Abstract
Lectins comprise a family of related proteins that mediate essential cell functions through binding to carbohydrates. Within this protein family, C-type lectins are defined by the requirement of calcium for optimal biologic activity. Using reverse transcription PCR, a cDNA corresponding to a putative C-type lectin has been amplified from the hookworm parasite Ancylostoma ceylanicum. The 550 nucleotide open reading frame of the Ancylostoma ceylanicum C-type Lectin-1 (AceCTL-1) cDNA corresponds to a 167 amino acid mature protein (18706 Da) preceded by a 17 amino acid secretory signal sequence. The recombinant protein (rAceCTL-1) was expressed in Drosophila S2 cells and purified using a combination of affinity chromatography and reverse phase HPLC. Using in vitro carbohydrate binding studies, it was determined that rAceCTL-1 binds N-acetyl-D-glucosamine, a common component of eukaryotic egg cell membranes. Using a polyclonal IgG raised against the recombinant protein, the native AceCTL-1 was identified in sperm and soluble protein extracts of adult male A. ceylanicum by immunoblot. Probing of adult hookworm sections with the polyclonal IgG demonstrated localization to the testes in males, as well as the spermatheca and developing embryos in females, consistent with its role as a sperm protein. Together, these data strongly suggest that AceCTL-1 is a male gender-specific C-type lectin with a function in hookworm reproductive physiology.
Keywords: hookworm, Ancylostoma ceylanicum, nematode, C-type lectin, sperm
1. Introduction
Lectins are single or multi-domained glycoproteins capable of binding sugar moieties through specific interaction with an intramolecular carbohydrate recognition domain (CRD) [1–3]. In certain physiologic settings, lectins act as recognition molecules, mediating cell adhesion and signal transduction events following binding to carbohydrates [3,4]. Lectins have been characterized in a multitude of species, including plants, mammals, viruses, and parasites, with specific functions that vary substantially. The C-type lectins (CTLs) are a diverse subset of lectins that bind carbohydrates in a calcium dependent manner [2,5]. Calcium is not only required for functional binding of CTLs, but also serves to stabilize their molecular structure.
In mammalian reproductive physiology, CTLs are involved in the molecular mechanisms underlying successful fertilization of embryos, a process through which sperm lectins recognize specific carbohydrate moieties on egg surface glycoproteins [6]. In the free living and hermaphroditic nematode Caenorhabditis elegans, some of the genes involved in fertilization (fer) and spermatogenesis (spe) have been characterized, and their role in progeny development defined [7,8]. By contrast, in parasitic nematodes, research on fertilization and the role of sperm proteins has focused largely on the Major Sperm Protein (MSP) family of molecules, which are cytoskeletal molecules that mediate the characteristic crawling type motility [9,10]. However, relatively little is known about other sperm proteins that might play a role in the reproductive physiology of parasitic nematodes.
Adult Ancylostoma and Necator hookworms, like most other soil transmitted nematodes, mate within the host intestine, where females release thousands of fertilized eggs per day. When excreted in the feces of an infected individual, hookworm eggs hatch and release first stage (L1) larvae that undergo successive molts to the infectious L3 stage. Upon skin contact or ingestion by a permissive host, hookworm larvae penetrate epithelium, invade small blood vessels or lymphatics, and migrate to the small intestine, where they undergo the final molts to male or female adult worms. To date, the mechanisms underlying hookworm sperm-egg interactions, or the molecular mechanisms of fertilization remain largely unknown. We report here the molecular cloning and characterization of a male-gender specific C-type lectin from the hookworm parasite Ancylostoma ceylanicum that likely plays a role in reproductive physiology.
2. Methods
2.1 Hookworm life cycle and parasite protein extracts
The A. ceylanicum life cycle was maintained by serial passage in Golden Syrian hamsters as previously described [11–13]. Soluble protein extracts of adult hookworms (HEX) were prepared by homogenizing adult worms harvested manually from the intestines of infected animals at day 18–21 post-infection in 50 mM Tris-HCl, pH 7.5, followed by centrifugation at 10,000 x g. Larval protein extracts (LEX) were prepared in a similar manner from third stage (L3) larvae cultured from the feces of infected animals using the Baermann method [14]. Egg and L1 larvae protein extracts were prepared by homogenizing A. ceylanicum eggs and newly hatched larvae harvested from live adult female worms cultured overnight in hookworm culture medium (HCM)[13], followed by centrifugation as described above. Sperm from 5 live adult males was extracted into 50 μl of SM buffer (50 mM HEPES, 50 mM NaCl2, 25 mM KCl, 5 mM CaCl2, 1 mM MgSO4 plus 1mg/ml bovine serum albumin; pH 7.0)[15] Briefly, the sperm was harvested by dissecting the testes from the caudal end of the adult worm under low magnification, and collecting the released sperm into a small volume of buffer. The resulting sperm solution was sonicated using a Branson (Danbury, CT) 450 Sonifier. Protein concentrations of all soluble protein preparations were determined using the BCA reagent (Pierce).
