Abstract
Although identified as an early-diverged protozoan, Giardia lamblia shares many similarities with higher eukaryotic cells, including an internal membrane system and cytoskeleton, as well as secretory pathways. However, unlike many other eukaryotes, Giardia does not synthesize lipids de novo, but rather depends on exogenous sources for both energy production and organelle or membrane biogenesis. It is not known how lipid molecules are taken up by this parasite and if endocytic pathways are involved in this process. In this investigation, we tested the hypothesis that highly regulated and selective lipid transport machinery is present in Giardia and necessary for the efficient internalization and intracellular targeting of ceramide molecules, the major sphingolipid precursor. Using metabolic and pathway inhibitors, we demonstrate that ceramide is internalized through endocytic pathways and is primarily targeted into perinuclear/endoplasmic reticulum membranes. Further investigations suggested that Giardia uses both clathrin-dependent pathways and the actin cytoskeleton for ceramide uptake, as well as microtubule filaments for intracellular localization and targeting. We speculate that this parasitic protozoan has evolved cytoskeletal and clathrin-dependent endocytic mechanisms for importing ceramide molecules from the cell exterior for the synthesis of membranes and vesicles during growth and differentiation.
Keywords: Giardia, Trophozoite, Ceramide, Endocytosis, Clathrin, Bodipy, NBD, Actin, Microtubule, ESV, Encystation
1. Introduction
Giardia lamblia is a major cause of waterborne illness worldwide (Adam et al., 2001). Molecular and genomic studies suggest that this pathogenic protozoan belongs to one of the earliest divergent eukaryotic lineages (Sogin et al., 1989; McArthur, 2000). It lacks typical eukaryotic organelles such as mitochondria, peroxisomes, lysosomes and the Golgi apparatus (Reiner et al., 1990; Lujan et al., 1995; Hehl, 2004). Interestingly, recent reports suggest that Giardia may have the vestige of a mitochondrion (Regoes et al., 2005) and the Golgi-like structures, which are induced during encystation (Lujan et al., 1995). This unicellular protozoan has very limited ability to synthesize membrane lipids, fatty acids and cholesterol de novo, therefore depending on exogenous sources for both energy production and membrane biogenesis (Jarroll et al., 1981; Das, et al., 2002). Proliferating trophozoite stages adhere to the microvilli lining the human small intestine below the bile duct and are exposed to bile salts, dietary lipids, cholesterol and fatty acids (Gillin et al., 1986). These biliary lipids regulate Giardia growth and encystation in the human small intestine (Farthing et al., 1985; Das et al., 1988, 1997; Lujan et al., 1996a). Previous reports suggest that Giardia is able to import both fatty acids and phospholipids and thus incorporate them into membrane lipids (Reiner et al., 1986; Rayan et al., 2005; Stevens et al., 1997; Gibson et al., 1999; Subramanian et al., 2000). Fatty acids and head groups were incorporated into giardial lipids by both deacylation/reacylation and base-exchange reactions (Das et al., 2001, 2002).
Hence, the questions of how Giardia import and traffic lipid molecules are key to understanding parasite proliferation and differentiation into transmissible form. The phenomena of endocytic membrane trafficking and inter-organelle lipid transport have yet to be characterized in this organism. In mammalian cells, vesicular transport has been observed to move along cytoskeletal ‘tracks’ that are powered by molecular motor proteins belonging to the kinesin, dynein and myosin super families (Kamal et al., 2000). It has been proposed that short-range vesicle transport takes place on actin filaments by myosin motors, whereas long-range transport occurs along microtubules using kinesin and dynein motors (Goode et al., 2000). Studies suggest that Giardia contains a single copy of the actin gene and its protein sequence reveals an approximate 58% nucleotide identity with other eukaryotic actin sequences (Elmendorf et al., 2003). A large set of kinesin homologues has been identified in Giardia (Iwabe and Miyata, 2002; Richardson et al., 2006), however, the presence of a putative homologue of myosin has not yet been reported (Elmendorf et al., 2003). On the other hand, microtubules are important cytoskeletal components in this organism and constitute several structures in trophozoites (i.e., the ventral disc, basal bodies, paraflagellar rods and median body) (Crossley et al., 1986). Additional studies have suggested that Giardia tubulins possess excessive post-translational modifications (Webber et al., 1997) that could affect the dynamic instability of microtubules and may not regulate vesicular movement as extensively as that observed in higher eukaryotes. These issues offer new challenges to the delineation of the mechanisms of intracellular lipid trafficking in Giardia trophozoites.
Since the giardial perinuclear region (composed of endoplasmic reticulum and primitive Golgi complex) can be labeled with 7-Nitro-2-(1, 3-Benzoxadiazol-4-yl) amino (NBD)-ceramide (Lanfredi-Rangel et al., 1999, 2003), it is conceivable that ceramide and other sphingolipids are important constituents of these endomembranes. Given the fact that Giardia possesses limited lipid synthesis ability, we asked how ceramide is transported and targeted by this parasite. Our results suggest that Giardia has evolved well-orchestrated ceramide endocytic pathways that involve clathrin-coated vesicles and cytoskeleton filaments. We propose some of these exogenously obtained ceramide molecules can undergo a further metabolic process for energy production and membrane/organelle biosynthesis in this early-diverging eukaryote.
2. Materials and methods
2.1. Materials
Unless otherwise specified, all chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) and were of the highest purity available. Tetramethyl Rhodamine (TMR)-conjugated goat anti-mouse and anti-rabbit antibodies, FM 4-64 (N-[3-triethylammoniumpropyl-4-6-4-diethylamino phenyl hexatrienyl]), Bodipy-ceramide [N-(4,4-difluoro-5, 7-dymethyl-4-bora-3a, 4a-diaza-s-indacene-3-pentanoyl)-sphingosine, Bodipy FLC5-ceramide] and NBD-phosphatidylethanolamine [1-Oleoyl-2-[12-[(7-nitro-2-1, 3-benzoxadiazol-4-yl) amino] dodecanoyl]-sn-Glycero-3-Phosphoethanolamine] analogues were purchased from Invitrogen Inc. and Avanti Polar Lipids (Alabaster, AL), respectively. Vinorelbine (navelbine) was obtained as a gift from Dr. Luis A. Adel, M.D., Centro Medico de Especialidades, Chihuahua, Mexico. Mouse monoclonal anti-β-tubulin antibodies (P14B4) were kindly donated by Isabel Barasoain, Ph.D., Centro de Investigaciones Biologicas, Madrid, Spain. Mouse monoclonal anti-α-tubulin antibody (Clone DM-1A) and taxol were obtained from ICN Biomedicals, Inc. (Aurora, OH).Giardia clathrin (GiCLH) antibody was produced as described previously (Marti et al., 2003a). Tritium [3H]-labeled ceramide (20 Ci/mmol) and arachidonoyl-[14C]-phosphatidylethanolamine (85 Ci/mmol) were purchased from American Radiochemicals (St. Louis, MO) and New England Nuclear (Boston, MA), respectively.
2.2. Organism and cell culture
Giardia lamblia trophozoites (strain WB, ATCC No. 30957) were cultivated following the method of Diamond et al. (1978) using TYI-S-33 medium supplemented with adult bovine serum and bovine bile (Keister et al., 1983). The antibiotic piperacillin (50 μg/ml) was added during routine culture of the parasite (Gillin et al., 1989). Parasites were harvested by centrifugation at 1,050 g for 6 min at 4°C, washed and counted using a hemocytometer under phase-contrast optics by light microscopy.
2.3. Potassium depletion
Giardia lamblia trophozoites were grown in culture flasks at 37°C until late log phase as described above. The attached cells were separated from non-attached trophozoites by decanting the medium and replacement with K+-depleted TYI-S-33 medium (i.e., KH2PO4 and K2HPO4 were replaced by NaH2PO4 and Na2HPO4). Cells were cultured for 1 h, 4 h and 12 h at 37°C and harvested by centrifugation. Trophozoites were washed with K+-depleted buffer (140 mM NaCl, 20 mM Hepes, 1 mM CaCl2, 1 mM MgCl2, 1 mg/ml D-glucose and 5 mM L-cysteine, pH 7.2) and then rinsed in hypotonic buffer (K+-free buffer diluted 1:1 with distilled water) for 5 min (Larkin et al., 1983; Hansen et al., 1993). Cells were quickly transferred to K+-free buffer and the uptake of radioactive and fluorescently labeled ceramide was measured.
