Abstract
Autism is a pervasive developmental disorder, with characteristics including impairments in reciprocal social interaction, impaired communication, and repetitive/stereotyped behaviors. Despite decades of research, the etiology of autism remains elusive. Thus, it is important that we pursue all avenues, in attempting to understand this complicated disorder. One such avenue is the development of animal models. While autism may be uniquely human, there are behavioral characteristics of the disorder that can be established in animal models. Evidence supports a genetic component for this disorder, and over the past few decades the mouse has been a highly valuable tool for the elucidation of pathways involved in many human disorders (e.g., Huntington’s disease). As a first step toward establishing a mouse model of autism, we studied same-sex social behavior in a number of inbred mouse strains. In Study 1, we examined intra-strain social behavior of male pairs after one mouse had 15 minutes prior exposure to the testing chamber. In Study 2, we evaluated intra-strain and inter-strain social behavior when both mice were naive to the testing chamber. The amount and type of social behavior seen differed between these studies, but overall there were general inbred strain differences in social behavior. Some strains were highly social (e.g., FVB/NJ, while others displayed low levels of social behavior (e.g., A/J, BTBR T+ tf/J). These strains may be useful in future genetic studies to determine specific genes involved in mouse social behavior, the findings of which should in turn help us to determine some of the genes involved in human social behavior and its disorders (e.g., autism).
Keywords: autism, mouse, inbred strain, social behavior, sniffing, huddling, wrestling, mouse model
1. Introduction
At least one child out of every 1,000 born in the United States will at a later developmental time point be diagnosed with autism spectrum disorder [22]. This estimate may be very conservative; in fact, some recent estimates are as high as 7 per 1,000 [26,55]. Autism spectrum disorders have recently been equated to Alzheimer’s disease in terms of the cost in “patient years” that they represent to our society [27]. First identified and described in the 1940s [3,30], autism is classified as one of the pervasive developmental disorders in the Diagnostic and Statistical Manual of Mental Disorders, with clinical symptoms including impairments in reciprocal social interaction, restlessness and distraction, difficulty with language, and repetitive and stereotyped motor behaviors [1]. The clinical phenotype of autism is complex and variable, making a simple therapy or solution problematic. Instead, a combination of therapeutic approaches may be the best solution for such a diverse phenotype. A number of brain regions are thought to be involved in this disorder, including the cerebellum, hippocampus, amygdala, basal ganglia, corpus callosum, and brainstem [4,15,44,53], and both the serotonergic and glutaminergic systems may play roles [14,16]. Unfortunately, the etiology of the disorder is still far from clear.
Autism is a complex disorder, likely to be influenced by a combination of genetic and environmental factors. Elucidation of autism-related genes is important for potential therapeutic interventions and, eventually, a cure. The first evidence for a genetic role in autism came from an epidemiological study of affected twins [24]. More recently attention has been focused on attempting to define the genetic component of autism [2,25,31,33,36]. A better understanding of the relationship between phenotype and genotype will provide new insights into the mechanisms involved. The use of non-human models of autism will be of great importance for elucidating the genotype-phenotype relationship, and for therapeutic testing. Advanced transgenic and recombinant technologies, and the recent sequencing of the mouse genome, have made the mouse the model of choice for many geneticists. While not all human behaviors can be easily translated to the mouse, we can, through careful operational definitions of the behavior being studied, use the mouse model to elucidate some of the fundamental elements of complex behaviors in humans. These components may not be disorder-specific (i.e., there may not be a perfect mouse model for disorders such as autism); however, we can use specific genetic findings in the mouse to search for candidate loci for human disorders [13,51]. With careful dissection of behavior, and with a thorough understanding of the components of the behavioral assay being utilized, we can enhance our potential to produce mouse models of social behavioral abnormalities such as autism [40]. In fact, most of the core symptoms of autism spectrum disorder can be evaluated in the mouse through an intensive battery of tests [20].
