SYNOPSIS
The Centers for Disease Control and Prevention recommends integrating viral hepatitis prevention services with services for adults evaluated for sexually transmitted diseases (STDs). The Denver Public Health STD clinic began hepatitis B vaccination in 1999, hepatitis C virus (HCV) antibody (anti-HCV) testing in 2000, and hepatitis A vaccination in 2002. Rapid human immunodeficiency virus (HIV) testing began in late 2004. Hepatitis B vaccinations peaked in 2003 (31/100 client visits) when a full-time nurse was hired to vaccinate and eligibility was expanded. The proportion of clients documented to have received their anti-HCV test results declined from an average of 71% in 2000–2003 to 22% in 2004–2005, coinciding with the introduction of rapid HIV testing. Viral hepatitis prevention services can be incorporated into a busy STD clinic if staff and resources are available. Rapid HIV testing may be associated with lower receipt of anti-HCV test results.
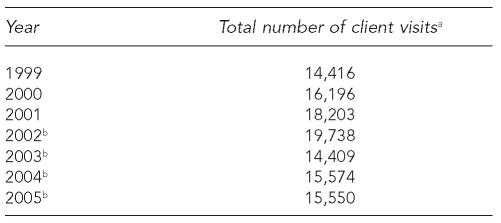
Hepatitis A, B, and C are serious public health problems, and efforts to prevent and control these infections by vaccination (hepatitis A and B) and counseling, testing, and referral (hepatitis C) should be included in programs that serve people at high risk for infection, including sexually transmitted disease (STD) clinics. This article describes the integration of viral hepatitis prevention services into the Denver Public Health (DPH) STD clinic, which averages more than 16,000 client visits annually (Table 1).
Table 1.
Total number of client visits, Denver Public Health STD Clinic, 1999–2005
Client visits refers to all visits, including multiple visits by the same individual.
Copayment for services from December 2002 to January 2005 required as a result of a state fiscal crisis.
STD = sexually transmitted disease
IMPLEMENTING VIRAL HEPATITIS INTEGRATION
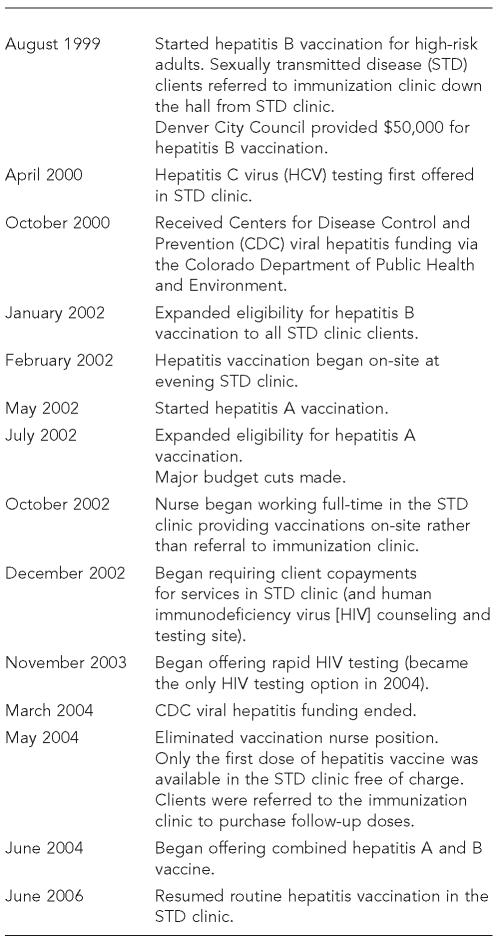
A 1996 survey of 224 DPH STD clinic clients found that 53% needed and 59% were interested in hepatitis B vaccination. The DPH STD clinic director used these findings to request funding from the Denver City Council for hepatitis B vaccination. In 1999, a one-time award of $50,000 was made to purchase hepatitis B vaccine specifically for high-risk clients (the Figure shows a timeline of viral hepatitis integration). In 1999, the STD clinic director and a local advocacy group appealed to the state legislature for funding for prevention of hepatitis C virus (HCV) infection, resulting in a $200,000 appropriation for an education and screening program.
Figure.
Timeline of viral hepatitis integration, Denver Public Health STD Clinic
In October 2000, the Centers for Disease Control and Prevention (CDC) funded the Colorado Department of Public Health and Environment (CDPHE) to expand viral hepatitis integration activities. CDPHE contracted with DPH to implement a project in the DPH STD clinic and other settings in the DPH system. Because the STD clinic had already implemented hepatitis B vaccination, funds were used to add HCV counseling, testing, and referral (April 2000) and hepatitis A vaccination (May 2002).
Viral hepatitis activities
Hepatitis A and B vaccination.