2.4. Adherence inhibition assays
Stock solutions of 5 mM colchicine and 1 mM vinorelbine were dissolved in 10 mM PBS (phosphate-buffered saline); stocks of 1 mM albendazole, 100 μM nocodazole, 1 μM taxol and 19.3 mM cytochalasin D were prepared in dimethyl sulfoxide (DMSO). The in vitro susceptibility of Giardia trophozoites to these anti-actin, anti-microtubule agents and other pathway inhibitors was assessed by exposing ∼107 trophozoites to various concentrations of the anti-cytoskeletal agents colchicine (250, 500, 750 and 1000 μM), vinorelbine (0.5, 1, 5 and 10 μM), albendazole (0.1, 1, 5 and 10 μM), nocodazole (0.5, 1, 5, 10, 25 and 50 μM), taxol (5, 10, 15 and 20 nM), cytochalasin D (5, 15, 25, 50 and 100 μM) and filipin-III (0.75, 1.5, 3, 7.5 and 15 μM) for 30 min at 37°C. Inhibitor-treated cells were then transferred to 8-ml culture tubes containing fresh culture medium. Non-attached cells were separated by decanting the medium and replacement by cold sterile PBS. The culture tubes were placed on ice for 15 min to allow trophozoite detachment. The cells were collected by centrifugation and the total number of adherent trophozoites was determined using a hemocytometer. Dose-response curves were constructed for all drugs and the concentrations that caused a 50% reduction in adherence (IC50) compared with controls were selected for further experiments. The working concentrations selected were as follows: 250 μM colchicine, 1 μM vinorelbine, 1 μM albendazole, 500 nM nocodazole, 5 nM taxol, 80 μM cytochalasin D and 1.5 μM filipin-III. Menadione (300 μM) and ouabain (200 μM) were used as described previously (Paget et al., 1989).
2.5. Lipid uptake experiments
Trophozoites were grown on complete TYI-S-33 growth medium and harvested as described above. Stock solution of Bodipy-ceramide (1 mM) in DMSO was prepared before each use. Fluorescently or radioactively labeled lipids were added directly to trophozoites suspended in the incubation buffer (i.e., PBS containing 5 mM glucose and 5 mM cysteine, pH 7.1) with or without inhibitors (final volume, 1 ml), as specified in the figure legends. For fluorescent lipid probes (200 nM-final concentration), cells were incubated in flaskette chambers (Nalge Nunc International, Naperville, IL) at 37°C for 30 min (or as otherwise specified), washed once in PBS and fixed in 4% methanol-free paraformaldehyde. Slides were thoroughly rinsed three times in PBS, cover slips were mounted using DAKO mounting media (DAKO Corp., Carpinteria, CA), and specimens examined using a Zeiss LSM5 PASCAL laser-scanning confocal microscope. For biochemical experiments, stock solutions of [3H]-ceramide was dried under a N2 stream, resuspended in a minimum volume of ethanol and mixed with cold ceramide (0.9 mM) before addition to the cell suspension (∼107 trophozoites in 1 ml of incubation buffer) as described previously (Stevens et al., 1997). Labeling was performed at either 4°C or 37°C for various time points; cells were collected by centrifugation (5,000 g, 10 min, 4°C), washed three times with cold PBS containing 0.5% BSA and measured in a liquid scintillation counter. For inhibitor experiments, trophozoites (∼107) were pre-treated with respective inhibitors for 30 min at 37°C. Cells were then separated by centrifugation, washed in PBS and resuspended in incubation buffer before labeling with [3H]-ceramide for 30 min (37°C).
2.6. Monitoring endocytosis by fluorescent membrane dye, FM 4-64 by live trophozoites
Live trophozoites (∼107) were incubated in the presence of 80 μM cytochalasin-D for 30 min at 25°C. FM 4-64 was added (3 μM, final concentration) directly to the incubation mixture, which was then vortexed and incubated at 4°C for 5 min. Next, cells were washed twice and resuspended in cold PBS and placed in a water bath at 30°C. Live cell images were obtained at different time points (5, 10, 15, 30 and 60 min).
2.7. Labeling with antibodies
Giardia lamblia trophozoites were allowed to attach to immunocytochemistry slides (Polysciences, Warrington, PA) for 30 min. After fixation in 4% paraformaldehyde, trophozoites were washed and blocked at 25°C in 5% normal goat serum (NGS) (Sigma) in PBS. Primary antibodies DM1A (anti-α-tubulin, 1:200), P14B4, β 1-13 (anti-β-tubulin, 1:500), and 1:7,000 anti-Giardia clathrin (GiCLH) were diluted in 1% NGS. The slides were incubated with these antibody dilutions overnight at 4°C, washed and exposed to TMR-conjugated goat anti-mouse or anti-rabbit IgG (purchased from Molecular Probes) for 1 h at 25°C, rinsed, mounted and examined by confocal microscopy. For colocalization experiments, live trophozoites were first labeled with Bodipy-ceramide, washed and allowed to attach to slides as described above. Attached cells were fixed in 4% paraformaldehyde and allowed to react with anti-clathrin antibody prior to development with TMR-conjugated goat anti-mouse antibody as described above.
2.8. Analysis of confocal images
The confocal images were analyzed using LSM 5 PASCAL-software version 3.2 (Zeiss). Each experiment was repeated four times. Cells were randomly selected from each slide and scanned twice using the same resolution, laser power and detector-gain. A single-factor analysis of variance (ANOVA) and the Dunnett’s multiple comparison tests were used to assess differences (means) between the treatment and control. P < 0.05 was considered significant.
2.9. Gel electrophoresis and immunoblotting
Total proteins from approximately 1 × 108 cells were extracted by disruption of either G. lamblia trophozoites or NIH 3T3 Swiss mouse embryo fibroblasts (ATCC No. CRL-1658) in cold lysis buffer (150 mM NaCl, 20 mM Hepes, pH 7.4, 1% Triton X-100, 5 mM EDTA, 10% glycerol, 10 mM NaF and 5 mM β-glycerophosphate plus a protease inhibitor cocktail consisting of 1 μM aprotinin, 1 μM leupeptin, 1 μM pepstatin, 0.25 mM PMSF, 1 μM E-64 and 1 mM EDTA, final concentrations). Proteins were separated by SDS-PAGE and subjected to immunoblot analysis. Blots were exposed to primary antibodies (1:1,000 anti-α-tubulin or 1:500 anti-β-tubulin). After treatment with secondary antibodies [alkaline phosphatase-conjugated goat anti-mouse IgG diluted in 1 X tris-buffered saline Tween 20 (TBST) (1:2,000 for α-tubulin; 1:1,000 for β-tubulin)], the membranes were treated with BCIP/NBT phosphatase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) to identify respective tubulin bands.