2.2 Cloning of the AceCTL-1 cDNA
Fifty live adult A. ceylanicum (equal numbers of males and females) were suspended in Trizol (Invitrogen, Carlsbad CA), and total RNA was isolated according to the manufacturer’s protocol. Total RNA was utilized as template in a reverse transcription reaction using previously described methods [16–18]. The first strand cDNA mixture was combined with PCR primers corresponding to the nematode spliced leader [19,20] and a conserved amino acid sequence previously identified in a family of nematode serine protease inhibitors [17,21]. Following amplification, resulting PCR products were ligated into the pCR 2.1 (Invitrogen) plasmid vector and One Shot E. coli INVαF’ cells (Invitrogen) were transformed with the ligation product. A partial cDNA sequence obtained by this method was then used to design a non-degenerate primer in order to isolate the mature coding sequence of the AceCTL-1 cDNA using a 3’RACE protocol [18]. The nucleotide and translated amino acid sequences were analyzed for homology to other known genes and proteins using the BLAST algorithm through the NCBI [22]. Multiple sequence alignment and analyses were carried out using the ClustalW algorithm [23].
2.3 Eukaryotic Expression of Recombinant Protein
For expression of rAceCTL-1, the cDNA corresponding to the mature protein was subcloned into the pMT/BiP/V5-His plasmid vector (Invitrogen), which was used to transform competent E. coli cells. Individual colonies containing the plasmid were identified by PCR screening, and the rescued plasmids used to transfect Drosophila S2 cells according to the manufacturer’s instructions. Protein expression was induced by the addition of CuSO4 (final concentration 500 μM) and assayed using immunoblotting with a HRP-labeled antibody against the polyhistidine fusion tag. Stable cell transfectants were established by co-transfection with the selection vector pCoBlast. Transfected cells were then selected by the addition of blasticidin (final concentration 25 μg/ml), and were subsequently established in a suspension culture of Drosophila Serum Free Media (Invitrogen). Recombinant protein expression was induced by the addition of CuSO4 and the recombinant AceCTL-1 protein (rAceCTL-1) was purified using nickel resin affinity chromatography and reverse phase HPLC as previously described for hookworm secretory proteins [19,24,25]. The protein concentration was estimated using the BCA assay.
2.4 Carbohydrate binding specificity of recombinant AceCTL-1
A solid phase binding assay was utilized in order to analyze the carbohydrate binding specificity of the rAceCTL-1 protein [26]. Aliquots (50 μl) of sepharose (Sigma) crosslinked to various carbohydrates (N-acetyl-D-galactosamine (GalNAC), β-D-Glucose (Glu), D-Mannose (Mann) or N-acetyl-D-glucosamine (GlcNAC)) were equilibrated in carbohydrate buffer (1.25 M NaCl, 25 mM Tris-HCl (pH 7.5), 2.5 mM CaCl2). Each bead mixture was incubated with 1μg of rAceCTL-1 in carbohydrate buffer/0.5% BSA at 25° C with constant mixing for 1 hr. After washing, the beads were resuspended in sample buffer containing β-Mercaptoethanol and subjected to SDS-PAGE followed by transfer to nitrocellulose. The presence of bound rAceCTL-1 was detected by immunoblot using the HRP-labeled antibody to the polyhistidine fusion tag as described above.
2.5 Preparation of a rabbit polyclonal α-rAceCTL-1 IgG
For the production of polyclonal antisera, a single rabbit was injected subcutaneously with 100 μg of rpHPLC purified rAceCTL-1 in complete Freund’s adjuvant. After 2 weeks, the rabbit was boosted with 100 μg of rAceCTL-1 in incomplete Freund’s adjuvant. The rabbit IgG fraction was purified according to manufacturer’s instructions using a Protein G affinity chromatography column (Amersham Pharmacia Biotech, Upsala, Sweden).
2.6 Immunoblot detection of recombinant and native AceCTL-1
The parasite life cycle stage specificity of AceCTL-1 was characterized using an immunoblot protocol. One μg each of A. ceylanicum HEX (prepared from either males or females), LEX, egg/L1 protein extracts, and male sperm were separated by SDS-PAGE, followed by electroblot transfer to a nitrocellulose membrane. The membrane was incubated at 4°C overnight with the purified α-rAceCTL-1 IgG (1 μg/ml), and bound antibody was detected using a purified HRP-labeled goat α-rabbit polyclonal IgG (0.5 μg/ml) (Sigma) with chemiluminescence. Control blots were performed using pre-immune rabbit IgG as the primary antibody.
2.7 Immunolocalization of AceCTL-1 using Immunohistochemistry
Adult A. ceylanicum hookworms were harvested from the intestine of a laboratory infected hamster at day 21 post-infection, separated by gender, and immediately placed in 10% formalin. Individual worms were embedded in paraffin and slides containing contiguous sagittal sections were blocked and probed with the α-rAceCTL-1 polyclonal IgG (1 μg/ml). After washing, the bound primary antibody was detected using a 1:500 dilution of Texas Red α-Rabbit IgG Histostain-Plus 2nd generation LAB-SA detection system from (Zymed Laboratories, South San Francisco, CA). Control slides were incubated with pre-immune rabbit IgG as the primary antibody.
