Abstract
The laboratory rat has been used extensively in auditory research but has had limited use in cochlear implant related research due mainly to the surgically restricted access to the scala tympani. We have developed a new surgical method for cochlear implantation in rats. The key to this protocol was cauterizing the stapedial artery (SA) and making a small cochleostomy near the round window in order to enlarge the surgical access to the scala tympani. Five normal hearing Hooded Wistar rats were used to investigate the effect of cauterizing the SA on hearing and auditory nerve survival. Results showed that cauterizing the SA was surgically feasible, afforded excellent exposure of the round window niche for cochleostomy, and did not adversely affect acoustic thresholds measured electrophysiologically. Moreover, there was no difference in spiral ganglion cell densities for any cochlear turn when compared with the contralateral control ears. Three deafened rats were subsequently implanted with a scala tympani electrode array using this new surgical approach. Electrically evoked auditory brainstem responses using bipolar stimulation, and subsequent cochlear histopathology demonstrated that the cochlear implantation using a custom-made rat electrode array was safe and effective. The surgical approach presented in this paper presents a safe and effective procedure for acute or chronic cochlear implantation in the rat model.
Keywords: Cochlear histopathology, cochlear implant, electrically-evoked auditory brainstem response, stapedial artery
1. Introduction
Research using experimental animals has played an important role in both the development and continued improvement of cochlear implants. Various animal models have been used to conduct this research, including mouse (Steel and Bock, 1984), gerbil (Hessel et al., 1997; Kadner and Scheich, 2000; Ryan et al., 1990), cat (Leake et al., 1995; Xu et al., 1997; Klinke et al., 1999; Smith et al., 1994), guinea pig (Miller et al., 1983, 1993, 2000; Shepherd et al., 2002; Shepherd et al., 2005), and primates (Parkins, 1989; Pfingst et al., 1995; Pfingst et al., 1979; Shepherd et al., 1995). The development of a rat model of cochlear implantation - given the low costs of this species, its low mortality rate following surgical intervention, the extensive auditory research already performed, and its applicability for molecular biological/proteomics research - is attractive, particularly in a chronic application.
Compared with other animal models, cochlear implantation in the rat is considered surgically difficult, and while there are a number of acute studies in this species (Nagase et al., 2000; Wu et al., 2003; Hsu et al., 2001; Vischer et al., 1997; Paolini and Clark, 1998; Shepherd et al., 2004), there has been no chronic cochlear implantation reported. The reasons for this difficulty includes: i) relatively smaller size of the rat cochlea; ii) the difficulty in developing such a small multiple channel scala tympani electrode array; and iii) the presence of the stapedial or pterygopalatine artery (SA), located in the middle ear cavity (Govaerts et al., 1993; Judkins and Li, 1997; Praetorius et al., 2001; Pinilla et al., 2001; Yamamoto et al., 2003), severely reducing surgical access to the scala tympani. Moreover, the majority of reports that described cochlear implantation in the rat used a single 75μm (dia.) platinum /iridium wire monopolar electrode. Monopolar electrical stimulation of the cochlea in laboratory animals can readily evoke non-auditory neural activity, severely restricting the useful dynamic range over which electrical stimulation of the auditory nerve, can be achieved (e.g. Xu et al., 1997). The objective of the present research is to describe a bipolar electrode array and a revised surgical technique for chronic cochlear implantation in the rat, and to present studies designed to demonstrate the safety and effectiveness of this procedure.
Anatomical knowledge of the middle and inner ear of rat is vital for this research. Description of the surgical anatomy of the middle ear of the rat has been published (Albiin et al., 1983; Albiin et al., 1985; Hellstrom et al., 1982); Judkins and Li, 1997), however, there is no report describing this anatomy with reference to cochlear implantation. In a preliminary study, we found that the SA blocks a large area of the round window (RW) and therefore obstructs electrode insertion through this structure (Fig. 1, 2). We therefore developed a new surgical approach for cochlear implantation in this species that included cauterizing the SA and performing a cochleostomy to enlarge the RW in order to provide sufficient access to the scala tympani (Fig. 2). We sought to assess the feasibility, safety and effectiveness of this surgical approach in the rat giving particular attention to the preservation of hearing and inner ear structures.
Fig. 1.
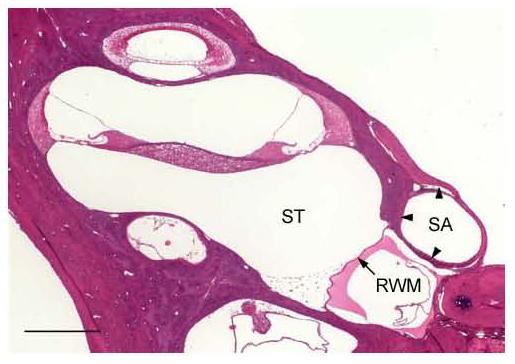
Histological section of the rat cochlea at the RW level showing the SA and its bony canal (arrowheads) nearly obstructing the entire RW promontory. SA – stapedial artery; RWM – round window membrane; ST – scala tympani, Scale bar = 0.5 mm.
Fig. 2.