On December 4, 2000, experts from the fields of autism, mouse genetics, and mouse behavior met at the Jackson Laboratory, Bar Harbor, ME, to discuss the utility of mouse models of autism. The consensus from that meeting was that mouse models of autism can make a significant contribution to our understanding of this complex disorder, although if we are to maximize the effectiveness of mouse models, we need to know more about the genetics involved [29]. Thus far, the effects of ablation or mutation of a number of genes have been examined in mouse models of autism, with mixed phenotypic results [23,34,35,39,49]. However, there is a great need for additional mouse models, especially those that are based on naturally occurring differences among groups of mice, i.e., differences among inbred strains. Crawley and colleagues have recently developed a social novelty/sociability automated assay that they are using to examine autism-like behaviors in inbred strains of mice [43]. Their studies illustrate that variability in social responsiveness exists among some of the most common inbred strains, with A/J, BALBcByJ, and BTBR T+ tf/J strains being less socially responsive [41,42]. Brodkin and colleagues also describe the BALB/cBy strain as displaying low levels of sociability, in contrast to strains such as C57BL/6J [12,50]. Our laboratory has been examining inbred strain behavior variability with a number of assays, including open field, fear conditioning, food preference, rotorod, and zero maze [6,8,10,17,37]. Since inbred mouse strain differences have been established for many behaviors, we decided to examine social behavior across a set of common inbred strains. This inbred strain survey is a first step in determining which of these strains will be the subject of future genetics research.
2. Materials and Methods
2.1. Mice
Male and female breeding pairs from seven inbred strains, 129S1/SvImJ (129S1), A/J (A), BALBcBy/J (CBy), C57BL/6J (B6), BTBR T+ tf/J (BTBR), DBA/2J (D2), and FVB/NJ (FVB), were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained in our colony at the Wadsworth Center. Male offspring of these breeders were used in all studies and were socially naïve to one other before the onset of testing. At weaning, mice were housed in same-sex groups of 2–4 per cage, at a temperature of 21 ± 2 °C, with a 12:12 hour light-dark cycle with lights on at 0700. Food and water were freely available. At the start of testing, mice were 60–90 days of age. All testing took place between 1230 and 1600. All procedures had prior approval by the Wadsworth Institutional Animal Care and Use Committee. In Study 1, mice from the following groups were tested in a 20-minute intra-strain test (6 male pairs per strain): 129S1, BTBR, B6, D2 and FVB. In Study 2, male mice (12 pairs per strain) from the following groups were tested in a 30-minute intra-strain test: 129, A, B6, BTBR, CBy, D2, and FVB. An additional 12 FVB and 12 BTBR males, which were used to examine inter-strain social behavior, were also tested in Study 2.
2.2. Apparatus
The chamber used for testing was a clear polypropylene cage (27.5 × 15.5 × 13.0 cm) with bedding covering the bottom. The cage was identical to the standard housing cages. All behavior during the sessions was videotaped with a Panasonic VHS camera. Videotapes were played back with a Panasonic VCR and monitor.
2.3. Procedure
In Study 1, designed to elicit all types of social interactions, one male mouse of a pair was placed in the cage and allowed to explore freely for 15 minutes. At the end of the 15-minute session, a second male mouse of the same strain was added to the cage, and the behavior of the pair of animals was recorded on videotape for 20 minutes. Thus, one mouse was the resident of the cage and the other an intruder. At the end of the session, both mice were removed from the chamber, and the chamber was cleaned and new bedding added. In Study 2, designed to reduce the number of aggressive behaviors and allow more positive social interactions, both mice (males of the same strain) were added to the chamber at the same time and the session was increased to 30 minutes so as to allow more time for social exchanges to occur. In addition, two strains known to display low levels of social behavior – A and CBy – were tested, so that we could compare our findings with those of recent inbred strain comparisons [12,41,50]. In Study 2, inter-strain social behavior during the 30-minute session was also studied in pairs comprised of one BTBR mouse and one FVB mouse (BTBR-FVB). The amount of time during which the mice engaged in social interactions (e.g., sniffing, following, allogrooming, biting, chasing, mounting, wrestling) was recorded from videotapes for each pair of mice, by a naïve observer. These behaviors have been routinely used to evaluate social behavior in rodents [5,9,45,47] and were coded according to established definitions [19].
2.4. Statistical Analysis
In all cases, the time engaged in social behavior was analyzed by one-way analysis of variance (ANOVA), with strain as the independent variable. The amount of time spent in specific categories of social behavior (e.g., sniffing, positive social behavior, negative social behavior, mounting/sexual behaviors) was analyzed by one-way ANOVA, with inbred strain as the independent variable. Fisher’s post-hoc tests were then used to make pairwise comparisons. All statistical analyses were completed with STATVIEW 5.0.