3. Results and Discussion
The CTLs are a family of related proteins that bind carbohydrates in a calcium dependent manner [1,3,4]. In mammals, CTLs have been shown to affect a variety of important functions mediated by cell-cell interactions, including inflammation, thrombosis, development, and reproductive physiology [6,27,28]. There are more than 120 C-type lectin domain containing proteins predicted from the genome sequence of the non-parasitic nematode C. elegans, some of which have been characterized for their physiologic roles [4,29]. In parasitic nematodes, it has been hypothesized that secreted C-type lectins function to subvert the host immune response by binding to specific cell-surface carbohydrate moieties [30,31]. Compelling evidence for a role of C-type lectins in the pathogenesis of parasitic nematode infections includes the fact that they are abundant protein components of pooled excretory/secretory products collected from live parasites, are expressed at very high levels in host-dwelling stages, and localize to the cuticle where they might interact with host immune effector cells [26,30,32,33]. Although it is likely that secreted lectins play an essential role in the biology of parasitic nematodes, thus far little experimental data exist to define the role(s) of specific members of this protein family in host-parasite interactions.
As part of an ongoing strategy aimed at identifying novel hookworm genes and proteins [17,18], a number of partial cDNAs were amplified from adult A. ceylanicum RNA using reverse transcription PCR (RT-PCR). Although an original 3’ oligonucleotide primer was designed in order to amplify nematode serine protease inhibitors [17,21], the translated amino acid sequence of a partial cDNA was found to have homology to the CTL family of proteins, including members from both the free living nematode C. elegans and other parasitic species [4,30,34]. Using standard techniques, the full length cDNA corresponding to Ancylostoma ceylanicum C-type lectin 1 (AceCTL-1) was successfully amplified from adult hookworm RNA. Analysis of the AceCTL-1 cDNA reveals an open reading frame of 550 nucleotides, which corresponds to a 167 amino acid mature protein with a 17-amino acid secretory signal sequence (Fig 1A). Based on sequence data, the mature AceCTL-1 protein is predicted to have a molecular mass of 18706 Da and a calculated pI of 8.14.
Figure 1.

A. The translated amino acid sequence of the AceCTL-1 cDNA predicts a 17-amino acid secretory signal sequence (in bold), followed by a mature protein of 167 amino acids with an expected mass of 18706 Da. The predicted Carbohydrate Recognition Domain (CRD) is boxed. Highly conserved amino acid residues based on sequences of previously characterized CRDs are marked with an asterix. The conserved WIG and Ca2+/Carbohydrate binding (EGN) motifs are underlined. Both nucleotide and amino acid sequences have been submitted to GenBank (Accession number AF172652). B. Alignment of the CTL domain of AceCTL-1 with previously identified lectins from the parasitic nematodes Necator americanus and Toxocara canis. Dark background denotes regions of conserved amino acid sequence between AceCTL-1 and one or more nematode lectins. Accession numbers for the lectins shown above: TES-70: AF126830; TES-32: AF040123; NaCTL-1: DQ058828; NaCTL-2: AF388311.
CTLs are distinguished from other lectins by structural homology in the carbohydrate-recognition domain (CRD), a 115–130 amino acid segment with a conserved spacing and number of cysteine residues, which form disulfide bonds that stabilize a double loop structure [1,2,5]. Using the Conserved Domain Search service (CD-Search) available through the NCBI [35], we confirmed the similarity of AceCTL-1 to other C-type lectins. As shown in Figure 1A, the predicted AceCTL-1 protein contains a characteristic WIG motif (amino acids W69 –I70 –G71), along with a predicted Carbohydrate Recognition Domain (spanning amino acid residues C102 to C145). Within the predicted CRD, there are a number of amino acid residues that are also conserved within the CTL family, including A103, W112, T114, A123, and M124. Additional residues predicted to mediate Ca2+ and carbohydrate binding include the tripeptide E94 –G95 –N96.[1,36,37]
Having confirmed that AceCTL-1 is most likely a C-type lectin, we then compared the translated amino acid sequence with those previously isolated by Loukas et al from the hookworm N. americanus (NaCTL-1, NaCTL-2) and the canine roundworm Toxocara canis (TES-70, TES-32) (Fig 1B) [26,32,38]). Using the Blast alignment tool available through the NCBI [22], the CTL domain of AceCTL-1 was found to have 26–33% amino acid sequence identity (36–50% amino acid similarity) to NaCTL-1, TES-70, and TES-32. Multi-sequence alignment using the ClustalW algorithm [23] shows that, despite the relatively modest sequence identity between AceCTL-1 and these three nematode CTLs, many of the residues that are essential for structural integrity and carbohydrate binding are conserved (Fig 1B). In contrast, the software analysis identified no significant amino acid sequence similarity with the N. americanus lectin NaCTL-2. These data confirm the broad divergence of amino acid sequences within the CTL family, and are consistent with potentially wide spectra of biological activities and functions in parasitic nematodes.