Initially, clients eligible for hepatitis A or B vaccination were referred to the DPH immunization clinic, located in the same building as the STD clinic. A chart review in 2001 found that approximately 30% of clients who accepted referral for vaccination were not vaccinated. In February 2002, the STD clinic began offering hepatitis B vaccination on-site one evening per week. Because on-site vaccination was well received by clients and staff, CDC funds were used in September 2002 to hire a full-time nurse to vaccinate clients on-site during regular clinic hours.
The vaccination nurse position was eliminated in May 2004 due in part to budget concerns, and was replaced by a nurse position that included responsibility for both vaccination and phlebotomy. By the end of CDC funding in mid–2004, only the first dose of vaccine was offered for free in the clinic, and clients were advised to purchase follow-up doses in the immunization clinic. In late 2004, approximately $75,000 of surplus funds was used to purchase monovalent and combined hepatitis A and B vaccine (hereafter referred to as combined vaccine). With a stable yet short-term hepatitis vaccine supply, the STD clinic's hepatitis vaccination program continued into early 2005 but was temporarily discontinued in March 2005 with the implementation of a new computerized charting system. Hepatitis vaccination has been reintroduced gradually as clinic flow stabilized and, with the addition of two new staff in June 2006, the clinic is once again offering vaccination routinely to all clients.
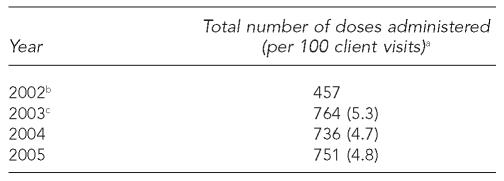
Eligibility criteria for hepatitis A vaccination initially included men who have sex with men (MSM), injection drug users (IDUs), and people testing positive for HCV antibody (anti-HCV). In September 2002, criteria were expanded to include sex workers. The average number of monovalent hepatitis A vaccinations between 2003, the first full year in which hepatitis A vaccination was offered, and 2005 was 750, with an average rate of 4.9 per 100 client visits during this time period (Table 2). The highest proportion of second doses relative to first doses, an approximation of series completion, was 15.8% (2003) (data not shown).
Table 2.
Number and rate of monovalent hepatitis A vaccinations per 100 client visits, Denver Public Health STD Clinic, 2002–2005
Includes first and second doses of monovalent and combined vaccine.
Hepatitis A vaccination began in May 2002; rate not calculated for this partial year.
Combined vaccine available in June 2003.
STD = sexually transmitted disease
Eligibility criteria for hepatitis B vaccination initially included MSM, IDUs, sex partners of MSM or IDUs, anti-HCV-positive individuals, people with a history of an STD, people having two or more sex partners in the last four months, and clients aged 18 or younger. A review of clinic data in 2001 showed that only about one-third of clients seen by clinicians were screened for hepatitis B risk factors. This finding, coupled with advance notice of the CDC 2002 STD treatment guidelines, which recommended that all STD clients—regardless of reported risk—be offered hepatitis B vaccination, led to a new policy in January 2002 to offer vaccination to all clients without a history of prior vaccination or infection.1
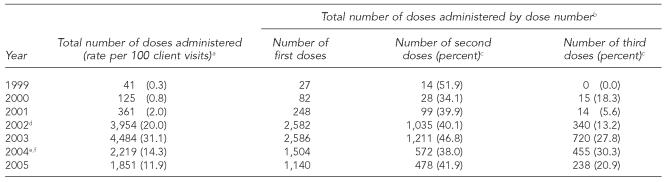
At its peak in 2003, the clinic administered 4,484 doses of hepatitis B vaccine (including monovalent and combined vaccine; 31.1 per 100 client visits). The number of hepatitis B vaccinations increased 11-fold from 361 in 2001 to 3,954 in 2002 (Table 3), coinciding with the hiring of a full-time nurse to vaccinate clients on-site and discontinuation of risk-based screening. In 2005, with the loss of the full-time vaccination nurse position, the number of doses dropped to 1,851, and the rate of hepatitis B vaccination per 100 client visits decreased 62% (from 31.1 in 2003 to 11.9 in 2005). The estimated rate of completion of the three-dose hepatitis B vaccine series ranged from 6% to 30% of clients who received a first dose (Table 3).
Table 3.
Number and rate of monovalent hepatitis B and combined hepatitis A and B vaccinations per 100 client visits, Denver Public Health STD Clinic, 1999–2005
Includes first, second, and third doses of monovalent vaccine and combined vaccine (starting in June 2004).
Includes monovalent and combined (starting in June 2004) hepatitis B vaccine doses.
Percentage is the number of second or third doses divided by the number of first doses multiplied by 100.
