SYNOPSIS
Hepatitis B vaccination is recommended for all clients of sexually transmitted disease (STD) clinics. Hepatitis A vaccination and hepatitis C testing are recommended for STD clinic clients who report specific risks for those viruses. In 1999, the Illinois Department of Public Health began working with local health departments in Illinois (excluding Chicago) to introduce hepatitis B testing and vaccination in public STD clinics. Hepatitis A vaccination and hepatitis C counseling and testing were introduced in 2001. Illinois state funding has covered more than one-third of the costs of offering these integrated viral hepatitis services to STD clients. Hepatitis A and B vaccination and hepatitis C counseling and testing are now the standard of care in almost all (35 of 41) Illinois public STD clinics (excluding Chicago). In 2005, 29.4% of STD client visits included a hepatitis B vaccination. In public STD clinics in Illinois, hepatitis A and B vaccinations and hepatitis C counseling and testing have increased from essentially no activity in 1999 to substantial levels of service in 2005.
In 1999, the Illinois Department of Public Health (IDPH) launched what became a statewide initiative to provide hepatitis B vaccination and testing in public sexually transmitted disease (STD) clinics. Because the City of Chicago receives separate federal public health funding, it was not part of this initiative. This IDPH initiative was a close collaboration of the IDPH STD and Immunization Programs. State funds and discretionary federal Vaccination Assistance Act funds paid for the hepatitis B vaccine used in the initiative.
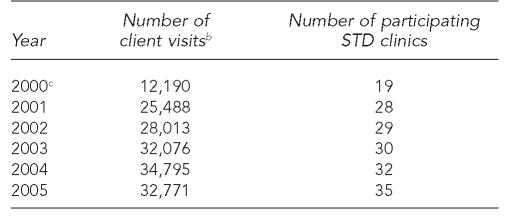
The initiative began in April 1999, with a pilot program in the STD clinics in Decatur and Peoria, Illinois. In six months, 47% of STD clinic client visits at these two pilot sites included a hepatitis B vaccination (926 doses/1,973 client visits). Because of the success of the pilot program, IDPH offered state support to add hepatitis B vaccination in the other Illinois public STD clinics. The STD clinics in Illinois are locally operated, usually by county health departments (CHDs). IDPH primarily supports the local STD clinics with in-kind provision of medications, pamphlets and educational materials, and laboratory testing. The number of Illinois STD clinics offering viral hepatitis services nearly doubled from 2000 (19) to 2005 (35) (Table 1). Six relatively small STD clinics, with a range of four to 48 (mean 21.4) STD clients per month, have not added viral hepatitis services as of 2005.
Table 1.
STD clinic client visits in Illinois (excluding Chicago),a by year, 2000–2005
Excluding Chicago, a separately funded Centers for Disease Control and Prevention project area.
Definition: all client visits including repeat visits by some clients during the calendar year.
Client visit data for 2000 were incomplete because of delays in setting up the reporting system.
STD = sexually transmitted disease
In 2000, as part of the hepatitis B vaccination initiative, participating STD clinics agreed to use a standard STD, human immunodeficiency virus (HIV), hepatitis behavioral risk assessment survey. STD clinic clients complete the risk assessment when they register, and completed surveys are submitted to IDPH for data entry and analysis. IDPH developed a Microsoft® Access database linking client-completed risk assessment data, laboratory results, and vaccinations performed. The database is used to generate clinic-specific and statewide reports and helps monitor STD clinic activities. Although CHDs do not have access to the database, IDPH provides quarterly reports to each STD clinic. The reports track a variety of program activities, such as hepatitis A and B vaccine acceptance and completion, and behavioral data such as clients who report injecting drugs. This data system allows analyses of individual factors such as prior history of an STD, drug and condom usage, and HIV testing history.
ADDING HEPATITIS A VACCINATION AND HEPATITIS C COUNSELING AND TESTING
In October 2000, the Centers for Disease Control and Prevention (CDC) awarded IDPH funds to expand viral hepatitis integration in Illinois STD clinics. IDPH staff selected six large STD clinics to use this funding to add hepatitis A vaccination and hepatitis C counseling and testing. The six clinics had high rates of STD morbidity and local leadership that supported hepatitis integration.
Hepatitis A and B vaccinations
Beginning in early 2001, in the six clinics that received additional funding, clients who reported injection drug use and/or self-identified as men who have sex with men (MSM) were offered hepatitis A vaccination. In 2001, the first full year in which hepatitis A vaccination was offered in the six funded clinics, 941 vaccinations were administered during 11,385 client visits for a rate of 8.2 hepatitis A vaccinations per 100 client visits (Table 2).