A cDNA corresponding to the mature AceCTL-1 protein was expressed in Drosophila S2 cells, and the recombinant protein purified using a combination of nickel resin affinity and rpHPLC. Following purification, we analyzed the binding of rAceCTL-1 to agarose beads crosslinked with individual carbohydrates [26]. As shown in Fig 2A, rAceCTL-1 effectively bound to only one (GlcNAC) of 4 carbohydrates tested. By comparison, rAceCTL-1 expressed in a prokaryotic (E. coli) system with a poly-histidine fusion tag failed to bind any of the carbohydrate containing beads (not shown), suggesting that post-translational modification is necessary for functional activity. It is also interesting to note that the carbohydrate binding signature sequence of AceCTL-1 (E94 –G95 –N96) is distinct from that of the mammalian Mannose Binding Lectin (EPN), which binds both GlcNAc and mannose [5,39]. Thus, it is possible that the substitution of Gly for Pro at residue 95 in AceCTL-1 accounts for the distinct binding pattern of the hookworm lectin.
Figure 2.
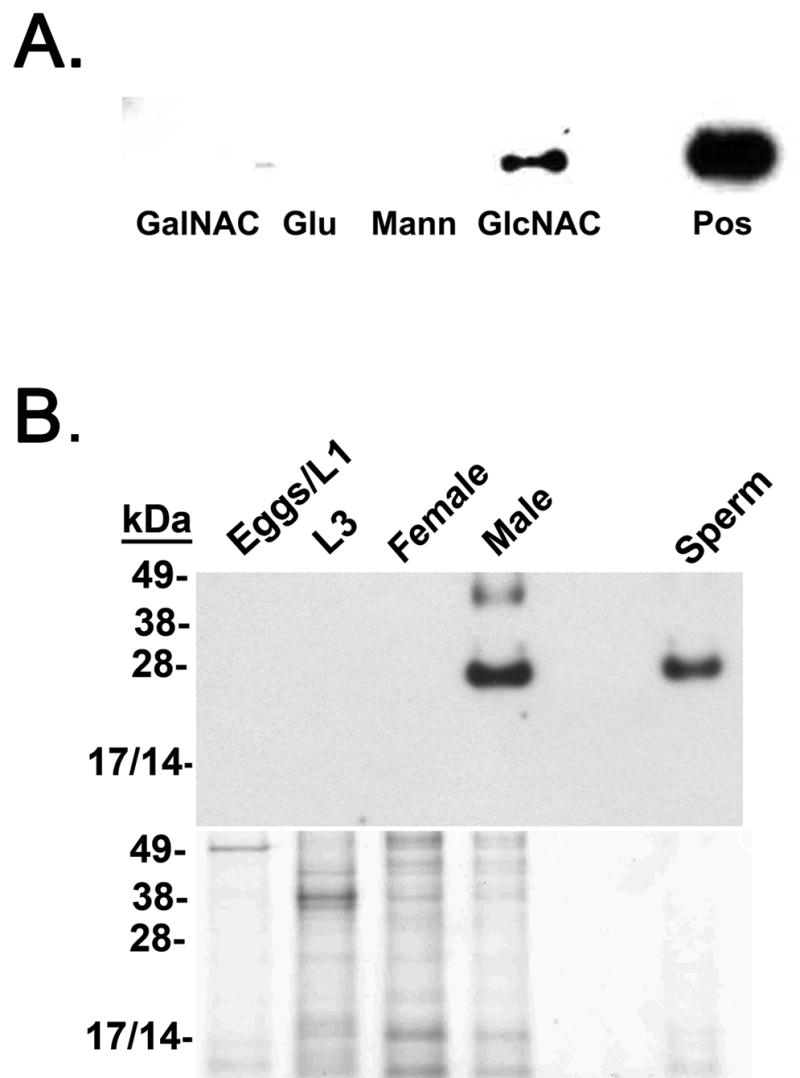
A. Carbohydrate binding specificity of recombinant AceCTL-1. The rAceCTL-1 protein was incubated with sepharose beads crosslinked to individual carbohydrates, followed by washing and separation of bound protein by SDS-PAGE. After blotting to nitrocellulose membrane, the presence of rAceCTL-1 bound to the sepharose beads was detected using an antibody against the poly-histidine residues located at the amino terminus of the recombinant fusion protein. As shown, rAceCTL-1 demonstrated specific binding to GlcNAC but not GalNAC, Glu, or Mann. The positive control lane (Pos) contains 1 μg of purified rAceCTL-1. B. Life cycle stage and gender specific expression of AceCTL-1. Soluble protein extracts (1 μg) from individual hookworm life stages (Eggs/L1, L3, adult males, adult females) or sperm were separated by SDS PAGE and electro-transferred to nitrocellulose membrane. The native protein was detected in adult male extracts and sperm using an α-rAceCTL-1 IgG.
