned, and whether any unintended consequences were noted.
RESULTS
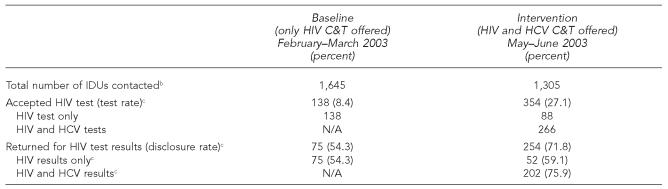
During the baseline phase, 1,645 IDUs were contacted by C&T staff across all five sites; 138 chose to be tested for HIV and 75 returned for their results (the Table). During the intervention phase, 1,305 IDUs were contacted across all five sites; 354 chose to be tested for HIV and 254 returned for their results.
Table.
HIV and HCV test and results disclosure ratesa in five California local health jurisdictions, 2003
Test rate equals total number of IDU tests/total number of IDUs contacted. Disclosure rate equals total number of disclosure sessions/total number of tests.
IDUs who were HIV-positive or who were recently tested for HIV (window period) were included as contacts but were not offered HIV tests.
p<0.05
HIV = human immunodeficiency virus
HCV = hepatitis C virus
C&T = counseling and testing
IDU = injection drug user
Aggregate HIV C&T rates among IDUs more than tripled from baseline (8.4%) to intervention (27.1%) (p<0.05), and HIV test results disclosure rates increased from 54.3% at baseline to 71.8% at intervention (p<0.05). Multiple logistic regression analyses indicated that IDUs who opted for HIV tests during the intervention phase were twice as likely to return for HIV test results as IDUs who tested for HIV during the baseline phase (odds ratio 1.93; 95% confidence interval 1.27, 2.94).
During the intervention stage, some HCV-positive IDUs chose to be tested for HIV and thus were offered this test alone. Among IDUs who tested for HIV alone during the intervention phase (n=88), only 59.1% (n=52) returned for their HIV test results. This test results disclosure rate, although slightly higher than the disclosure rate among IDUs during the baseline phase (54.3%), was not statistically significant. The disclosure rate was significantly higher among IDUs who accepted both HCV and HIV C&T (75.9%, p<0.05) than among IDUs who tested for HIV alone during the baseline phase.
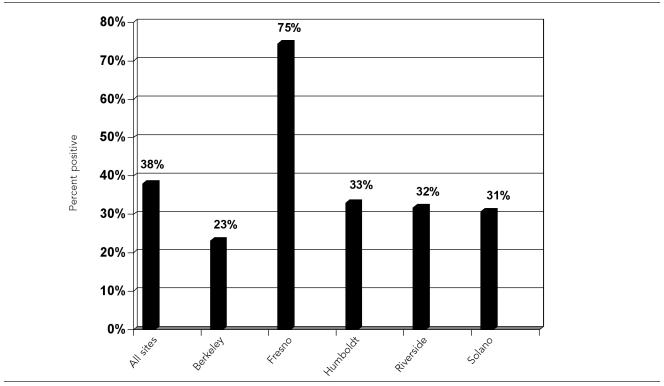
Across all five sites, overall HCV prevalence among IDUs tested was 38% (the Figure). Humboldt (33%), Riverside (32%), Solano (31%), and Berkeley (23%) all had similar HCV prevalence rates for IDUs who were tested, while Fresno had an HCV prevalence rate of 75%.
Figure.
HCV antibody test positive rates among IDUs tested during the intervention phase: May–June 2003
NOTE: HCV antibody tests were performed to detect HCV serostatus; confirmatory ribonucleic acid (RNA) tests, which are used to determine active chronic infection, were not performed because of high cost.
HCV = hepatitis C virus
IDU = injection drug user
Participating sites reported that incorporating HCV into HIV C&T took more time to administer than HIV C&T alone (i.e., an estimated 40 minutes compared with 20 minutes for an HIV C&T session alone). We found that integrated HIV and HCV C&T also increased emotional stress on the test counselors because of the number of HCV-positive results they had to give. Counselors gave a total of 101 HCV-positive test results to clients during the intervention compared to four HIV-positive results.
The blood sample required for the HCV test (i.e., enough blood to stain a half-dollar-sized circle) was difficult to draw from some participants. As a result, the time spent by C&T staff and IDUs during a risk-assessment session was sometimes extended.
In addition, it was sometimes challenging to obtain both HIV and HCV lab results in time for the test results disclosure session. Although this only occurred on a few occasions, it presented challenges for local C&T staff members who then had to ask their IDU clients to return for a second disclosure session to receive their HCV test results.
Finally, site staff members reported that HCV-specific service referrals and resources for clients identified with HCV-positive lab test results were severely limited.