Table 2.
Monovalent hepatitis A vaccinations at STD clinics in Illinois,a by year, 2000–2005
Excluding Chicago.
Includes first and second doses of monovalent hepatitis A vaccine.
Six STD clinics supported in part by Centers for Disease Control and Prevention funding began using combined hepatitis A and B vaccine in January 2002. The other participating clinics began using combined vaccine in October 2002.
STD = sexually transmitted disease
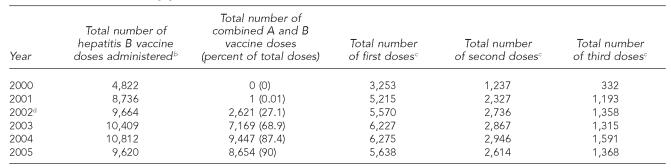
During 2000 and 2001, the six funded clinics and the other participating STD clinics offered hepatitis B vaccination to all clients at least 18 years of age who had not been previously infected or completed the three-dose series. In January 2002, the six funded clinics began using combined hepatitis A and B vaccine (hereafter referred to as “combined vaccine”) for all STD clinic clients regardless of risk factors. IDPH began primarily using combined vaccine for two reasons: (1) the belief that hepatitis A vaccination was indicated for many STD clients who were non-injecting drug users and (2) having one vaccine simplified clinic procedures. In October 2002, combined vaccine was made available to the other participating STD clinics. Currently, most clients who receive hepatitis vaccinations receive combined vaccine. Monovalent hepatitis A and B vaccines are available for people who are already known to be immune to either hepatitis A or B.
From 2001 to 2005, the total number of hepatitis B vaccinations per year (combining monovalent hepatitis B and combined vaccine doses) reported by participating STD clinics ranged from 8,736 (2001) to 10,812 (2004) (Table 3). In that period, the number of hepatitis B vaccinations per 100 client visits (data not shown) dropped from 34.3 (2001) to 29.4 (2005). Two STD clinics (DuPage and Winnebago counties) reported peak rates of 56 and 55.8 vaccine doses per 100 client visits in 2002 (data not shown).
Table 3.
Monovalent hepatitis B and combined hepatitis A and B vaccinations in STD clinics in Illinois,a by year, 2000–2005
Excluding Chicago.
Includes first, second, and third doses of monovalent hepatitis B and combined hepatitis A and B vaccines.
Includes monovalent hepatitis B and combined hepatitis A and B vaccines.
Six STD clinics supported in part by Centers for Disease Control and Prevention funding began using combined hepatitis A and B vaccine in January 2002. The other participating clinics began using combined vaccine in October 2002.
STD = sexually transmitted disease
Even though STD clinics used a combination of appointment cards, reminder letters, and chart reviews to promote completion of the three-dose hepatitis B vaccination series, there were steep declines from first to second and second to third vaccine doses. For example, in 2004, 6,275 first doses of hepatitis B vaccine, 2,946 second doses, and 1,591 third doses were reported, and third doses were 25.3% (1,591/6,275) (Table 3) of first doses.
As part of the risk assessment, STD clinic clients were asked if they had ever been vaccinated against hepatitis B. The percentage of clients who reported having already received at least one vaccination increased each year, from 27% in 2000 to 44% in 2005.
Hepatitis B and C counseling, testing, and referral services
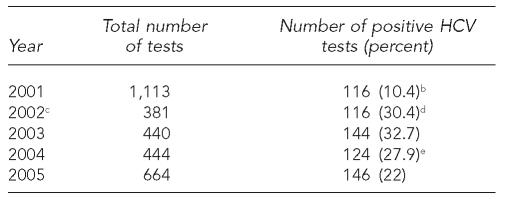
In 2001, STD clinic clients who reported snorting or injecting drugs were eligible for hepatitis B and C testing. Serologic testing was done by the IDPH laboratory. Because of concern about possible chronic hepatitis B virus (HBV) infection, IDPH tested for HBV surface antigen (HBsAg). Initially in 2001, hepatitis C testing used an enzyme immunoassay (EIA) test for antibody to hepatitis C virus (HCV) (anti-HCV). Only four of 1,113 (0.36%) clients tested positive for HBsAg, and 116 of 1,113 clients (10.4%) had positive anti-HCV test results. More than 95% of the 116 clients with positive anti-HCV tests reported injecting drugs. As a result, in 2002, serologic testing procedures were changed: hepatitis B serologic testing was discontinued, and anti-HCV testing was limited to clients who reported injecting drugs.