3. Results
3.1. Mechanisms of [3H]-ceramide uptake
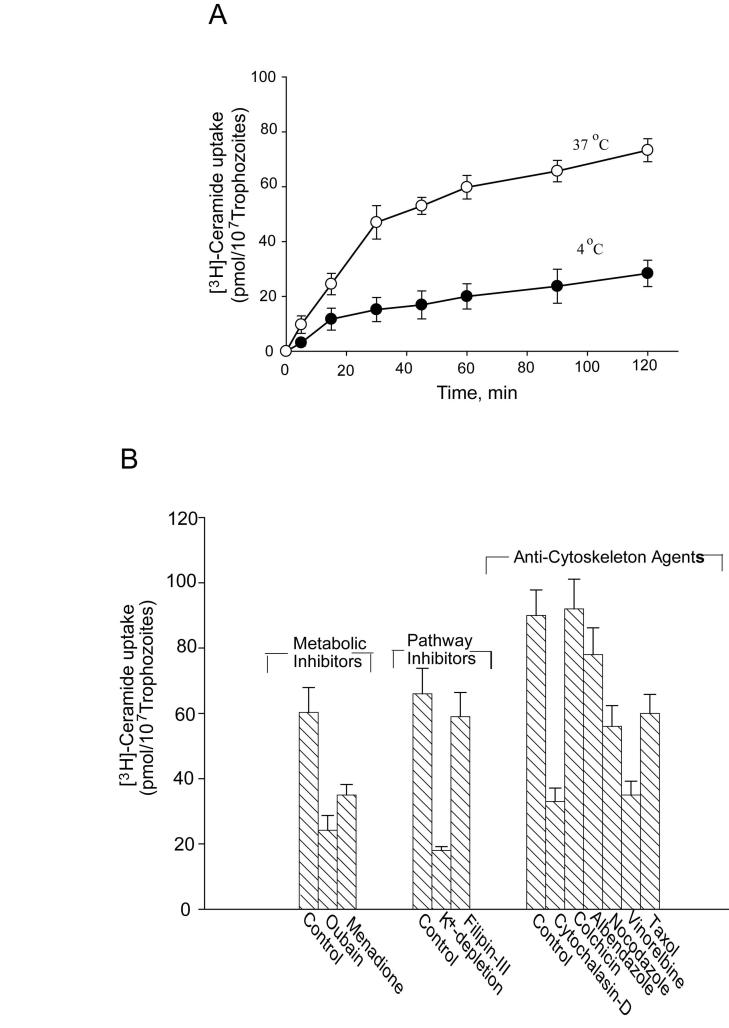
Endocytic and non-endocytic transport of ceramide and other lipids in eukaryotic cells are important for both energy production and membrane biosynthesis (Li et al., 1999). To delineate the mechanism of ceramide uptake, we used radioactive ceramide. We have also included arachidonoyl-[14C]-phosphatidylethanolamine (PE), an important membrane lipid (structurally and functionally different from ceramide), for comparison (not shown). Fig. 1A shows that radiolabeled ceramide can be taken up by trophozoites at 37°C; some non-specific internalization at 4°C was also observed. Internalization at 37°C followed saturable kinetics. For additional parameters, the effects of several inhibitors [ouabain (a Na+-K+-pump inhibitor), menadione (a redox inhibitor), K+ depletion (interferes with clathrin-dependent receptor-mediated endocytosis) and filipin-III (an inhibitor of caveolae-based endocytosis)] were tested on ceramide uptake (Fig. 1B). Ouabain (200 μM) and menadione (300 μM) reduced the internalization of [3H]-ceramide by 60% and 42%, respectively. These results indicated that ceramide import by Giardia was linked to cellular respiration and the adenosine 5′-triphosphate (ATP) generation/synthesis machinery. Cytochalasin-D (80 μM/∼107 cells), an actin depolymerizing agent also interfered with [3H]-ceramide internalization processes, reducing uptake by approximately 60%. Fig. 1B also shows that various anti-microtubule agents interfered with [3H]-ceramide uptake by Giardia. Maximum inhibition was noted with vinorelbine (∼62%), followed by taxol (∼38%) and nocodazole (∼40%). None of these agents, however, had any influence on [14C]-arachidonoyl-PE uptake (not shown), indicating that PE is taken up through a different mechanism.
Fig. 1.
Uptake of [3H]-ceramide by Giardia lamblia trophozoites. Approximately 107 trophozoites were suspended in incubation buffer and labeled with [3H]-ceramide (100 μM, 100,000 cpm) as described in Materials and methods. Labeled trophozoites were then separated by centrifugation (1,500 g, 4°C), washed in PBS containing 0.05% BSA and the incorporation was measured in a liquid scintillation counter. A) The time-course of ceramide uptake by Giardia. Cells were suspended in incubation buffer, mixed with [3H]-ceramide (100 μM, ∼100,000 cpm) and incubated for various time points at 4° and 37°C respectively. B) Effects of various metabolic/pathway inhibitors and anti-cytoskeleton agents on ceramide uptake. Trophozoites were pre-treated with respective inhibitors for 30 min at 37°C. Inhibitor-treated cells were then separated by centrifugation, washed in PBS and resuspended in the incubation buffer before labeling with [3H]-ceramide for 30 min (37°C). Results (pmol incorporated/∼107cells) presented here are the mean (± S.E.M.) of four separate experiments, where each experiment was carried out in duplicate.
3.2. Endocytosis and intracellular targeting of Bodipy-ceramide
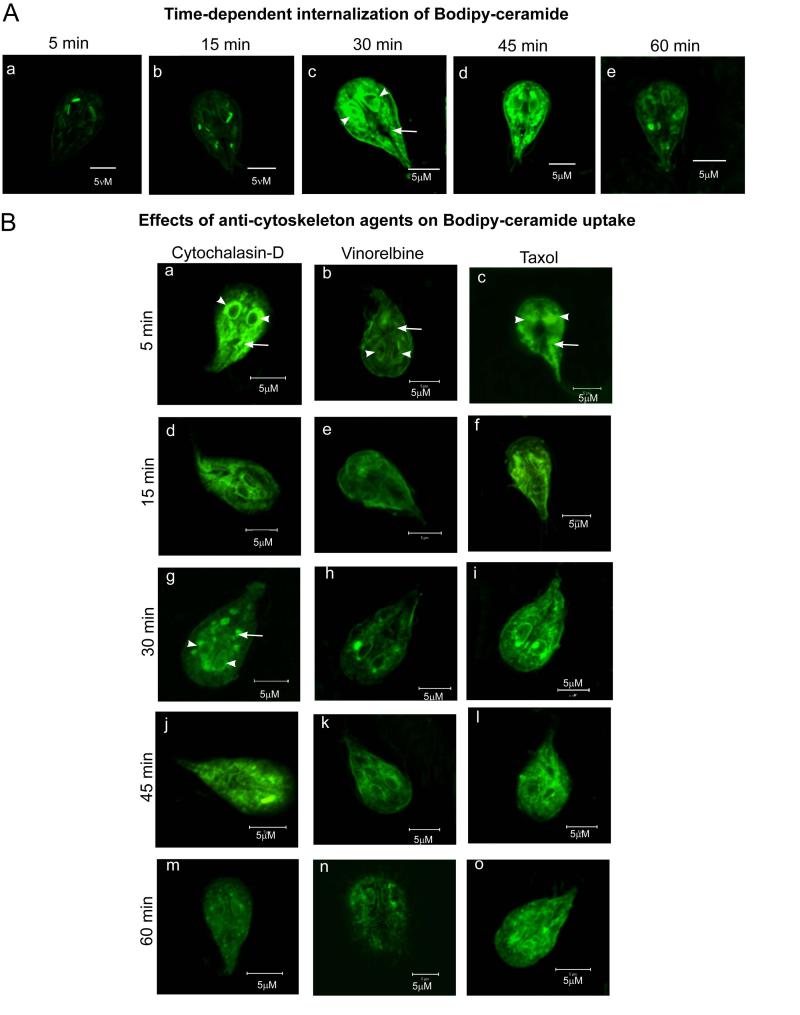
Our next goal was to investigate the mechanism of uptake and targeting of fluorescently (Bodipy)-labeled ceramide by Giardia. Fig. 2A shows time-dependent uptake of Bodipy-ceramide and its intracellular accumulation, especially in endoplasmic reticulum (ER)/perinuclear membranes. Maximum accumulation observed around 30 min of incubation (a-e). Interestingly, the intensity of labeling decreased with time (i.e., 45-60 min), indicating the recycling of ceramide through exocytic pathways. Fig. 2B shows that uptake and intracellular localization of ceramide in trophozoites was affected significantly by cytochalasin-D. The changes of labeling pattern can be seen at 15 min (d). The formation of large ceramide aggregates is clearly visible at 30 min of incubation in the presence of this actin-depolymerizing agent (g). Further incubation decreased the intensity of labeling significantly (j and m).
Fig. 2.