A full-time nurse was hired in August 2002 and began to vaccinate STD clinic clients on-site in October 2002.
Clients advised to purchase follow-up doses in the immunization clinic in mid–2004.
Full-time vaccination nurse position eliminated in May 2004.
STD = sexually transmitted disease
In June 2004, the STD clinic introduced combined vaccine. Initially, eligibility criteria included all clients who were eligible for hepatitis B vaccination, although clients who were documented in the clinic's database to have been vaccinated for hepatitis A virus (HAV) or hepatitis B virus (HBV) were offered the appropriate monovalent vaccine. Starting in November 2005, all STD clinic clients without a documented history of vaccination for HAV and HBV were offered combined vaccine. In 2005, the first full year in which combined vaccine was used, nearly half of all hepatitis B vaccinations were combined vaccine (916 combined hepatitis A and B/1,851 hepatitis B vaccinations) (data not shown).
Hepatitis C counseling, testing, and referral.
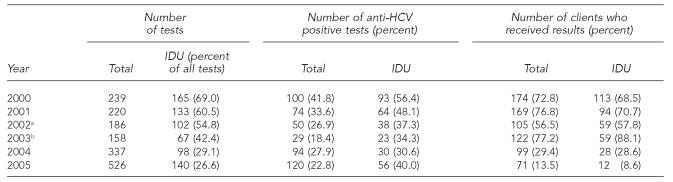
HCV counseling, anti-HCV testing, and referral services were introduced in April 2000. IDUs, sex partners of HCV-infected individuals, and people who received a blood transfusion before 1992 were eligible for anti-HCV testing. From 2000 to 2005, the annual average number of anti-HCV tests was 278, ranging from 158 (2003) to 526 (2005) (Table 4). The proportion of people tested who reported injection drug use declined steadily from 2000 (69%) to 2005 (27%), suggesting that providers did not adhere to eligibility criteria for anti-HCV testing.
Table 4.
HCV testing and outcomes, Denver Public Health STD Clinic, 2000–2005
Phone notification of HCV test results began.
Rapid human immunodeficiency virus testing was introduced in November 2003 and became the only testing option in 2004.
HCV = hepatitis C virus
STD = sexually transmitted disease
IDU = injection drug user
The prevalence of anti-HCV among clients tested decreased from 42% in 2000 to 18% in 2003, increased to 28% in 2004, and decreased slightly to 23% in 2005. Among people tested who reported injection drug use, the prevalence of anti-HCV declined steadily from 56% in 2000 to 31% in 2004, but increased to 40% in 2005. Among people tested who did not report injection drug use, or for whom this risk factor was not documented, the prevalence of anti-HCV ranged from 7% (2003) to 27% (2004) (data not shown).
The proportion of clients tested who received their anti-HCV test results (in person or by telephone) increased from 57% in 2002 to 77% in 2003, and then dropped to 29% in 2004 and 14% in 2005. This decrease coincided with increasing use of rapid human immunodeficiency virus (HIV) testing, which began in November 2003, and telephone reporting of results beginning in 2002, which may not have been well documented.
Referrals and follow-up of people with positive anti-HCV tests.
People with positive anti-HCV tests are provided a guide to the referral process for the local public hospital and insurance carriers for indigent and low-income populations. Denver County residents can be referred to the public hospital, and members of the STD clinic staff help residents complete financial paperwork to initiate this process. Nonresidents can be referred to a university hospital-based gastroenterology clinic.
In 2002, 65 clients who received a positive anti-HCV test result were interviewed by telephone three to six months later to determine if they had received recommended follow-up services (e.g., medical evaluation, vaccination, substance abuse counseling). Less than 20% of anti-HCV-positive clients followed through with recommended services. Barriers reported by clients included financial obstacles and lack of motivation to seek follow-up care.
HIV counseling, testing, and referral
Most clients interested in HIV testing made appointments with the HIV counseling and testing site, which has been co-located with the DPH STD clinic since 2000. The STD clinic provided clients at risk for HIV with the option of confidential testing or referral to the HIV counseling and testing site for anonymous testing. Two recent changes were made to the clinic's HIV testing policy to improve identification of clients with undiagnosed infections. Rapid HIV testing, for which the specimen is collected with a finger stick, was introduced in late 2003 and has been the only test option since November 2004. Beginning in 2005, all clients seen in the STD clinic were offered HIV testing regardless of reported risk factors.
From 2001 to 2005, the mean annual number of HIV tests was 8,745 and ranged from 7,992 (2000) to 10,194 (2004) (data not shown). Among clients tested, the number of positive HIV test results ranged from 42 (2003), with a 0.55% positivity rate, to 77 (2005), with a 0.91% positivity rate. Rates of post-test counseling gradually improved from approximately 57% in 2000–2002 to 62% in 2003, 78% in 2004, and 88% in 2005.