Selective binding of rAceCTL-1 to GlcNAC is consistent with that described for C-type lectins that mediate sperm-egg recognition and fusion during fertilization, including a related sperm membrane protein from the sea urchin, Strongylocentrotus purpuratus [40,41]. Although little is known about molecular mechanisms of fertilization in hookworms, the in vitro binding specificity of rAceCTL-1 provided the first evidence of its potential role in reproductive physiology, as GlcNAC is a likely to be a major component of the nematode egg membrane [6,28,41].
The rAceCTL-1 protein was used to immunize a rabbit with Freund’s adjuvant, and the purified α-rAceCTL-1 IgG was then used to characterize the life cycle stage and gender specificity of the lectin in A. ceylanicum. As shown in Fig 2B, AceCTL-1 protein was detected in sperm and soluble extracts of adult males, but not females, eggs/L1, or L3 stages of the parasite. We also did not detect the native AceCTL-1 protein in pooled adult worm ES products (not shown), suggesting the lectin is not secreted. Taken together, the data confirm that AceCTL-1 is primarily an adult male specific protein, and that sperm represent at least one source of its production in vivo.
Further evidence for a role of AceCTL-1 in hookworm reproductive function was obtained using immunohistochemistry. As shown in Fig 3, sagittal sections of adult A. ceylanicum probed with the polyclonal α-rAceCTL-1 IgG exhibited a distinct gender-specific staining pattern. In male worms, the α-rAceCTL-1 IgG localized exclusively to the testes. At higher magnification (100X), prominent staining of the rachis, a nematode structure that generates spermatocytes in the male, was clearly visualized [10]. The rachis serves as a site of early sperm maturation, ultimately releasing spermatocytes that develop to mature sperm prior to insemination of the female via copulation. These data suggest that AceCTL-1 is incorporated at a very early stage into the developing spermatid. Of note, adult worm sections probed with a pre-immune rabbit IgG showed no staining of any structures. Preliminary data suggest that the antibody raised against the rAceCTL-1 protein also localizes to the male testes of the dog parasite A. caninum (not shown), suggesting the presence of conserved epitopes in related lectins from at least two hookworm species. In fact, a review of the A. caninum EST database [42] identified multiple nucleotide sequences that predicted proteins with CTL domains.
Figure 3.
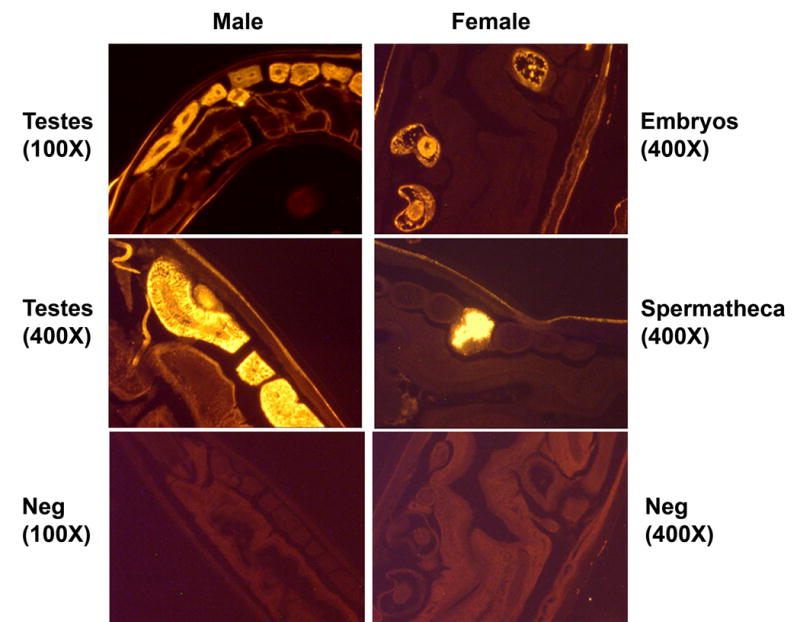
Immunolocalization of AceCTL-1 in adult hookworms. Sagittal sections of adult males (left panels) or females (right panels) were probed with the α-AceCTL-1 IgG, followed by detection with a fluorescent labeled α-rabbit IgG. The native AceCTL-1 protein localizes to the male testes (top and middle left panels). In females, the protein was found in embryos (top right panel) and the spermatheca (middle right panel), both of which are thought to contain male sperm proteins.