3. Results and Discussion
3.1. Study 1
There were significant differences in the amount of time spent engaged in social interactions (F(4,25) = 8.441, p=0.0002; see Figure 1), with BTBR mice spending the least and FVB spending the most time engaged in social behavior, among the five strains (all comparisons p<0.05; except the comparison between B6 and BTBR, where p<.12). When we examined specific types of social behavior, it was clear that some behaviors were more common than others and were seen in all strains tested, whereas for other behaviors strain differences existed (see Table 1). For instance, sniffing behavior was seen in all male pairs, regardless of strain. Allogrooming and huddling were never seen in male pairs of any strain during this 20-minute session. Mounting occurred in 129S1, D2, and FVB, but not in B6 and BTBR strains. Wrestling was only seen in B6 and FVB strains and then only for a few pairs, whereas following was seen for all strains except BTBR. The high level of social behavior observed in FVB mice was consistent with the sociability and preference for social novelty levels typically seen in this strain [41]. The level of social behavior in the BTBR strain observed in our study is similar to that observed by Crawley and colleagues for both adult and juvenile age groups of this strain [38,42]. Thus, BTBR and FVB represent opposite ends of the social behavior spectrum and may be good choices for future genetic studies.
Figure 1.
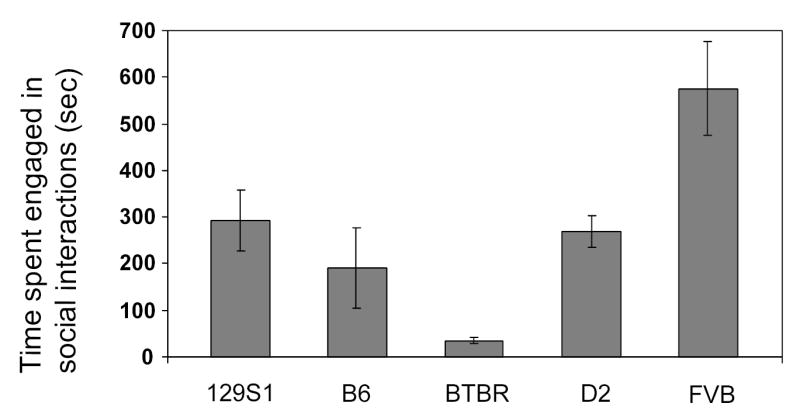
Intra-strain social interaction, when one mouse of the pair had prior experience in the testing chamber. Mean (± SEM) time spent engaged in any type of social interaction (12 pairs per strain of 129S1, B6, BTBR, D2, and FVB) during the 20-minute session.
Table 1.
Study 1: Percentage (number of pairs) of mice of each inbred strain displaying various types of social behaviors during the 20-minute test session.
| Strain | Type of Social Behavior | |||||
|---|---|---|---|---|---|---|
| Mounting | Wrestling | Following | Allogrooming | Sniffing | Huddling | |
| 129S1 | 50%(3) | 0%(0) | 100%(6) | 0%(0) | 100%(6) | 0%(0) |
| B6 | 0%(0) | 17%(1) | 100%(6) | 0%(0) | 100%(6) | 0%(0) |
| BTBR | 0%(0) | 0%(0) | 0%(0) | 0%(0) | 100%(6) | 0%(0) |
| D2 | 17%(1) | 0%(0) | 100%(6) | 0%(0) | 100%(6) | 0%(0) |
| FVB | 50%(3) | 50%(3) | 100%(6) | 0%(0) | 100%(6) | 0%(0) |
3.2. Study 2
To focus on non-aggressive social behaviors, we modified our procedure so that both mice of the pair were added to the chamber simultaneously. In this situation we found significant inbred strain differences in the amount of time spent engaged in social interactions (F(6,77) = 11.307, p<0.0001; see Figure 2), with FVB mice spending more time engaged in social activity than any other strain (all p<0.01). The D2 strain displayed the second-highest length of time engaged in social behavior (all comparisons p<0.5).
Figure 2.