Internalization of Bodipy-ceramide by Giardia trophozoites. A) Approximately 107 trophozoites were resuspended in incubation buffer (final volume, 1 ml) and incubated in flaskette chambers for 15 min (37°C) before incubating with Bodipy-ceramide (200 nM). Labeling was carried out for various time points, i.e., 5, 15, 30, 45 and 60 min at 37°C. Trophozoites were washed in PBS and fixed in 4% methanol-free paraformaldehyde (4%) before subjected to confocal microscopy (Zeiss). Parts a-e show perinuclear (arrowheads) and cytoplasmic (arrows) localizations of Bodipy-ceramide. It is clear that Bodipy-ceramide is taken up by trophozoites in a time-dependent manner and maximum accumulation occurs around 30 min of incubation. Longer incubation (i.e., 45-60 min) reveals the lowering of labeling intensity at the endoplasmic reticulum/perinuclear membranes, which could be due to the recycling of ceramide through exocytic pathways. B) Effects of cytochalasin-D, vinorelbine and taxol on ceramide uptake. Trophozoites were allowed to attach in flaskette chambers as described in (A) and treated with cytochalasin-D (80 μM/∼107 trophozoites), vinorelbine (1 μM/∼107 cells) or taxol (5 nM/∼107 trophozoites) for various time points as indicated. At the end of each treatment, cells were washed in PBS, resuspended in incubation buffer and incubated with Bodipy-ceramide for 30 min at 37°C as described in Materials and methods. Results show that anti-cytoskeleton agents either inhibit and/or alter the distribution of Bodipy-ceramide. The formation of large ceramide aggregates in cytochalasin-D treated cells is noticeable (g). Results also show that vinorelbine reduces the uptake and intracellular targeting of Bodipy-ceramide. Arrow indicates endoplasmic reticulum/perinuclear membranes; arrowhead indicates vesicle-like structures. Bar: 5 μm.
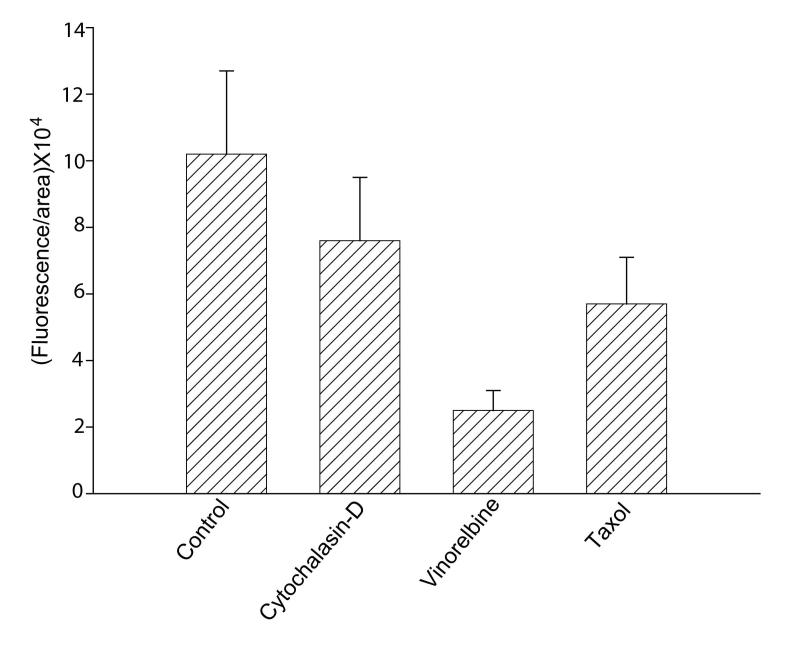
Since vesicular trafficking is dependent on the microtubule network, we tested the possibility that tubulin/microtubules are involved in ceramide uptake and targeting in Giardia. Fig. 2B demonstrates that the internalization of Bodipy-ceramide appears significantly reduced in the presence of vinorelbine. Taxol, which stabilizes microtubules and is used in cancer therapy, also altered the uptake and localization of ceramide in perinuclear membranes but not as dramatically as vinorelbine. Again, both uptake and intracellular localization of NBD-PE were not perturbed by vinorelbine and taxol (not shown). Fig. 3 measures the quantitative changes of fluorescence intensities in the presence of cytochalasin-D, vinorelbine and taxol. Results show that vinorelbine is a potent inhibitor of Bodipy-ceramide uptake and targeting in G. lamblia trophozoites (∼80%). Cytochalasin-D and taxol also reduced the uptake but not as much as vinorelbine (Fig. 3).
Fig. 3.
Quantitative analysis of confocal images. Fluorescence intensities for Bodipy-ceramide in the presence and absence of anti-cytoskeleton agent were measured by Zeiss LSM 5 PASCAL software version 3.2. Labeled trophozoites were randomly selected and analyzed as described Materials and methods. Results (fluorescence/area × 104) show that vinorelbine reduces ceramide uptake significantly.
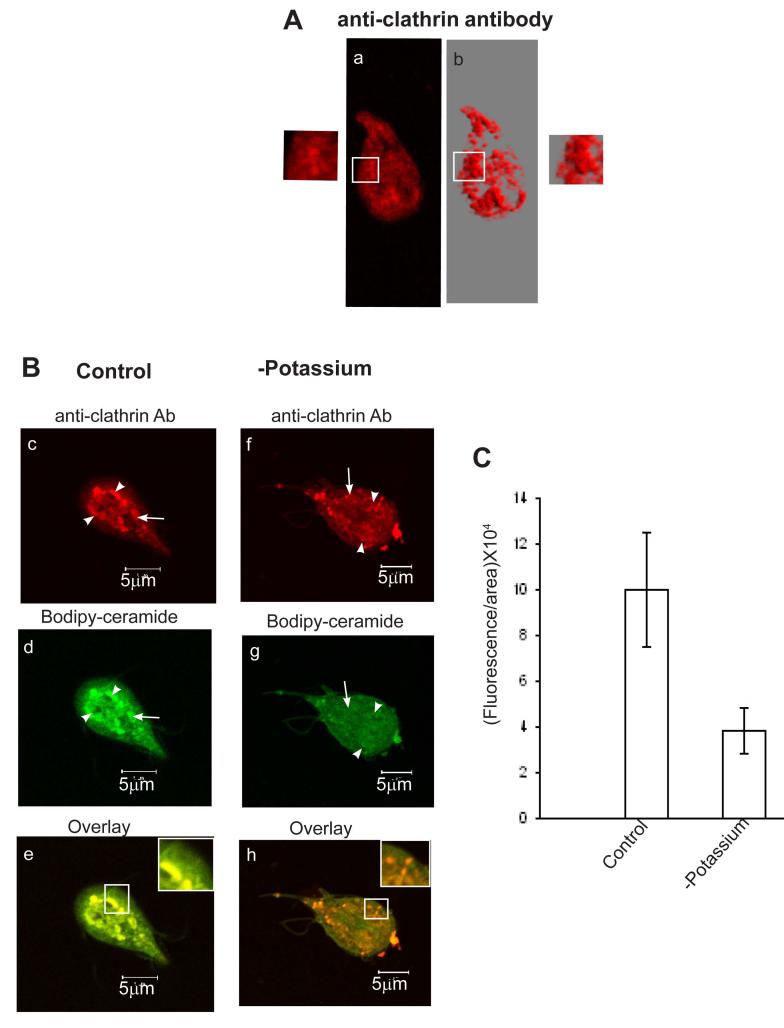
The inhibition of [3H]-ceramide uptake (Fig. 1B) by K+-depletion suggests the involvement of clathrin-dependent pathways in this process. To ensure that Bodipy-ceramide is indeed internalized by clathrin-dependent pathways trophozoites were subjected to colocalization experiments with giardial anti-clathrin antibody (Gi CLH). Fig. 4A suggests that Giardia anti-clathrin antibody reacts brightly and distinctly against vesicle-like structures in non-encysting trophozoites. Anti-clathrin antibody-positive structures are visible in regions contiguous with the plasma membrane. In some cases, the budding of vesicles from the plasma membrane are also visible (a and b). Interestingly, in the presence of ceramide clathrin-like structures become aggregated, enlarged and migrate towards the perinuclear ER membranes (Fig. 4B, see c and d). Earlier studies by Li et al. (1999) demonstrated that exogenous C6 ceramide induces endocytic vesicle formation and causes enlarged late endosomes and lysosomes in mouse fibroblasts. Fig. 4 (e) shows that Bodipy-ceramide labeling (green) overlaps extensively with clathrin labeling (red) in the cytoplasm, as well as in the perinuclear regions. Fig. 4 (f-h) reveal that K+-depleted condition alters cell morphology and affects the synthesis of vesicle-like structures. K+ depletion also inhibits (Fig. 4C) the uptake and perinuclear localization of Bodipy-ceramide significantly (∼60%). In a separate study, we found that K+-depletion did not alter NBD-PE labeling of trophozoites (not shown).
Fig. 4.