In contrast to the recognition pattern of male A. ceylanicum, two very distinct structures were highlighted upon probing of adult female hookworm sections with α-AceCTL-1 (Fig 3). One is the spermatheca, which lies between the nematode ovary and uterus [10,43,44]. Both hermaphroditic (eg C. elegans) and non-hermaphroditic nematodes utilize the spermatheca, a specialized structure located at the junction of the uterus and oviduct, to store sperm following copulation but prior to fertilization. Soon after deposition within the female genital tract, nematode sperm migrate up the uterus to the spermatheca, eventually anchoring themselves until binding a passing oocyte [10,45]. Nematode eggs are sequentially fertilized by stored sperm as they pass through the spermatheca, a process in Ancylostoma that likely occurs and requires a series of sperm-egg interactions based on recognition of specific cell surface molecules.
Once fertilization is initiated, the amoeboid sperm extend pseudopodia around the oocyte. In nematodes and other invertebrate species, successful fertilization results in fusion of the cell membranes, with the resulting zygote cell wall comprised of proteins derived from both sperm and egg [45]. Consistent with this phenomenon, strong signal was also noted by immunohistochemistry in hookworm embryos visualized within adult females (Fig 3). It is likely that these images represent recently fertilized oocytes that have passed through the spermatheca. These data suggest that Ancylostoma eggs, similar to other nematode ova, incorporate sperm proteins into the egg membrane upon fertilization.
Our observation that AceCTL-1 protein can be detected in female worms and embryos by immunohistochemistry, but not by immunoblot, deserves comment. First, it is possible that there is significantly more AceCTL-1 contained in extracts of adult males, due to the fact that the testes are large structures literally filled with spermatocytes. In contrast, in adult females AceCTL-1 appears only in scattered embryos and within the spermatheca, a relatively small structure. Alternatively, it is possible that while native AceCTL-1 is present in the recently fertilized embryos, at the time of excretion by the adult female worm the protein has been degraded to the point that it is no longer present in quantities sufficient to be detected by immunoblot. Lastly, it is also possible that once incorporated into the egg membrane following fertilization, the native AceCTL-1 protein is no longer present in soluble protein extracts. Work is underway to determine the kinetics of AceCTL-1 expression, and potentially degradation, following fertilization of hookworm embryos.
In summary, the data presented here describe the molecular characterization of a C-type lectin with a putative role in the reproductive physiology of the hookworm A. ceylanicum. While secreted lectins from parasitic nematodes, including hookworm, also function as secreted immunomodulatory proteins [30,46], to our knowledge, this is the first member of the CTL family with a potential role in nematode sperm-egg recognition and fertilization. Work is currently underway to more clearly define the function of AceCTL-1 in the biology of Ancylostoma, as well as to target hookworm reproductive mechanisms as a means of reducing the risk of infection. Strategies to reduce nematode fecundity have previously been suggested as a means to control human and veterinary nematode disease, including hookworm [47]. Considering that female hookworms pass tens of thousands of fertilized eggs per day, strategies aimed at decreasing hookworm fecundity may ultimately lead to reduced transmission, as well as disease, within highly endemic communities.
Acknowledgments
The authors would like to thank Gerhard Schad and Alan Scott for thoughtful interpretation of the immunohistochemistry data, and Richard Bungiro for advice during the course of this work. This work was supported by grant AI47929 from the National Institutes of Health (MC). MC is a recipient of a Hellman Family Fellowship from the Office of the President of Yale University.
Abbreviations
- IPTG
isopropyl-β-D-thiogalactopyranoside
- RT-PCR
reverse transcription polymerase chain reaction
- NCBI
National Center for Biotechnology Information
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- HRP
horseradish peroxidase
- 3’RACE
3’-rapid amplification of cDNA ends
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. Febs J. 2005;272:6179–217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 2.Ebner S, Sharon N, Ben-Tal N. Evolutionary analysis reveals collective properties and specificity in the C-type lectin and lectin-like domain superfamily. Proteins. 2003;53:44–55. doi: 10.1002/prot.10440. [DOI] [PubMed] [Google Scholar]