After early 2002, when HCV screening was limited to clients reporting injecting drugs, the number of anti-HCV tests decreased to 381 (2002) and then gradually increased to 664 (2005) (Table 4). The proportion of anti-HCV positive tests ranged from 32.7% (2003) to 22% (2005).
Table 4.
HCV testing and outcomes in STD clinics in Illinois,a by year, 2001–2005
Excluding Chicago.
An HCV test was defined as anti-HCV positive if it was reactive to both an enzyme immunoassay (EIA) and a recombinant immunoblot assay (RIBA).
In 2002, new screening criteria was introduced: only injecting drug users are eligible for HCV testing.
In July 2002, the Illinois Department of Public Health (IDPH) began using anti-HCV antibody signal-to-cutoff (S/CO) ratio to reduce testing costs. The new definitions of anti-HCV positive were (1) EIA reactive with a S/CO ratio >3.8 or (2) EIA reactive with a S/CO ratio ≤3.8 and RIBA reactive.
In 2004, IDPH began using an Ortho Vitros assay. The new definitions of anti-HCV positive were (1) EIA reactive with a S/CO ratio >8.0 or (2) EIA reactive with a S/CO ratio ≤8.0 and RIBA reactive.
HCV = hepatitis C virus
STD = sexually transmitted disease
Local health departments send blood for anti-HCV testing to the IDPH state laboratory. The IDPH STD Program pays the state laboratory from $5,000 to $10,000 a year for anti-HCV testing.
SERVICES FOR CLIENTS WITH POSITIVE HEPATITIS C TESTS
Because of limited funding, the IDPH STD Program does not monitor referrals and follow-up for clients with positive anti-HCV tests. However, STD clinic staff reported substantial difficulties finding follow-up medical evaluation and care for STD clients with positive anti-HCV tests who had limited or no health insurance.
FUNDING FOR VIRAL HEPATITIS INTEGRATION
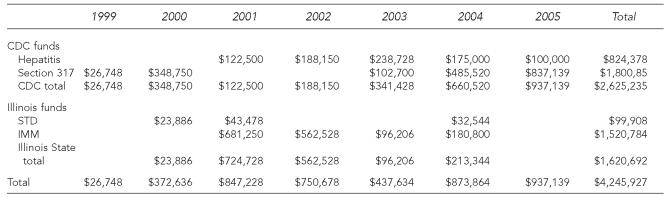
Partial funding data, including expenditures for vaccine and some hepatitis personnel, were available for 1999–2005 (Table 5). The total funding for these viral hepatitis components from 1999 to 2005 was $4,245,927. Of the total funds, $3,321,641 (78.2%) was used for vaccine purchases and $265,922 (6.3%) for personnel costs. Overall, Illinois state funding ($1,620,692) covered 38% of these costs. The IDPH Immunization Program contributed 45.8% of the funds for vaccine purchase ($1,800,857 of $3,321,641).
Table 5.
Funding of viral hepatitis prevention services in STD clinics in Illinois,a 1999–2005
Excluding Chicago.
STD = sexually transmitted disease
CDC = Centers for Disease Control and Prevention
IMM = immunizations
The local health departments pay for the staff time needed to manage hepatitis activities, screen and vaccinate clients, and provide pre- and post-test counseling for HCV testing.
DISCUSSION
In STD clinics, hepatitis B vaccination is recommended for all clients,1 and hepatitis A vaccination is recommended for all MSM, illegal drug users (both injecting and non-injecting drugs), and people with chronic liver disease.2 Hepatitis B vaccination at STD clinics is a cost-effective public health strategy when long-term consequences of these diseases are considered.3–5 Providing integrated viral hepatitis services to all STD clients could contribute to expanding viral hepatitis prevention.6 However, the introduction of hepatitis B vaccination into STD clinics in the United States has been slow.4,7,8
In Illinois, state and local public health departments and staff have worked together to integrate viral hepatitis services into the majority of public STD clinics. Viral hepatitis integration efforts began in 1999, when IDPH introduced hepatitis B testing and vaccination in STD clinics as an IDPH initiative. CDC funding and technical assistance for viral hepatitis integration helped catalyze the addition of hepatitis A vaccination and HCV counseling and testing in IDPH-supported local STD clinics.
Hepatitis A and B vaccination and HCV counseling and testing are now the standard of care in almost all (in 2005, 35 of 41) Illinois public STD clinics. Illinois state funding has covered more than one-third of the costs of offering these integrated viral hepatitis services to STD clinic clients.
The findings of this report are subject to the limitation that they are from a single state and may not be generalizable to other states.