Potassium-depletion affects the colocalization of Bodipy-ceramide with anti-clathrin antibody. Both control and K+-depleted cells were first treated with Bodipy-ceramide (200 nM) for 30 min before reacting with anti-clathrin antibody. A) a) identifies clathrin-positive structures in trophozoites cultured in normal growth medium. b) is the 3D reconstruction of the same picture (a) using VIS-ART software (Carl Zeiss). Insets (a and b) show the association of vesicles with plasma membranes. B) c) demonstrates the localization of clathrin-positive vesicles in the presence of ceramide, which reveals enlargement of vesicles and migration towards endoplasmic reticulum/perinuclear regions. d) depicts Bodipy-ceramide labeling of trophozoites; and e) is the colocalization of ceramide with clathrin-coated vesicles in the endoplasmic reticulum/perinuclear regions. f, g) show clathrin-positive structures and Bodipy-ceramide labeling of K+-depleted cells. K+-depletion causes puffiness of trophozoites, illuminates discrete and isolated clathrin-like vesicles (f), and lowers ceramide uptake significantly (g). h) is the merger of images of (f) and (g). Histograms of fluorescence images (fluorescence/area × 104, measured by Zeiss LSM5 PASCAL software version 3.2) in (C) show the relative amount of Bodipy-ceramide internalized by parasites in control and K+-depleted cells. Arrowheads denote endoplasmic reticulum/perinuclear membranes and arrows indicate vesicle-like structures. Bars: 5 μM.
It is possible that because of different acyl chain lengths and bulky fluorescent groups, Bodipy labeled lipids will behave differently in terms of metabolism and cellular localizations. However our result with fluorescently-labeled ceramide is in good agreements with radioactive data and therefore, should be taken into consideration. Furthermore, we found that Giardia is unable to metabolize Bodipy-ceramide (unpublished data) and therefore, it can be expected that the labeling of plasma or endomembranes are due to actual probes and not because of their metabolites.
3.3. Endocytosis of FM 4-64
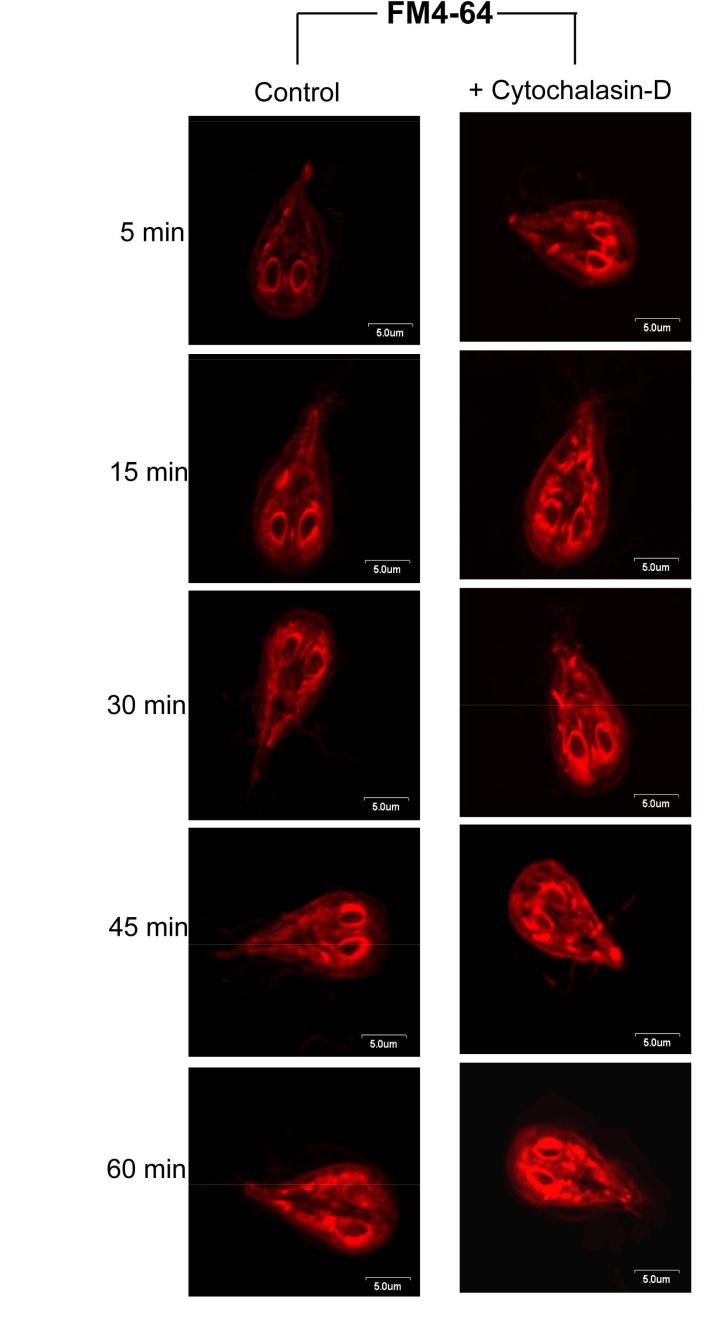
Figs. 1 and 2 indicate that actin-filament may be involved in radiolabeled and Bodipy-ceramide uptake by Giardia, since it is influenced by cytochalasin-D. Confirming our observation that giardial actin, which shares 58% nucleotide identity with actin sequences from other organisms is involved in endocytosis-we used FM 4-64, a fluid phase exocytic/endocytic marker (Bolte et al., 2004) to stain live trophozoites and examined its localization at different time points. FM 4-64 is taken up by trophozoites in a time-dependent manner (Fig. 5). An immediate staining of the plasma membrane and nuclei periphery was noticed, followed by dye internalization and staining of nuclear envelopes as well as cytoplasmic tubular/vesicular structures. Signs of internalized dye could be visualized as early as ∼5 min. Subsequently, the nuclear envelopes became brighter and numerous small cytoplasmic vesicle-like structures were stained (∼15 min). With the increase in time (∼60 min), staining of perinuclear membranes decreased and labeling of peripheral vesicles were prominent, indicating the recycling/exocytosis of the tracer. Internalization of FM 4-64 in the presence of cytochalasin-D exhibited irregular but intense labeling of perinuclear and plasma membranes as well as the tubular/vesicular structures throughout the cytoplasm. These findings indicated that cytochalasin-D does not affect FM 4-64 endocytosis; however, longer periods of treatment with the drug (30-60 min) induce large aggregates and affect the distribution of dye in G. lamblia trophozoites. Thus, an intact actin filament network is needed for the continuous and regulated endocytic flow and recycling of exogenous molecules back to the plasma membranes.
Fig. 5.
Endocytosis of FM-4-64 is interrupted by cytochalasin-D. FM-4-64 was added to live trophozoites pre-treated with cytochalasin-D (80 μM/∼107cells), which were then mixed for 5 min at 4°C before monitoring the internalization at 30°C. Staining is visible at plasma membrane and nuclei periphery, suggesting a rapid internalization of the dye. Incorporation of the perinuclear membranes increases with time. Labeling of small vesicle-like structures is also visible throughout the cytosol. At 60 min post-labeling, staining of peripheral cytoplasmic vesicles is more prominent, indicating the possible recycling of the dye back to the plasma membrane. Exposure of cells to cytochalasin-D (80 μM/107 trophozoites), which depolymerizes the actin filaments, increases the staining of unorganized cytoplasmic tubular structures and reduces the release of FM-4-64 from perinuclear membranes most likely by affecting the recycling/exocytosis pathways. Bar: 5 μm.