- 3.Taylor ME, Drickamer K. Structure-function analysis of C-type animal lectins. Methods Enzymol. 2003;363:3–16. doi: 10.1016/S0076-6879(03)01039-5. [DOI] [PubMed] [Google Scholar]
- 4.Dodd RB, Drickamer K. Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology. 2001;11:71R–9R. doi: 10.1093/glycob/11.5.71r. [DOI] [PubMed] [Google Scholar]
- 5.Zelensky AN, Gready JE. Comparative analysis of structural properties of the C-type-lectin-like domain (CTLD) Proteins. 2003;52:466–77. doi: 10.1002/prot.10626. [DOI] [PubMed] [Google Scholar]
- 6.Rodeheffer C, Shur BD. Characterization of a novel ZP3-independent sperm-binding ligand that facilitates sperm adhesion to the egg coat. Development. 2004;131:503–12. doi: 10.1242/dev.00937. [DOI] [PubMed] [Google Scholar]
- 7.Singson A. Sperm activation: time and tide wait for no sperm. Curr Biol. 2006;16:R160–2. doi: 10.1016/j.cub.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 8.Geldziler B, Kadandale P, Singson A. Molecular genetic approaches to studying fertilization in model systems. Reproduction. 2004;127:409–16. doi: 10.1530/rep.1.00009. [DOI] [PubMed] [Google Scholar]
- 9.Tarr DE, Scott AL. MSP domain protein-1 from Ascaris suum and its possible role in the regulation of major sperm protein-based crawling motility. Mol Biochem Parasitol. 2005;143:165–72. doi: 10.1016/j.molbiopara.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Scott AL. Nematode sperm. Parasitol Today. 1996;12:425–30. doi: 10.1016/0169-4758(96)10063-6. [DOI] [PubMed] [Google Scholar]
- 11.Held M, Bungiro RD, Harrison LM, Hamza I, Cappello M. Host dietary iron mediates hookworm pathogenesis in vivo. Infect Immun. 2006 doi: 10.1128/IAI.74.1.289-295.2006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bungiro RD, Jr, Greene J, Kruglov E, Cappello M. Mitigation of hookworm disease by immunization with soluble extracts of Ancylostoma ceylanicum. J Infect Dis. 2001;183:1380–7. doi: 10.1086/319867. [DOI] [PubMed] [Google Scholar]
- 13.Cappello M, Bungiro RD, Harrison LM, Bischof LJ, Griffitts JS, Barrows BD, Aroian RV. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0607002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foreyt WJ. Diagnostic parasitology. Vet Clin North Am Small Anim Pract. 1989;19:979–1000. doi: 10.1016/s0195-5616(89)50107-4. [DOI] [PubMed] [Google Scholar]
- 15.Nelson GA, Ward S. Vesicle fusion, pseudopod extension and amoeboid motility are induced in nematode spermatids by the ionophore monensin. Cell. 1980;19:457–64. doi: 10.1016/0092-8674(80)90520-6. [DOI] [PubMed] [Google Scholar]
- 16.Bungiro RD, Jr, Solis CV, Harrison LM, Cappello M. Purification and molecular cloning of and immunization with Ancylostoma ceylanicum excretory-secretory protein 2, an immunoreactive protein produced by adult hookworms. Infect Immun. 2004;72:2203–13. doi: 10.1128/IAI.72.4.2203-2213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison LM, Nerlinger A, Bungiro RD, Cordova JL, Kuzmic P, Cappello M. Molecular characterization of Ancylostoma inhibitors of coagulation factor Xa. Hookworm anticoagulant activity in vitro predicts parasite bloodfeeding in vivo. J Biol Chem. 2002;277:6223–9. doi: 10.1074/jbc.M109908200. [DOI] [PubMed] [Google Scholar]
- 18.Milstone AM, Harrison LM, Bungiro RD, Kuzmic P, Cappello M. A broad spectrum Kunitz type serine protease inhibitor secreted by the hookworm Ancylostoma ceylanicum. J Biol Chem. 2000;275:29391–9. doi: 10.1074/jbc.M002715200. [DOI] [PubMed] [Google Scholar]
- 19.Cappello M, Hawdon JM, Jones BF, Kennedy WP, Hotez PJ. Ancylostoma caninum anticoagulant peptide: cloning by PCR and expression of soluble, active protein in E. coli Mol Biochem Parasitol. 1996;80:113–7. doi: 10.1016/0166-6851(96)02658-8. [DOI] [PubMed] [Google Scholar]
- 20.Krause M, Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987;49:753–61. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babin DR, Peanasky RJ, Goos SM. The isoinhibitors of chymotrypsin/elastase from Ascaris lumbricoides: the primary structure. Arch Biochem Biophys. 1984;232:143–61. doi: 10.1016/0003-9861(84)90530-7. [DOI] [PubMed] [Google Scholar]
- 22.McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucl Acids Res. 2004;32:W20–25. doi: 10.1093/nar/gkh435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li KB. ClustalW-MPI: ClustalW analysis using distributed and parallel computing. Bioinformatics. 2003;19:1585–6. doi: 10.1093/bioinformatics/btg192. [DOI] [PubMed] [Google Scholar]
- 24.Mieszczanek J, Harrison LM, Cappello M. Ancylostoma ceylanicum anticoagulant peptide-1: role of the predicted reactive site amino acid in mediating inhibition of coagulation factors Xa and VIIa. Mol Biochem Parasitol. 2004;137:151–9. doi: 10.1016/j.molbiopara.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Del Valle A, Jones BF, Harrison LM, Chadderdon RC, Cappello M. Isolation and molecular cloning of a secreted hookworm platelet inhibitor from adult Ancylostoma caninum. Mol Biochem Parasitol. 2003;129:167–77. doi: 10.1016/s0166-6851(03)00121-x. [DOI] [PubMed] [Google Scholar]