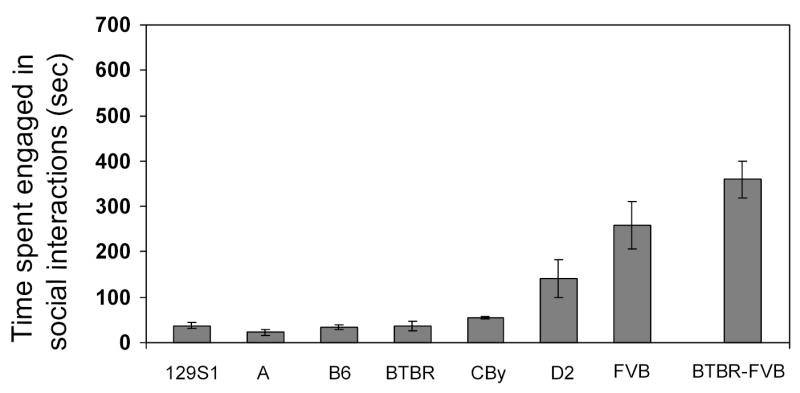
Intra-strain and inter-strain social interaction, when both mice of a pair were naïve to the testing chamber. Mean (± SEM) time spent engaged in any type of social interaction (12 pairs per strain of 129S1, A, B6, BTBR, CBy, D2, and FVB, and 12 BTBR-FVB pairs) during the 30-minute session.
In general, the time spent in social interactions was low in this study for most of the inbred strains tested, although the general trends are similar to that obtained in other studies [41,42]. Furthermore, the time spent engaged in social interactions is lower in Study 2 than it was in Study 1. The lower overall values seen in Study 2 may be due, at least in part, to the testing situation, as experimental manipulations can influence the social behavior of some strains more than others [21]. The new cage and clean bedding may have provided a conflict between cage exploration and social exploration. Perhaps the low level of social interaction was the result of increased exploratory activity, as it was noted that the mice spent a large percentage of their time running around the cage, rearing, and digging in the bedding. In contrast, one member of each pair had prior exposure to the chamber in Study 1, which might have increased the time spent in social interactions initiated by the “resident” mouse.
However, since the overall social behavior analysis included all types of social behavior, it does not differentiate those types of behavior most appropriate to autism spectrum disorders. To address this issue, we next calculating the time spent engaged in each type of social behavior. We divided the social behaviors into four main categories: sniffing; positive behaviors such as huddling or allogrooming; negative behaviors such as wrestling and biting; and mounting behavior [19]. Surprisingly, although we modified our procedure to encourage positive social interactions and discourage negative ones, this did not happen. There was no decrease in negative behaviors between the two sessions and the levels of positive behaviors remained low. We did find that some behaviors occurred more frequently than others. For instance, sniffing behavior was seen in all male pairs regardless of strain. However, there were inbred strain differences in the amount of time that males spent engaged in sniffing behavior (F(6,77) = 21.617, p<0.0001; see Figure 3). FVB spent more time engaged in this type of social behavior than any other strain (all p<0.0001). With the exception of D2, which engaged in this behavior significantly longer than A or BTBR (all p<.05), there were no differences among the other inbred strains. However, as can be seen in Figure 3, other differences may have emerged, if strains had been examined on a pairwise basis. This is especially true for the two strains – A and BTBR- that displayed this type of social behavior least often. Negative social behaviors such as wrestling and biting were not common and were only seen in B6 (mean time (sec) = 8.3 ±5.4) and FVB (29.8±13.1) strains. However, even in those cases, they were observed in four pairs (33%) of FVB mice and in three pairs (25%) of B6. For positive social behaviors such as allogrooming and huddling, inbred strain differences were evident (F(6,77) = 2.497, p=0.0292; see Figure 4). D2 was the only strain that differed from the other strains (all p<0.05), and it displayed the most variability in performance of positive behaviors. Examination of the raw data revealed that some D2 mice displayed a lot of these behaviors, while others displayed them only rarely. It is unknown at this time why there was so much variation in the D2 strain; the variation was not related to litter or age factors. Finally, mounting behavior was rare and was only observed in one 129S1 male pair (8%).
Figure 3.