3.4. Immunofluorescence labeling of tubulin/microtubule filaments
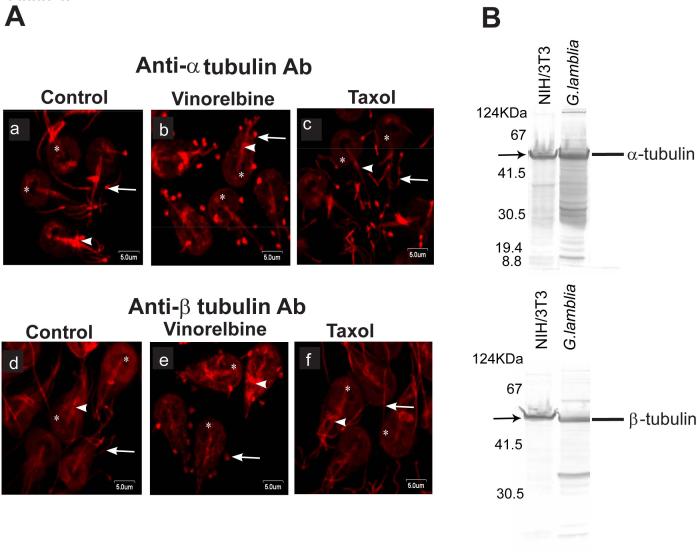
Immunofluorescence was carried out to assess the organization of α- and β-tubulins in trophozoites and how anti-microtubule agents altered their organization. Staining by α-tubulin antibody (DM1A) was observed at the ventral disc, flagella and median body (Fig. 6A). Similarly, reactivity to β-P14B4 was also prominent at the ventral disc, flagellar axonemes and median body. Close observation reveals greater cytoplasmic labeling with anti-β-tubulin antibody than with anti-α-tubulin antibody. To further comprehend how anti-microtubule agents alter the organization of microtubule filaments, trophozoites were treated with either vinorelbine or taxol prior to staining with anti-α-tubulin (DM1A) or β-tubulin (P14B4) antibodies (Fig. 6, a-f). Taken together our results indicate that vinorelbine effectively disrupted microtubule filaments in Giardia trophozoites. The microtubule network collapses in the presence of this vinca alkaloid drug and formed paracrystal-like structures in trophozoites, which appeared as red spots. On the other hand, taxol slightly distorted the flagellar ends as seen by DM1A labeling. Interestingly, the organization of β-tubulin was unaltered in the presence of taxol.
Fig. 6.
Immunolabeling of microtubules in the presence of vinorelbine and taxol. A) Trophozoites were first exposed to anti-microtubule agents and subsequently labeled with anti-α and -β-tubulin antibodies. Vinorelbine (b and e) interacts with microtubule filaments and form paracrystalline structures, especially in flagella (arrow) and in ventral disc (asterisk), and to a lesser degree in the median body (arrowhead). In contrast, taxol (c and f) stabilizes microtubule; slightly distorted the flagellar ends (arrowhead), as evidenced by anti α-tubulin antibody labeling, whereas no effect was seen by this drug in trophozoites labeled with P14B4, β1-13 (anti β-tubulin antibody). Arrowhead indicates median body of trophozoites and asterisk shows the ventral disc. Bar: 5 μm. B) demonstrates the specificity of α- and β-tubulin antibodies used in immunolocalization experiments shown in (A).
Fig. 6B shows the specificity of antibodies used in this investigation. Using whole cell extracts of G. lamblia trophozoites, the presence of α- and β-tubulin was evaluated by polyacrylamide gel electrophoresis and compared with extracts from NIH 3T3 cells. Immunoblot analyses were performed using DM1A and P14B4 antibodies. All antibodies reacted with Giardia trophozoites and mammalian cells. Immunoblots displayed a band that migrated at ∼55 kDa, validating the specificities of antibodies used in the current investigation (Fig. 6B).
4. Discussion
The current study investigated the mechanism of ceramide import by Giardia. We show that Giardia uses clathrin-dependent pathways and actin filaments for ceramide endocytosis and a microtubule network for perinuclear targeting. Our study also demonstrates the presence of a sophisticated lipid transport and targeting machinery in this early-diverging eukaryote.
The coordination between actin, microtubules and target organelles are essential for delivering membrane lipids via endocytic pathways in higher eukaryotic cells (Apodaca, 2001). However, it is unknown whether the actin/microtubule cytoskeleton in Giardia is involved in vesicular trafficking. Earlier studies from various laboratories primarily focused on the role of actin and microtubule filaments on Giardia attachment to glass or cell surfaces (Sandhu et al., 2004; Hausen et al., 2006). More recent reports reveal that long-term exposure of trophozoites to cytochalasin-D, nocodazole and colchicine induced the fragmentation of the ventral discs of trophozoites and the formation of large vacuoles. The blocking of cytokinesis and membrane blebbing is also possible (Correa and Benchimol, 2006; Mariante et al., 2005). In the current study, trophozoites were treated with anti-cytoskeletal agents for a short time period, that is, 30 min to avoid damage by long-term exposure, before examining the transport of radioactively or fluorescently labeled ceramide. This short-term treatment did not produce disc fragmentation or morphological changes (Figs. 2 and 4). In a separate experiment, we found that internalization and recycling of FM 4-64, an endocytic/exocytic marker (Bolte et al., 2004) is affected by cytochalasin-D (Fig. 5). This result suggests that despite the substantial sequence divergence of Giardia actin (∼58% nucleotide identical to actin sequences from other species) (Elmendorf et al., 2003), it participates in an endocytic process and is sensitive to cytochalasin-D.
In mammalian cells, clathrin polymerization and coat assembly are required for receptor-mediated internalization of lipid and protein molecules, which is inhibited by K+-depletion (Puri et al., 2001; Cupers et al., 1994), therefore, ceramide internalization in Giardia may also be linked to a receptor-mediated endocytosis. Like clathrin vesicles, lipid raft-like membrane domains were also shown to participate in endocytosis of lipids and other molecules in parasites and higher eukaryotes. Disruption of lipid rafts by filipin III and methyl-β-cyclodextrin in Entamoeba histolytica inhibits the process of pinocytosis (Loughlin et al., 2004). Using Vibrant Alexa Fluor lipid-raft labeling kits (Molecular Probes), we confirmed the presence of raft-like microdomains in Giardia (not shown). However, these microdomains do not appear to be involved in ceramide endocytosis, since filipin-III is ineffective in blocking [3H]-ceramide uptake (Fig. 1B).
There are few reports regarding lipid endocytosis by parasitic cells. Receptor-mediated internalization of low-density lipoprotein (LDL) and cholesterol has been demonstrated in Schistosoma japonicum and other parasites (Rogers et al., 1989; Coppens et al., 2000). In fact, it has been shown that an LDL-like receptor is present in Giardia trophozoites and is involved in internalizing phospholipids and cholesterol (Lujan et al., 1996b). Although, in the current study, no attempt was made to test the role of cholesterol on ceramide internalization, it is not unlikely that ceramide uptake in Giardia is linked to cholesterol/LDL endocytosis (Lujan et al., 1996b). Similarly, taurocholic acid, which is taken up by parasites through a sodium-dependent active process (Das et al., 1997), may also regulate ceramide internalization in Giardia. Earlier, Crawford et al. (1991) reported that taurocholate induces perinuclear localization of C6-ceramide in isolated hepatocytes. This could also be true for Giardia.
Among various anti-microtubule agents tested, we found that vinorelbine was the most effective inhibitor of ceramide uptake. Vinorelbine was chosen because it is unique among other vinca alkaloids and demonstrates a spectrum of activity in human tumors (Boiron et al., 1991). It inhibits mammalian cell proliferation by affecting the dynamics of spindle microtubules and also induces the formation of vinblastin-tubulin paracrystals at 1 μM concentration in cultured HeLa S3 cells (Jordan et al., 1992). Our data suggest (Fig. 6A) that like HeLa cells, paracrystal-like structures are produced in Giardia after treatment with vinorelbine, suggesting that this drug interacts with Giardia tubulins in a similar fashion.