DISCUSSION
This project illustrated that integrating HIV and HCV testing can improve viral surveillance efforts while providing enhanced services to IDUs. When HCV and HIV C&T were offered in tandem at the five demonstration project sites in California, HIV C&T rates more than tripled and HIV test results disclosure rates increased from 54.4% to 71.8% (p<0.05).
Test results disclosure rates
Although test results disclosure rates did not increase as markedly as C&T rates, the increase in disclosure rates suggests that HIV/HCV testing integration can encourage more IDUs to return for test results. This became particularly evident when we compared HIV test results disclosure rates among IDUs who tested for HIV only during the intervention phase (i.e., because they had previously tested positive for HCV and did not need to be tested for HCV in this project) with IDUs who tested for both HCV and HIV. IDUs who tested only for HIV during the intervention phase had a test results disclosure rate only slightly higher than that of IDUs who tested for HIV during the baseline phase (59.1% vs. 54.3%, not statistically significant). IDUs who tested for both HCV and HIV during the intervention phase, however, had a significantly higher test result disclosure rate than IDUs who tested for HIV alone during the baseline phase (75.9% vs. 54.3%, p<0.05).
Although implementation of rapid testing at HIV C&T sites across the state is likely to further increase participation in HIV test results disclosure sessions, it may have a detrimental effect on test results disclosure rates for HCV C&T. It may be important to consider offering combined HIV and HCV rapid testing if an HCV rapid test is available in the future.
HCV prevalence rates
It is important to note that actual HCV prevalence at each of the five project sites is likely to be even higher than project HCV prevalence rates (38% overall) because site staff members were instructed to conduct HCV tests with only those IDUs who had not reported previously testing positive for HCV. As a result, a number of IDUs could not be offered the HCV test because they already knew they were HCV-positive. However, if these IDUs were interested in receiving an HIV test, it was provided.
Increased testing time
The additional time required to conduct integrated C&T creates challenges for both C&T staff members and IDU clients. For staff members, increased testing time often equates to less time in the field recruiting IDUs for C&T. This may, in fact, explain why fewer IDUs were contacted during the intervention phase of the project. Despite this fact, the number and rate of IDUs tested still increased significantly from baseline to intervention. For some clients, integrated HIV and HCV C&T may take more time than they are willing to spend. Sites considering HIV and HCV C&T integration should set up C&T sites and staffing schedules in a manner that is most time-efficient for IDU clients and staff.
Counselor stress factor
Compared with other geographic areas of the United States, California has a relatively low HIV prevalence rate (2.0%) among IDUs who seek testing at publicly funded sites.5 Thus, many of the counselors who work with IDUs were not accustomed to giving a large number of positive test results during a relatively short time frame. The following quote from a staff coordinator in one of the sites helps put this challenge in perspective:
… even though we expected a high rate of (HCV) positives, we didn't foresee the toll that it would take on the testers, emotionally, to give so many positives …
As illustrated by this feedback, test counselor support is key when offering integrated HIV and HCV C&T to IDUs. In Riverside County, a therapeutic counselor met with C&T staff weekly to provide them the opportunity to debrief from their experience providing so many HCV-positive results to clients. This example may be helpful for other programs planning to integrate HIV and HCV C&T.
Expansion of integrated services
Throughout the demonstration project, it became increasingly evident that HCV follow-up evaluation and treatment services are direly needed in these California communities. Sites that implement integrated HCV and HIV C&T activities at the local level should plan to work with service providers to find ways of enhancing HCV evaluation and treatment services to individuals.
Based on results of this demonstration project, CDHS/OA opted to expand integration activities for a longer period of time and across a much larger number of LHJs. In 2004, CDHS/OA developed and implemented a new IDU-focused high-risk initiative to attempt to further increase HIV C&T rates among IDUs while providing improved HIV and HCV viral surveillance among this often hard-to-reach population. Currently, integrated C&T services are offered to IDUs in 24 LHJs across California.
Study limitations
This study had several limitations. First, we did not note, among IDUs contacted, the number of IDUs who were contacted and turned away because they were already known to be HIV-positive or because they had been tested recently (and were within the window period). This would have reduced the actual number of eligible IDUs contacted in our calculation for test rates. Thus, our calculations for HIV test rates may actually be more conservative than they might have been otherwise. Second, we did not collect demographic or risk behavior data from IDUs who were recruited but who opted not to test. Thus, we were unable to determine whether demographic and risk behaviors influenced whether IDUs opted to test or not. We were, however, able to conduct logistic regression analyses to compare disclosure rates among testing IDUs during the baseline and intervention phase while controlling for demographic and risk factors. These analyses indicated that disclosure remained higher among intervention participants regardless of gender, race, syringe sharing, and syringe exchange program access. Third, the baseline and intervention phases were relatively short (two months each), and seasonal fluctuations in testing may have influenced differential testing and disclosure rates. A review of statewide HIV C&T trends, however, indicates that there is typically little fluctuation in the number of C&T tests conducted between February and June.5 Finally, because of limited resources, we were unable to directly assess why HCV testing appears to be attractive to IDUs. Future research on integrated C&T should include research questions and methodologies that explore IDUs' motivations related to HCV and HIV testing.