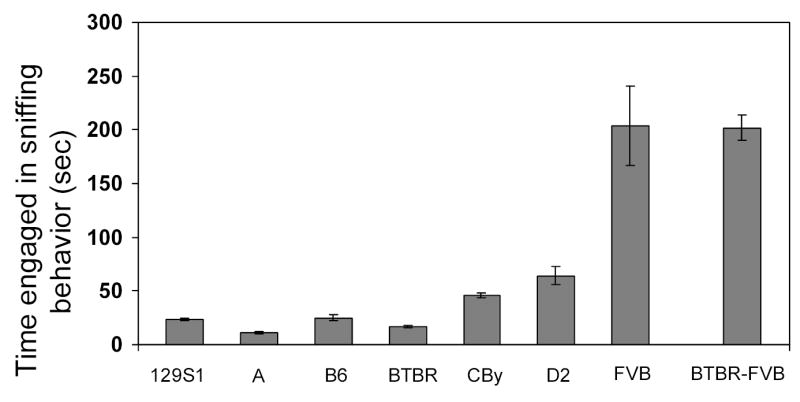
Mean (± SEM) time spent engaged in sniffing behavior (12 pairs per strain of 129S1, A, B6, BTBR, CBy, D2, and FVB, and 12 BTBR-FVB pairs) when both mice of a pair were naïve to the testing chamber during the 30-minute session.
Figure 4.

Intra-strain and inter-strain social interaction, when both mice of a pair were naïve to the testing chamber. Mean (± SEM) time spent engaged in positive social behaviors (e.g., huddling, allogrooming) (12 pairs per strain, 129S1, A, B6, BTBR, CBy, D2, and FVB, and 12 BTBR-FVB pairs) during the 30-minute session.
We next examined the effect of placing highly social FVB and less social BTBR males together in pairs. These strains were selected for several reasons. First, FVB was consistently the most socially active of all the strains in intra-strains studies and BTBR displayed a low level of social interaction. In contrast to some of the strains also displaying low levels of social behavior (e.g., A), the activity levels of BTBR and FVB mice are both high [7,42]. Thus, in BTBR-FVB pairings, social behavior differences should not merely be a consequence of low activity levels, and the two mice should be well matched in terms of exploratory behavior ability. The inter-strain BTBR-FVB pairs were compared to the FVB and BTBR intra-strain pairs. As can be seen in Figure 2, there were significant differences in time spent engaged in social interactions among the groups (BTBR-BTBR, FVB-FVB, and BTBR-FVB; F(2,33) = 18.498, p<0.0001). The BTBR-FVB pairs displayed significantly more social behavior than did the BTBR-BTBR pairs (p<0.0001), and there was a tendency for the inter-strain pairs to be even more social than FVB-FVB pairs (p=0.0741).
The three types of pairings, BTBR-BTBR, BTBR-FVB, and FVB-FVB, differed in the amount of time spent engaged in sniffing behavior (F(2,33) = 22.407, p<0.0001; see Figure 3). The BTBR-BTBR combination displayed significantly less of this type of social behavior than did the FVB-FVB and BTBR-FVB pairs (all p<0.0001); the latter two types of pairings did not differ from one another. There was no difference in the amount of time engaged in positive social behaviors among BTBR-FVB, BTBR-BTBR and FVB-FVB pairs (see Figure 4). In terms of negative social interactions, there were differences among the combinations (F(2,33) = 11.068, p=0.0002; see Figure 5). Pairs of BTBR-FVB mice displayed significantly more wrestling and biting than did pairs of BTBR-BTBR and FVB-FVB mice (p<.0.01). Observations of the videotapes often revealed an interesting sequence of events, with the BTBR mouse initiating the negative social behavior. During the first few minutes, little social behavior occurred, as both males explored the cage. Then, the FVB mouse approached the BTBR mouse. Most of this behavior consisted of sniffing. Little aggressive behavior was observed during this time. At this point the BTBR mouse moved away from the FVB, started digging, or simply remained relatively unresponsive to the social attention. After 8–10 minutes of this forced social interaction, the BTBR suddenly displayed aggressive behavior. The FVB mouse eventually responded by fighting; however, it is often clear from the videotapes that the BTBR mouse was the actual initiator of the aggression. It is noteworthy that this negative social behavior did not occur until after the FVB mouse’s continual attempts to initiate social contact with the BTBR mouse. This sequence was seen in over 50% of the BTBR-FVB pairs. Although aggression is not one of the core symptoms of autism, it has been reported to occur in response to sensory overload [46]. Perhaps, the persistent attempts at social contact by the FVB causes a sensory overload for the BTBR mouse, and aggression resulted. Further studies are needed to dissect the complex set of interactions seen between these two mouse strains.