- 26.Loukas A, Mullin NP, Tetteh KK, Moens L, Maizels RM. A novel C-type lectin secreted by a tissue-dwelling parasitic nematode. Curr Biol. 1999;9:825–8. doi: 10.1016/s0960-9822(99)80366-2. [DOI] [PubMed] [Google Scholar]
- 27.Shur BD, Rodeheffer C, Ensslin MA, Lyng R, Raymond A. Identification of novel gamete receptors that mediate sperm adhesion to the egg coat. Mol Cell Endocrinol. 2006;250:137–48. doi: 10.1016/j.mce.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Shur BD, Rodeheffer C, Ensslin MA. Mammalian fertilization. Curr Biol. 2004;14:R691–2. doi: 10.1016/j.cub.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 29.Drickamer K, Dodd RB. C-Type lectin-like domains in Caenorhabditis elegans: predictions from the complete genome sequence. Glycobiology. 1999;9:1357–69. doi: 10.1093/glycob/9.12.1357. [DOI] [PubMed] [Google Scholar]
- 30.Loukas A, Maizels RM. Helminth C-type lectins and host-parasite interactions. Parasitol Today. 2000;16:333–9. doi: 10.1016/s0169-4758(00)01704-x. [DOI] [PubMed] [Google Scholar]
- 31.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 32.Loukas A, Doedens A, Hintz M, Maizels RM. Identification of a new C-type lectin, TES-70, secreted by infective larvae of Toxocara canis, which binds to host ligands. Parasitology. 2000;121:545–54. doi: 10.1017/s0031182099006721. Pt 5. [DOI] [PubMed] [Google Scholar]
- 33.Tetteh KK, Loukas A, Tripp C, Maizels RM. Identification of abundantly expressed novel and conserved genes from the infective larval stage of Toxocara canis by an expressed sequence tag strategy. Infect Immun. 1999;67:4771–9. doi: 10.1128/iai.67.9.4771-4779.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daub J, Loukas A, Pritchard DI, Blaxter M. A survey of genes expressed in adults of the human hookworm, Necator americanus. Parasitology. 2000;120 :171–84. doi: 10.1017/s0031182099005375. Pt 2. [DOI] [PubMed] [Google Scholar]
- 35.Marchler-Bauer A, Bryant SH. CD-Search: protein domain annotations on the fly. Nucl Acids Res. 2004;32:W327–331. doi: 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1992;360:183–6. doi: 10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- 37.Drickamer K. C-type lectin-like domains. Curr Opin Struct Biol. 1999;9:585–90. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 38.Loukas A, Brown AP, Pritchard DI. Na-ctl-2, a cDNA encoding a C-type lectin expressed exclusively in adult Necator americanus hookworms. DNA Seq. 2002;13:61–5. doi: 10.1080/10425170290019900. [DOI] [PubMed] [Google Scholar]
- 39.Agah A, Montalto MC, Young K, Stahl GL. Isolation, cloning and functional characterization of porcine mannose-binding lectin. Immunology. 2001;102:338–43. doi: 10.1046/j.1365-2567.2001.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galindo BE, Moy GW, Vacquier VD. A third sea urchin sperm receptor for egg jelly module protein, suREJ2, concentrates in the plasma membrane over the sperm mitochondrion. Dev Growth Differ. 2004;46:53–60. doi: 10.1111/j.1440-169x.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- 41.Mengerink KJ, Vacquier VD. Glycobiology of sperm-egg interactions in deuterostomes. Glycobiology. 2001;11:37R–43R. doi: 10.1093/glycob/11.4.37r. [DOI] [PubMed] [Google Scholar]
- 42.Wylie T, Martin JC, Dante M, Mitreva MD, Clifton SW, Chinwalla A, Waterston RH, Wilson RK, McCarter JP. Nematode.net: a tool for navigating sequences from parasitic and free-living nematodes. Nucleic Acids Res. 2004;32 doi: 10.1093/nar/gkh010. Database issue:D423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding L, Candido EP. HSP43, a small heat-shock protein localized to specific cells of the vulva and spermatheca in the nematode Caenorhabditis elegans. Biochem J. 2000;349:409–12. doi: 10.1042/0264-6021:3490409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lysek H, Ondrus J. Morphology of the uterus of Ascaris lumbricoides in the region where fertilization and formation of egg-shell occur. Folia Parasitol (Praha) 1992;39:41–50. [PubMed] [Google Scholar]
- 45.Justine J-L. Male and Female Gametetes and Fertilisation. In: Lee DL, editor. The Biology of Nematodes. Taylor & Francis; London: 2002. pp. 73–119. [Google Scholar]
- 46.Loukas A, Constant SL, Bethony JM. Immunobiology of hookworm infection. FEMS Immunol Med Microbiol. 2005;43:115–24. doi: 10.1016/j.femsim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Bakker N, Vervelde L, Kanobana K, Knox DP, Cornelissen AW, de Vries E, Yatsuda AP. Vaccination against the nematode Haemonchus contortus with a thiol-binding fraction from the excretory/secretory products (ES) Vaccine. 2004;22:618–28. doi: 10.1016/j.vaccine.2003.08.025. [DOI] [PubMed] [Google Scholar]