CONCLUSIONS
Integration of HIV and HCV C&T targeting IDU populations in California is effective at increasing HIV C&T rates and test results disclosure rates while enhancing surveillance of HCV. Capitalizing on existing disease screening centers, trained field staff, and public health infrastructures allows for HIV and HCV C&T integration to take place without substantial budgetary increases and with minimal extra training required. It is important, however, for program managers to consider appropriate staff scheduling and support given the increased time necessary to conduct integrated C&T and the increased number of positive test results (HCV) that will likely need to be disclosed to clients. Both of these issues, if not adequately addressed, can be cause for increased stress among counselors. The dearth of HCV evaluation and treatment options for clients with positive HCV test results also indicates that it is necessary to consider ways to enhance surveillance activities and clinical services to better meet local needs. Finally, the advent of rapid testing may improve HIV C&T rates and test results disclosure rates among IDUs in the future, but without a similar rapid HCV test, the benefits of integrated HIV and HCV C&T may be diminished.
Acknowledgments
The HCV-HIV C&T Integration Team would like to thank the site coordinators and project staff at all five project sites for their guidance, feedback, and diligence throughout this project. The team also particularly acknowledges the contributions of Jena Adams (Fresno), Amity Balbutin-Burnham (Berkeley), Eric Huling (Riverside), Cynthia Packard and Lara Weiss (Humboldt), and Peter Turner (Solano). The team also thanks Heather Lusk, Lori Fries, Fred Molitor, and Allison Precht for their contributions during various aspects of the demonstration project.
REFERENCES
- 1.California Department of Health Services, Office of AIDS. Sacramento: California Department of Health Services; 2001. Dec, Consensus meeting on HIV/AIDS incidence and prevalence in California. [Google Scholar]
- 2.California Department of Health Services. Sacramento: California Department of Health Services; 2001. Hepatitis C strategic plan. Recommendations for the prevention and control of hepatitis C in California. [Google Scholar]
- 3.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1–39. [PubMed] [Google Scholar]
- 4.Molitor F, Bell RA, Truax SR, Ruiz JD, Sun RK. Predictors of failure to return for HIV test result and counseling by test site type. AIDS Educ Prev. 1999;11:1–13. [PubMed] [Google Scholar]
- 5.California Department of Health Services. Sacramento: California Department of Health Services; 2005. [cited 2006 Dec 8]. California HIV counseling and testing annual report: January–December 2002. Also available from: URL: http://www.dhs.ca.gov/AIDS/Reports/PDF/2002HIVCTAnnualReport091605.pdf. [Google Scholar]
- 6.Prevalence of hepatitis C virus infection among clients of HIV counseling and testing sites—Connecticut, 1999. [cited 2005 May 25];MMWR Morb Mortal Wkly Rep. 2001 50(27):577–81. Also available from: URL: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5027a2.htm. [PubMed]
- 7.Strauss SM, Falkin GP, Vassilev Z, Des Jarlais DC, Astone J. A nationwide survey of hepatitis C services provided by drug treatment programs. J Subst Abuse Treat. 2002;22:55–62. doi: 10.1016/s0740-5472(01)00213-6. [DOI] [PubMed] [Google Scholar]
- 8.Astone J, Strauss SM, Vassilev ZP, Des Jarlais DC. Provision of hepatitis C education in a nationwide sample of drug treatment programs. J Drug Educ. 2003;33:107–17. doi: 10.2190/YEGL-GX4W-HGRA-EDC7. [DOI] [PubMed] [Google Scholar]
- 9.Strauss SM, Astone J, Vassilev ZP, Des Jarlais DC, Hagan H. Gaps in the drug-free and methadone treatment program response to hepatitis C. J Subst Abuse Treat. 2003;24:291–7. doi: 10.1016/s0740-5472(03)00037-0. [DOI] [PubMed] [Google Scholar]
- 10.Fraser MR, Buffington J, Lipson L, Meit M. Hepatitis C prevention programs: assessment of local health department capacity. J Public Health Manag Pract. 2002;8:46–9. doi: 10.1097/00124784-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen H, Chen M, Myrick R, Truax S. An evaluation of California's Neighborhood Intervention Geared to High-Risk Testing (NIGHT) outreach program. Proceedings of the XIV International AIDS Conference; Jul 7–12; Barcelona, Spain. 2002. [cited 2006 Aug 8]. Available from: URL: http://www.cdc.gov/outreach/resources/InternationalAIDSConference-night.ppt. [Google Scholar]