From 2000 to 2005, between 28.4 (2005) and 49.8 (2000) of every 100 client visits to participating Illinois public STD clinics included a hepatitis B vaccination (data not shown). These vaccinations contributed to the increasing proportion of STD clients who reported receiving at least one prior hepatitis B vaccination (44% in 2005) and will help protect adults at risk of hepatitis B who have not been previously vaccinated.9
Several factors contributed to the successful integration of viral hepatitis services into Illinois STD clinics, including: (1) availability of vaccine that was free of charge to the local health department-operated STD clinics; (2) commitment and involvement of local STD clinic staff and supervisors; (3) close collaboration between the IDPH Immunization and STD Programs; and (4) existence of a statewide data system that included information on client characteristics and risk behaviors, vaccinations performed, and serology test results.
The Illinois experience identified three problems associated with integrating viral hepatitis services into STD clinics: (1) inadequate resources available to support the evaluation and follow-up of STD clients who have positive anti-HCV screening tests; (2) the low rates of completing the recommended vaccination series; and (3) limited funding to cover the additional staff time needed for viral hepatitis activities, which has limited the ability of some Illinois STD clinics to offer all clients the full range of viral hepatitis services.
Our experience in Illinois indicates that it is possible to provide substantial levels of viral hepatitis services for clients in the majority of locally operated STD clinics throughout the state (excluding Chicago). Our experience supports the feasibility of implementing the recommendation that all STD clients receive hepatitis B vaccination.2 We believe that federal support is needed to help provide states and local governments with vaccines for adults at high risk of viral hepatitis. A system for adult vaccines modeled on the excellent Vaccines for Children program10 would help STD clinics achieve and maintain high levels of vaccination of STD clients. In addition, financial support for the evaluation and treatment of clients who have positive anti-HCV test results, perhaps modeled on the Ryan White CARE Act11 for human immunodeficiency virus (HIV) care, is urgently needed.
REFERENCES
- 1.Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), part II: immunization of adults. [cited 2007 Jan 2];MMWR Recomm Rep. 2006 55(RR-16):1–33. Also available from: URL: http://www.cdc.gov/mmwr/PDF/rr/rr5516.pdf. [PubMed]
- 2.Centers for Disease Control and Prevention (US) Sexually transmitted diseases treatment guidelines, 2006. [cited 2007 Jan 2];MMWR Recomm Rep. 2006 55(RR-11):1–94. Also available from: URL: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5511a1.htm. [PubMed]
- 3.Weinstock HS, Bolan G, Moran JS, Peterman TA, Polish L, Reingold AL. Routine hepatitis B vaccination in a clinic for sexually transmitted diseases. Am J Public Health. 1995;85:846–9. doi: 10.2105/ajph.85.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handsfield HH. Hepatitis A and B immunization in persons being evaluated for sexually transmitted diseases. Am J Med. 2005;118(Suppl 10A):69S–74S. doi: 10.1016/j.amjmed.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 5.CDC (US) Hepatitis B vaccination among high-risk adolescents and adults—San Diego, California, 1998–2001. [cited 2007 Jan 2];MMWR Morb Mortal Wkly Rep. 2002 51(28):618–21. Also available from: URL: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5128a3.htm. [PubMed]
- 6.National Viral Hepatitis Roundtable (US) Eliminating hepatitis: a call to action. [cited 2007 Jan 2];April 2006 Available from: URL: http://www.nvhr.org/pdf/NVHR_CalltoAction.pdf.
- 7.Wilson BC, Moyer L, Schmid G, Mast E, Voigt R, Mahoney F, et al. Hepatitis B vaccination in sexually transmitted disease (STD) clinics: a survey of STD programs. Sex Transm Dis. 2001;28:148–52. doi: 10.1097/00007435-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert LK, Bulger J, Scanlon K, Ford K, Bergmire-Sweat D, Weinbaum C. Integrating hepatitis B prevention into sexually transmitted disease services: U.S. sexually transmitted disease program and clinic trends—1997 and 2001. Sex Transm Dis. 2005;32:346–50. doi: 10.1097/01.olq.0000154503.41684.5d. [DOI] [PubMed] [Google Scholar]
- 9.CDC (US) Hepatitis B vaccination coverage among adults—United States, 2004. [cited 2007 Jan 2];MMWR Morb Mortal Wkly Rep. 2006 55(18):509–11. Also available from: URL: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5518a3.htm?s_cid=mm5518a3_e. [PubMed]
- 10.CDC (US) Vaccines for Children Program. [cited 2006 Dec 6]; Available from: URL: http://www.cdc.gov/nip/vfc.
- 11.Department of Health and Human Services (US) Ryan White CARE Act. Health Resources and Services Administration. [cited 2006 Oct 20]; Available from: URL: http://hab.hrsa.gov/history.htm.