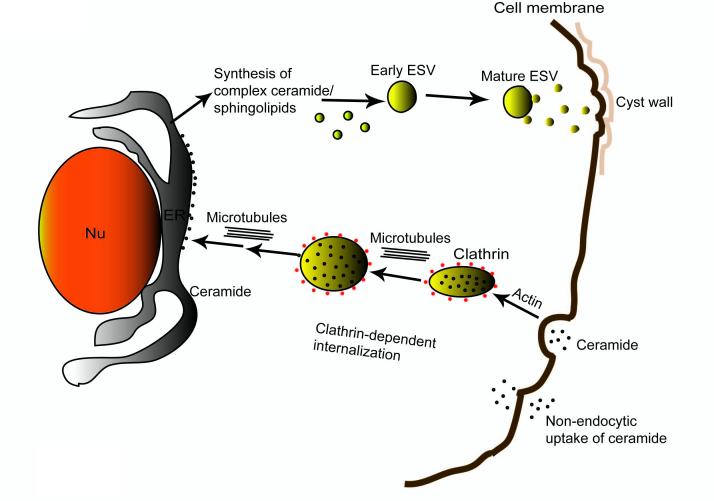
Many investigators have confirmed the involvement of primitive Golgi, ER and other internal membrane systems in the transport of cyst wall proteins and glycans in encysting Giardia trophozoites (Lujan et al., 1995; Hehl and Marti, 2004; Lanfredi-Rangel et al., 1999, 2003). Giardia synthesizes large encystation-specific vesicles (ESVs), in which the cyst wall materials accumulates and matures before progressing along a regulated secretory pathway to the cell surface (Reiner et al., 1990). Further examination suggested that these vesicles constituted Golgi-like cisternae (Marti et al., 2003b; Stefanic et al., 2006) and those ESVs and the nuclear envelopes could be stained with C6-NBD-ceramide (Lanfredi-Rangel et al., 1999, 2003). The function of ceramide in these endomembranes is still unclear, however. In Fig.7, we proposed a model summarizing our observations on ceramide internalization and targeting by Giardia. In this model, exogenous ceramide is internalized and trafficed with the help of clathrin-dependent pathways. Giardial actin and microtubule cytoskeleton also play roles in this event. Ceramide taken up by this waterborne pathogen is targeted and stored in the ER/perinuclear membranes. At the onset of encystation, stored ceramide can act as a scaffold for the synthesis of complex saccharide-containing ceramide/sphingolipid for cyst wall biosynthesis. Nonetheless, more in-depth studies are required to support this notion. Currently, we are engaged in analyzing sphingolipid genes/enzymes and their possible functions in giardial growth and encystation.
Fig. 7.
Summary of overall results. The model proposes that Giardia internalizes the majority of its ceramide through clathrin-dependent endocytic pathways. A small portion of ceramide can also be internalized by non-endocytic mechanisms. Actin and microtubule cytoskeletons are involved in ceramide endocytosis and targeting to ER/perinuclear membranes. It is possible that Giardia uses exogenous ceramide as a scaffold to synthesize complex ceramide/sphingolipids that either constitutes ESVs or transported by ESVs to plasma membranes for cyst wall biosynthesis during encystation. Nu, nucleus; ER, endoplasmic reticulum: ESV, encystation-specific vesicles.
Acknowledgement
We are grateful to Dr. Isabel Barasoain of Centro de Investigaciones Biologicas, Madrid, Spain, for providing us with anti-β-tubulin antibodies. Microscopy experiments were performed in the Analytical Cytology Facility at UTEP. This work was supported by grants (506 GM 008012-34 to SD) from the National Institutes of Health and an infrastructure development grant (5G112RR08124-09 to UTEP) from NCRR (RCMI). Ms. Yunuen Hermandez was partially supported by a pre-doctoral fellowship from the State of Chihuahua, Mexico. Supports from the Hazel Harvey and Coldwell Foundations (Texas) are also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–75. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Taffic. 2001;2:149–59. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Boiron M. Navelbine (vinorelbine): Update and New Trends (Solae-Celilgny, P., Scientific Advisor) Montrouge; France: 1991. Published by John Libbey Eurotext Ltd. [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice, O. O, Read ND, Satiat-Jeunemaitre B. FMdyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 2004;214:159–73. doi: 10.1111/j.0022-2720.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- Coppens I, Sinai AP, Joiner KA. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 2000;149:167–80. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa G, Bencimol M. Giardia lamblia behavior under cytochalasins treatment. Parasitol. Res. 2006;98:250–56. doi: 10.1007/s00436-005-0065-z. [DOI] [PubMed] [Google Scholar]
- Crawford JM, Vinter DW, Gollan JL. Taurocholate induces pericanalicular localization of C6-NBD-ceramide in isolated hepatocyte couplets. Am J Physiol. 1991;260:G119–32. doi: 10.1152/ajpgi.1991.260.1.G119. [DOI] [PubMed] [Google Scholar]
- Crossley R, Marshall J, Clark JT, Holberton DV. Immunocytochemical differentiation of microtubules in the cytoskeleton of Giardia lamblia using monoclonal antibodies to alpha-tubulin and polyclonal antibodies to associated low molecular weight proteins. J. Cell Sci. 1986;80:233–52. doi: 10.1242/jcs.80.1.233. [DOI] [PubMed] [Google Scholar]
- Cupers P, Veithen A, Kiss A, Baudhuin P, Courtoy PJ. Clathrin polymerization is not required for bulk-phase endocytosis in rat fetal fibroblasts. J. Cell. Biol. 1994;127:725–35. doi: 10.1083/jcb.127.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Reiner DS, Zenian J, Hogan DL, Koss MA, Wang CS, Gillin FD. Killing of Giardia lamblia trophozoites by human intestinal fluid in vitro. J. Infect Dis. 1988;157:1257–60. doi: 10.1093/infdis/157.6.1257. [DOI] [PubMed] [Google Scholar]
- Das S, Gillin FD. Giardia lamblia: Increased UDP-N-acetyl-D-glucosamine and N-acetyl-D-galactosamine activities during encystation. Exp. Parasitol. 1996;84:18–31. doi: 10.1006/expr.1996.0045. [DOI] [PubMed] [Google Scholar]
- Das S, Schteingart CD, Hofmann AF, Reiner DS, Aley SB, Gillin FD. Giardia lamblia: evidence for carrier-mediated uptake and release of conjugated bile acids. Exp. Parasitol. 1997;87:133–41. doi: 10.1006/expr.1997.4197. [DOI] [PubMed] [Google Scholar]
- Das S, Castillo C, Stevens T. Phospholipid generation/remodeling by Giardia: the role of Lands Cycle. Trends Parasitol. 2001;17:317–21. doi: 10.1016/s1471-4922(01)01901-8. [DOI] [PubMed] [Google Scholar]
- Das S, Stevens T, Castillo C, Villasenõr A, Arredondo H, Reddy K. Lipid metabolism in mucus-dwelling amitochondriate protozoa. Int. J. Parasitol. 2002;32:655–75. doi: 10.1016/s0020-7519(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 1978;27:487–88. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Ellis JE, Wyder MA, Jarroll EL, Kaneshiro ES. Changes in lipid composition during in vitro encystation and fatty acid desturase activity of Giardia lamblia. Mol. Biochem. Parasitol. 1996;81:13–25. doi: 10.1016/0166-6851(96)02677-1. [DOI] [PubMed] [Google Scholar]
- Elmendorf HG, Dawson SC, McCaffery JM. The cytoskeleton of Giardia lamblia. Int. J. Parasitol. 2003;33:3–28. doi: 10.1016/s0020-7519(02)00228-x. [DOI] [PubMed] [Google Scholar]
- Farthing MJG, Keusch GT, Carey MC. Effects of bile and bile salts on growth and membrane lipid uptake by Giardia lamblia. J. Clin. Invest. 1985;76:1727–32. doi: 10.1172/JCI112162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Ramirez D, Maier J, Castillo J, Das, S. C. Giardia lamblia: Incorporation of free and conjugated fatty acids into glycerol-based phospholipids. Exp. Parasitol. 1999;92:1–11. doi: 10.1006/expr.1999.4389. [DOI] [PubMed] [Google Scholar]
- Gillin FD, Gault MJ, Hofman AF, Gurantz FD, Sauch JF. Biliary lipids support serum-free growth of Giardia lamblia. Infect. Immun. 1986;53:641–46. doi: 10.1128/iai.53.3.641-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin FD, Boucher SE, Rossi SS, Reiner DS. Giardia lamblia: The roles of bile, lactic acid and pH in the completion of the life-cycle in vitro. Exp. Parasitol. 1989;69:164–74. doi: 10.1016/0014-4894(89)90185-9. [DOI] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Cur. Opin. Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypotonic medium, and cytosol acidification. J. Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausen MA, Freitas JCM, Jr., Monteiro-Leal LH. The effects of metronidazole and furazolidone during Giardia differentiation into cysts. Exp. Parasitol. 2006;113:135–41. doi: 10.1016/j.exppara.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hehl AB, Marti M. Secretory protein trafficking in Giardia intestinalis. Mol. Micro. 2004;53:19–28. doi: 10.1111/j.1365-2958.2004.04115.x. [DOI] [PubMed] [Google Scholar]