Figure 5.
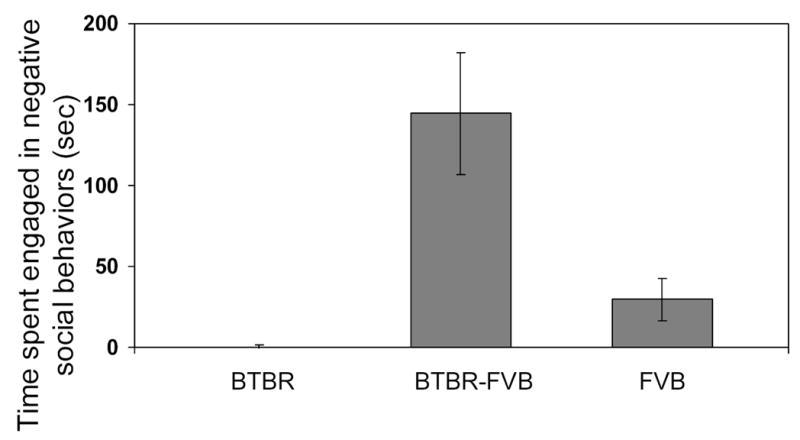
Effects of pairing male mice from inbred strains with high and low levels of social interaction behavior (12 pairs BTBR-BTBR, BTBR-FVB, FVB-FVB). Mean (± SEM) time spent engaged in negative social behavior (e.g., wrestling and biting) during the 30-minute session.
4. General Discussion
It is clear from these studies that inbred mouse strains can differ markedly in their levels of social behavior. This conclusion is in agreement with findings of previous studies [41,50]. Some strains (e.g., FVB) are very social, whereas others (e.g., A, BTBR) are not. Furthermore, some social behaviors, like sniffing, are common in all strains, although the time spent engaged in this behavior varies across strains. Other social behaviors (e.g., mounting of same-sex mouse) are relatively rare, at least in the strains that we tested. Thus, there do exist differences in social behavior that may be useful in the search for the genetic components of social behavior.
Of particular interest in these studies is the low level of social behavior of the BTBR mouse. Crawley and colleagues have also reported low levels of social behavior in both adult and juvenile BTBR mice [38,42]. This low level of social behavior does not correlate with low locomotor activity levels or high levels of anxiety [7,38,42], which may play a role in the low social activity in strains such as A/J [6,18,42]. Until recently the BTBR strain has been one of the least studied inbred strains. Originally, Mary Lyon crossed mice bearing the tufted mutation to mice from a stock carrying the T gene; the result was BTBR [48]. BTBR is most similar genetically to the various 129 substrains [48], but this may be the result of crossing the stock with one of the 129 strains for hardiness. Until a few years ago, BTBR was best known in its role as the parental strain for the mutant BTBR-Pahenu2 mouse, a model of phenylketonuria developed by germline ethylnitrosourea [52]. It wasn’t until the Mouse Phenome Project (MPP; http://jax.org/phenome), a coordinated effort of over a dozen laboratories to examine 40 inbred strains maintained by The Jackson Laboratory, that the behavioral genetics community took note of the BTBR strain. Initially, it was placed among the inbred strains in the category with top priority for testing, but was later dropped to lowest priority. Fortunately, some researchers had already started their studies with this mouse and had become intrigued with BTBR. The performance of BTBR mice on the accelerating rotorod is superior to many strains [48]. They display poor social learning in the transmission of food preference assay [38]; this learning deficit may be related to low levels of sniffing behavior. These mice also have noticeable abnormalities in brain structure. There is a virtual absence of the corpus callosum, and severe reduction of the hippocampal commissure [48]. Since a number of studies have reported corpus callosum deficits in autistics [11,15,28,54], this corpus callosum deficit in the BTBR strain makes it even more fascinating for further study as a possible mouse model of autism. We have recently mapped the corpus callosum deficiency seen in the BTBR mouse to two quantitative trait loci on the X Chromosome [32]. It is not yet known whether the genes on the X Chromosome that underlie these loci are related to autism, however, ongoing research in our laboratory is addressing that issue.