DISCUSSION
Hepatitis A and B vaccination and HCV counseling, antibody testing, and referral have become an accepted part of services in the Denver STD clinic. However, these services were reduced because of budget cuts. Viral hepatitis services have been well received by clinic clients and staff, although most clients do not come in specifically for these services. However, in a busy clinic environment, hepatitis prevention services may not be prioritized when demand exceeds available resources. To sustain integration efforts, viral hepatitis services must be part of the daily clinical routine, with appropriate resources available, including free vaccination and staff who are trained and able to vaccinate clients on-site.
Making hepatitis B vaccine available to all clients and hiring a full-time nurse to provide hepatitis vaccinations in the STD clinic, both occurring in 2002, contributed to an increase in hepatitis B vaccination in 2003. However, hepatitis B vaccination decreased in 2004, coinciding with the elimination of the full-time nurse position.
Full measurement of completion of the hepatitis B vaccine series was not possible due to inability to track individual client data; however, data on annual first, second, and third dose numbers suggest completion rates similar to rates found in other STD clinics.2 A postcard reminder system was implemented in June 2001 in an effort to increase the number of clients who return for follow-up doses, but the reminder system was discontinued because it was too labor intensive.
Although clinicians are asked to screen clients for HCV risk factors (e.g., injection drug use), testing appeared to be increasingly offered to low-risk people requesting the test. The proportion of clients tested for anti-HCV who did not report injection drug use or for whom this risk was not documented increased from 31% in 2000 to 73% in 2005. From 2000 to 2003, the proportion of positive anti-HCV test results decreased, consistent with the decrease in the proportion of documented IDU clients tested. However, in 2004 and 2005, the proportion of positive anti-HCV test results increased, despite the continued decrease in the proportion of documented IDUs tested. Furthermore, 60% of clients with positive anti-HCV test results in 2004 did not report injection drug use as a risk behavior. Under-reporting or poor documentation of injection drug use among STD clinic clients may be contributing to these results.
Although it was not possible to directly measure the impact of hepatitis service delivery on existing clinic services, integration did not appear to adversely affect core STD services. However, the transition to rapid HIV testing likely contributed to the dramatic increase in the number of clients receiving HIV post-test counseling.
Although anti-HCV testing requires a separate venipuncture blood draw, the number of anti-HCV tests did not decrease with implementation of HIV rapid testing. However, the proportion of clients who were documented to have received their anti-HCV test results decreased after 2003. Two factors may have contributed to this decrease. First, rapid HIV test results are available within 20 minutes, whereas anti-HCV test results are not available for several weeks. Clients who opt to receive both an HIV and an anti-HCV test may not be motivated to return for anti-HCV test results only. Second, poor recording of results delivered over the telephone, which began in 2002, instead of in person also may have contributed to the apparent drop in documented receipt of anti-HCV test results.
The DPH STD clinic adopted innovative policies and practices to address some of the challenges encountered as viral hepatitis prevention services were integrated. First, clinicians initially did not routinely screen clients for HBV risk factors to determine eligibility for hepatitis B vaccination. In response, the STD clinic dropped eligibility screening and offered hepatitis B vaccination to all clients who were not already immune.
Second, many STD clients need basic information about viral hepatitis. As a result, clinicians had to spend a significant amount of time educating clients about hepatitis before they would accept recommended services. Brochures are available in English and Spanish describing the hepatitis virus types. Vaccinating clients and educating them about viral hepatitis before they are seen by clinicians who conduct the STD exam decreases the time those clinicians need to spend educating clients.
Third, the clinic hired a full-time nurse to vaccinate clients in the STD clinic because 30% of clients who accepted referral to the immunization clinic were not vaccinated. Although this full-time position in part contributed to a large increase in the number of clients getting vaccinated, the position was eliminated in mid–2004, largely because of funding cuts. Although there was still a staff person who could vaccinate clients, vaccination was one of multiple tasks assigned. As of June 2006, hepatitis vaccination is once again routinely offered as part of the registration process, and clients can return to the clinic just to receive subsequent vaccine doses.
This article demonstrates that viral hepatitis prevention services can be incorporated into a busy STD clinic, provided that leadership, staff, and resources are available. Members of the STD clinic staff remain committed to providing viral hepatitis prevention services to STD clients as resources allow.
REFERENCES
- 1.Centers for Disease Control and Prevention (US). Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep. 2002;51(RR-6):1–78. [PubMed] [Google Scholar]
- 2.CDC (US) Hepatitis B vaccination among high-risk adolescents and adults—San Diego, California, 1998–2001. MMWR Morb Mortal Wkly Rep. 2002;51(28):618–21. [PubMed] [Google Scholar]