- Iwabe N, Miyata T. Kinesin-related genes from diplomonad, sponge, amphioxus, and cyclostomes: divergence pattern of kinesin family and evolution of giardial membrane-bounded organella. Mol Biol Evol. 2002;19:1524–33. doi: 10.1093/oxfordjournals.molbev.a004215. [DOI] [PubMed] [Google Scholar]
- Jarroll EL, Muller PJ, Meyer PJ, Morse, S.A. EA. Lipid and carbohydrate metabolism of Giardia lamblia. Mol. Biochem. Parasitol. 1981;2:187–96. doi: 10.1016/0166-6851(81)90099-2. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Thrower D, Wilson L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles: Implications for the role of microtubule dynamics in mitosis. J. Cell Sci. 1992;102:401–16. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- Kamal A, Goldstein LS. Connecting vesicle transport to the cytoskeleton. Curr. Opin. Cell Biol. 2000;12:503–8. doi: 10.1016/s0955-0674(00)00123-x. [DOI] [PubMed] [Google Scholar]
- Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc Trop. Med. Hyg. 1983;77:487–88. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Larkin JM, Brown MS, Goldstein JL, Anderson RG. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–85. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Lanfredi-Rangel A, Kattenbach WM, Diniz JA, Jr., de Souza W. Trophozoites of Giardia lamblia may have a Golgi-like structure. FEMS Microbiol Lett. 1999;181:245–51. doi: 10.1111/j.1574-6968.1999.tb08851.x. [DOI] [PubMed] [Google Scholar]
- Lanfredi-Rangel A, Attias M, Reiner DS, Gillin FD, De Souza W. Fine structure of the biogenesis of Giardia lamblia encystation secretory vesicles. J. Struct. Biol. 2003;143:153–63. doi: 10.1016/s1047-8477(03)00123-0. [DOI] [PubMed] [Google Scholar]
- Loughlin RC, McGugan GC, Powell RR, Welter BH, Temesvari LA. Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Inf. Immun. 2004;72:5349–57. doi: 10.1128/IAI.72.9.5349-5357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Blanchette-Mackie EJ, Ladisch S. Induction of endocytic vesicles by exogenousC(6)-ceramide. J. Biol.Chem. 1999;274:21121–27. doi: 10.1074/jbc.274.30.21121. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Marotta A, Mowatt MR, Sciaky N, Lippincott-Schwartz J, Nash TE. Developmental induction of Golgi structure and function in the primitive eukaryote, Giardia lamblia. J. Biol. Chem. 1995;270:4612–18. doi: 10.1074/jbc.270.9.4612. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Byrd RG, Nash TE. Cholesterol starvation induces differentiation of the intestinal parasite Giardia lamblia. Proc. Natl. Acad. Sci. USA. 1996a;93:7628–33. doi: 10.1073/pnas.93.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Nash TE. Lipid requirements and lipid uptake by Giardia lamblia trophozoites in culture. J. Euk. Micro. 1996b;43:237–42. doi: 10.1111/j.1550-7408.1996.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Marti M, Regos A, Li Y, Schraner EM, Wild P, Muller N, Knopf LG, Hehl A,B. An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J. Biol. Chem. 2003a;278:24837–48. doi: 10.1074/jbc.M302082200. [DOI] [PubMed] [Google Scholar]
- Marti M, Li Y, Schraner EM, Wild P, Kohler P, Hehl AB. The Secretory apparatus of an ancient eukaryote: protein sorting to separate export pathways occurs before formation of transient Golgi-like compartments. Mol. Biol. Cell. 2003b;14:1433–47. doi: 10.1091/mbc.E02-08-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariante RM, Vancini RG, Melo AL, Benchimol M. Giardia lamblia: evaluation of the in vitro effects of nocodazole and colchicine on trophozoites. Exp. Parasitol. 2005;110:62–72. doi: 10.1016/j.exppara.2005.01.007. [DOI] [PubMed] [Google Scholar]
- McArthur AG, Morrison HG, Nixon JE, Passamaneck NQ, Kim U, Hinkle G, Crocker MK, Holder ME, Farr R, Reich CI, Olsen GE, Aley SB, Adam RD, Gillin FD, Sogin ML. The Giardia genome database. FEMS Microbiol. Lett. 2000;189:271–73. doi: 10.1111/j.1574-6968.2000.tb09242.x. [DOI] [PubMed] [Google Scholar]
- Paget TA, Jarroll EL, Manning P, Lindmark DG, Lloyd D. Respiration in the cysts and trophozoites of Giardia muris. J. Gen. Microbiol. 1989;135:145–54. doi: 10.1099/00221287-135-1-145. [DOI] [PubMed] [Google Scholar]
- Puri V, Watanabe R, Singh RD, Dominguez M, Brown JC, Wheatley CL, Marks DL, Pagano RE. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 2001;154:535–47. doi: 10.1083/jcb.200102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayan P, Stenzel D, McDonnell PA. The effects of saturated fatty acids on Giardia duodenalis trophozoites in vitro. Parasitol Res. 2005;97:191–200. doi: 10.1007/s00436-005-1432-5. [DOI] [PubMed] [Google Scholar]
- Regoes A, Zourmpanou D, Leon-Avila G, van der Giezen M, Tovar J, Hehl AB. Protein import, replication, and inheritance of a vestigial mitochondrion. J. Biol.Chem. 2005;280:30557–63. doi: 10.1074/jbc.M500787200. [DOI] [PubMed] [Google Scholar]
- Reiner DS, Wang CS, Gillin FD. Human milk kills Giardia lamblia by generating toxic lipolytic products. J. Infect. Dis. 1986;154:825–32. doi: 10.1093/infdis/154.5.825. [DOI] [PubMed] [Google Scholar]
- Reiner D,S, McCaffery M, Gillin FD. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur. J. Cell Biol. 1990;53:142–53. [PubMed] [Google Scholar]
- Richardson DN, Simmons MP, Reddy AS. Comprehensive comparative analysis of kinesins in photosynthetic eukaryotes. BMC Genomics. 2006;7:18. doi: 10.1186/1471-2164-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MV, Henkle KJ, Fidge NH, Mitchell GF. Identification of a multispecific lipoprotein receptor in adult Schistosoma japonicum by ligand blotting analyses. Mol. Biochem. Parasitol. 1989;35:79–88. doi: 10.1016/0166-6851(89)90145-x. [DOI] [PubMed] [Google Scholar]
- Sandhu H, Mahajan RC, Ganguly NK. Flowcytometric assessment of the effect of drugs on Giardia lamblia trophozoites in vitro. Mol. Cellu. Biochem. 2004;265:151–60. doi: 10.1023/b:mcbi.0000044392.01704.5f. [DOI] [PubMed] [Google Scholar]
- Sharma DK, Choudhury A, Singh RD, Wheatley CL, Marks DL, Pagano RE. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 2003;278:7564–72. doi: 10.1074/jbc.M210457200. [DOI] [PubMed] [Google Scholar]
- Sogin ML, Gunderson JH, Elwood HJ, Alonso RA, Peattie DA. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989;243:75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Stefanic S, Palm D, Svärd SG, Hehl AB. Organelle proteomics reveals cargo maturation mechanisms associated with Golgi-like encystations vesicles in the early-diverged protozoan Giardia lamblia. J. Biol. Chem. 2006;28:7595–04. doi: 10.1074/jbc.M510940200. [DOI] [PubMed] [Google Scholar]
- Stevens TL, Gibson GR, Adam R, Maier J, Allison-Ennis M, Das S. Uptake and cellular localization of exogenous lipids by Giardia lamblia, a primitive eukaryote. Exp. Parasitol. 1997;86:133–43. doi: 10.1006/expr.1997.4162. [DOI] [PubMed] [Google Scholar]
- Subramanian AB, Navarro S, Carrasco RA, Marti M, Das S. Role of exogenous inositol and phosphatidylinositol in glycosylphosphatidylinositol anchor synthesis of GP49 by Giardia lamblia. Biochim. Biophys ACTA. 2000;1483:69–80. doi: 10.1016/s1388-1981(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Weber K, Schneider A, Westermann S, Muller N, Plessmann U. Posttranslational modifications of alpha- and beta-tubulin in Giardia lamblia, xf. an ancient eukaryote. FEBS Lett. 1997;419:87–91. doi: 10.1016/s0014-5793(97)01436-1. [DOI] [PubMed] [Google Scholar]