Acknowledgments
This work was supported by NIH grants MH067850 and MH068013 to Valerie Bolivar. The authors would like to thank Rene Macy and Alexis Santiago for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 2.Andres C. Molecular genetics and animal models in autistic disorders. Brain Res Bull. 2002;57:109–119. doi: 10.1016/s0361-9230(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 3.Asperger H. Autistic psychopathology in childhood. In: Frith U, editor. Autism and Asperger syndrome. Cambridge University Press; Cambridge: 1944. [Google Scholar]
- 4.Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SCR. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard DC, Blanchard RJ. Ethoexperimental approaches to the biology of emotion. Ann Rev Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- 6.Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: a survey of inbred strains and F1 hybrids. Behav Genet. 2000;30:285–293. doi: 10.1023/a:1026545316455. [DOI] [PubMed] [Google Scholar]
- 7.Bolivar VJ, Flaherty L. Assessing autism-like behaviors in inbred strains of mice. Society for Neuroscience Abstract. 2003;318.13 [Google Scholar]
- 8.Bolivar VJ, Flaherty L. Genetic control of novel food preference in mice. Mamm Genome. 2004;15:193–198. doi: 10.1007/s00335-003-2307-7. [DOI] [PubMed] [Google Scholar]
- 9.Bolivar VJ, Ganus JS, Messer A. Behavioral abnormalities in the motor neuron degeneration (mnd) mouse. Brain Res. 2002;937:74–82. doi: 10.1016/s0006-8993(02)02470-8. [DOI] [PubMed] [Google Scholar]
- 10.Bolivar VJ, Pooler O, Flaherty L. Inbred strain variation in contextual and cued fear conditioning behavior. Mamm Genome. 2001;12:651–656. doi: 10.1007/s003350020039. [DOI] [PubMed] [Google Scholar]
- 11.Brambilla P, Hardan A, Ucelli di Nemi S, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. 2003;61:557–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Bucan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet. 2002;3:114–23. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson Ml. Is infantile autism a hypoglutamatergic disorder? Relevance of glutamate-serotonin interactions for pharmacotherapy. J Neural Transm. 1998;105:525–535. doi: 10.1007/s007020050076. [DOI] [PubMed] [Google Scholar]
- 15.Cody H, Pelphrey K, Piven J. Structural and functional magnetic resonance imaging of autism. Int J Dev Neurosci. 2002;20:421–438. doi: 10.1016/s0736-5748(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 16.Cook EH, Leventhal B. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–611. [PubMed] [Google Scholar]
- 18.Cook MN, Williams RW, Flaherty L. Anxiety-related behaviors in the elevated zero-maze are affected by genetic factors and retinal degeneration. Behav Neurosci. 2001;115:468–476. [PubMed] [Google Scholar]
- 19.Crawley JN. What's wrong with my mouse? John Wiley & Sons, Inc; New York: 2000. p. 329. [Google Scholar]
- 20.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 21.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 22.Di-Cicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 24.Folstein S, Rutter M. Genetic influences and infantile autism. Nature. 1977;265:726–728. doi: 10.1038/265726a0. [DOI] [PubMed] [Google Scholar]
- 25.Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 26.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Aut Dev Disorders. 2003;33:365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- 27.Gerlai J, Gerlai R. Autism: a large unmet medical need and a complex research problem. Physiol Behav. 2003;79:461–470. doi: 10.1016/s0031-9384(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 28.Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55:1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- 29.Insel TR. Mouse models for autism: report from a meeting. Mamm Genome. 2001;12:755–7. doi: 10.1007/s00335-001-4006. [DOI] [PubMed] [Google Scholar]
- 30.Kanner L. Autistic disturbance of affective contact. Nerv Child. 1943;2:216–250. [Google Scholar]
- 31.Korvatska E, Van de Water J, Anders TF, Gershwin ME. Genetic and immunologic considerations in autism. Neurobiol Dis. 2002;9:107–25. doi: 10.1006/nbdi.2002.0479. [DOI] [PubMed] [Google Scholar]
- 32.Kusek G, Wahlsten D, Herron B, Bolivar VJ, Flaherty L. Localization of two new X-linked quantitative trait loci controlling corpus callosum size in the mouse. Genes Brain Behav. doi: 10.1111/j.1601-183X.2006.00264.x. (in press) [DOI] [PubMed] [Google Scholar]
- 33.Lamb JA, Moore J, Bailey A, Monaco AP. Autism: recent molecular genetic advances. Hum Mol Genet. 2000;9:861–868. doi: 10.1093/hmg/9.6.861. [DOI] [PubMed] [Google Scholar]
- 34.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 35.Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav. 2004;3:51–62. doi: 10.1046/j.1601-183x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 36.Maestrini E, Paul A, Monaco AP, Bailey A. Identifying autism susceptibility genes. Neuron. 2000;28:19–24. doi: 10.1016/s0896-6273(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 37.McFadyen MP, Kusek G, Bolivar VJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–9. doi: 10.1034/j.1601-183x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 38.McFarlane HG, Bolivar VJ, Crawley JN. Autism-like behaviors in BTBR T+tf/J mice, (in preparation) [Google Scholar]
- 39.Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Moy SS, Nadler JJ, Magnuson TR, Crawley JN. Mouse models of autism spectrum disorders: The challenge for behavior genetics. Am J Med Genet. 2006;142C:40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- 41.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 42.Moy SS, Nadler JJ, Young NB, Perez A, Hollowoy LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of ten inbred strains. Behav Brain Res. 2006 doi: 10.1016/j.bbr.2006.07.030. this volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 44.Nicolson R, Szatmari P. Genetic and neurodevelopmental influences in autistic disorder. Can J Psychiatr. 2003;48:526–537. doi: 10.1177/070674370304800804. [DOI] [PubMed] [Google Scholar]
- 45.Parmigiani S, Brain PF, Palanza P. Ethoexperimental analysis of different forms of intraspecific aggression in the house mouse (Mus musculus) In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental approaches to the study of behavior. Kluwer Academic Publishers; Dordrechi: 1989. pp. 418–431. [Google Scholar]
- 46.Powers MD. Children with autism: a parents' guide. Woodbine House; Bethesda: 2000. [Google Scholar]
- 47.Roubertoux PL, LeRoy I, Mortaud S, Perez-Diaz F, Tordjman S. Measuring aggression in the mouse. Handbook of molecular-genetic techniques for brain and behavior research. In: Crusio WE, Gerlai RT, editors. Techniques in the behavioral and neural sciences. Vol. 13. Elsevier; Amsterdam: 1999. pp. 696–709. [Google Scholar]
- 48.Rustay NR, Wahlsten D, Crabbe JC. Influence of task parameters on rotorod performance and sensitivity to ethanol in mice. Behav Brain Res. 2003;141:237–249. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- 49.Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of Reln gene dosage and social isolation. Behav Neurosci. 2003;117:1257–1275. doi: 10.1037/0735-7044.117.6.1257. [DOI] [PubMed] [Google Scholar]
- 50.Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2005;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 51.Seong E, Seasholtz AF, Burmeister M. Mouse models for psychiatric disorders. Trends Genet. 2002;18:643–650. doi: 10.1016/s0168-9525(02)02807-x. [DOI] [PubMed] [Google Scholar]
- 52.Shedlovsky A, McDonald JD, Symula D, Dove WF. Mouse models of human phenylketonuria. Genetics. 1993;134:1205–1210. doi: 10.1093/genetics/134.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweeten TL, Posey DJ, Shekhar A, McDougle CJ. The amygdala and related structures in the pathophysiology of autism. Pharmacol Biochem Behav. 2002;71:449–455. doi: 10.1016/s0091-3057(01)00697-9. [DOI] [PubMed] [Google Scholar]
- 54.Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drosts DJ, Williamson PC, Rajakumar N, Sui Y, Dutton RA, Toga AW, Thompson PM. Mapping corpus callosum deficits in autism: An index of aberrant cortical connectivity. Biol Psychiatry. 2006;60:218–25. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Wing L, Potter D. The epidemiology of autistic spectrum disorders. Ment Retard Dev Disabil Res Rev. 2002;8:151–161. doi: 10.1002/mrdd.10029. [DOI] [PubMed] [Google Scholar]