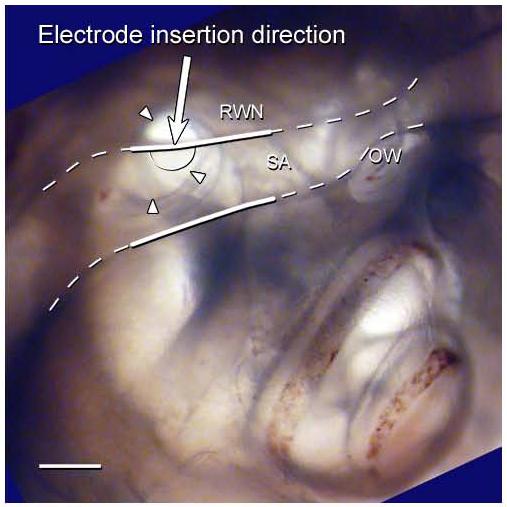
A photograph of right cochlea of a rat embedded in resin and orientated to represent the surgical view. The SA and its bony canal (white dashed line) lie partially over the RWN (arrow heads), severely restricting surgical access to the RW. The white solid lines showing the region of the SA cauterised in the surgical procedure described in this paper. The proposed cochleostomy site is shown on this figure (semi-circle solid line). The arrow represents the direction of electrode insertion. RWN – round window niche; SA – stapedial artery; OW – oval window site; Scale bar = 0.5 mm.
2. Materials and Methods
2.1. Study 1 – The effect of cauterizing the stapedial artery on hearing status and spiral ganglion cell survival
2.1.1. Surgical procedure for cauterizing the stapedial artery
Five normal hearing Hooded Wistar rats (250-350g) with normal tympanic membranes were used in this study. Hearing status was confirmed using Auditory Brainstem Responses (ABRs; see 2.1.2 below). Animals were anesthetized by a single intra-peritoneal (i.p.) injection of ketamine (75mg/kg) and xylazine (10mg/kg). They were then placed on a heating pad and maintained at 37 ±1°C. Using sterile surgical techniques, a post-auricular incision was made on the right side following application of local anaesthetic (Lignocaine, 4mg subcutaneously). A well-defined sternocleidomastoid muscle and facial nerve, extending anteriorly above the bulla, were easy identified. The bony bulla was exposed, and the dorsal region drilled using a high-speed cutting bur, providing a clear view onto the SA with its medial margin laying over the edge of the round window niche and coursing anterior-superiorly towards the oval window. With the aid of a Zeiss OPMI-1 operating microscope using a x16 objective, the SA was carefully cauterized using 100 mm Jeweller's forceps (300-800-C; Trewavis Surgical Instruments, Australia) and a bipolar coagulator (Model MF1, Zencor Pty. Ltd., Australia). The wound was sutured in two layers. Identical sham surgery was performed on the contralateral side, except that the SA was kept intact.
2.1.2. Hearing status
The hearing status of each animal was evaluated using click and frequency-specific ABRs prior to surgery, and three weeks following surgery. The ABR procedure used to evaluate hearing status has previously been described in detail for cat (Xu et al. 1997) and rat (McGuinness and Shepherd, 2005). Briefly, animals were anaesthetized with a single i.p. injection of 75mg/kg ketamine and 10mg/kg xylazine in a sound-attenuated, electrically shielded Faraday room. An otoscopic examination was performed to ensure that the external auditory canal and tympanic membrane were free from infection or perforation. Computer generated, 100μs duration rarefaction clicks or tone-pips (1-msec rise/fall time, 3-msec plateau) at frequencies of 0.5, 1, 2, 4, 8, and 16 kHz were fed to a Richard Allen DT-20 loudspeaker placed 10cm from the ipsilateral ear. The contralateral ear was plugged with an ear-mould compound (OTOFORM-K/c; Dreve-Otoplastik GMBH, Unna, Germany). The system was calibrated so that the maximum sound level of the click or tone pips generated by the loudspeaker at the pinna was determined for each stimulus. ABRs were then recorded using sterile stainless steel electrodes at the vertex (positive) and the neck (negative). Responses were amplified by a factor of 105, and band-pass filtered using a Krohn Hite 3750 R (high-pass 150 Hz, 24 dB/octave; low pass 3 kHz, 6 dB/octave). The filter output was fed to a 10 bit analogue-to-digital converter and sampled at 20 kHz for a period of 12.5 ms following the onset of the click stimulus. The stimulus level was varied in 5dB increments and two recordings were made at each level. Threshold was defined as the first visual detected response consistent in both recordings within the wave III latency window (2.5-3.5 ms). ABR audiograms were constructed from those thresholds over a frequency range of 0.5-16 kHz.
All results were presented as means ± SEM. The ABR data was subject to statistical analysis using two-tailed t-test (Sigma Stat, 2000). The 0.05 level of probability was used as the criterion of statistical significance. The mean ABR audiograms of both pre-operative and 3 weeks post-operative were plotted against frequency.
2.1.3. Spiral ganglion cell densities
Upon completion of the 3 week postoperative ABR, each animal was killed by an overdose of sodium pentobarbitone and perfused intra-cardially with 100ml of 0.9% saline solution at 37C° (containing heparin, 1000 units/kg and 0.3 ml of 10% sodium nitrite), followed by 100 ml of paraformaldehyde in 0.1M phosphate buffer at 4C° (pH 7.4). The temporal bones were harvested and the oval and round windows were opened to allow penetration of the fixative over night. Temporal bones were then decalcified (4% EDTA and 2.5% glutaraldehyde, buffered to a pH of 7.35 with 0.1 M phosphate buffer), trimmed, embedded in Spur's resin and sectioned at a thickness of 2μm in the horizontal plane. Sections were collected every 60 μm and stained with haematoxylin and eosin.
Spiral ganglion cell density within RC was measured for the lower basal, upper basal, lower middle and upper middle turns in four randomly selected mid-modiolus sections for each cochlea using techniques described in detail previously (Xu et al., 1997; McGuinness and Shepherd, in press). The area of RC was measured using NIH Image software (http://rsb.info.nih.gov/nih-image/). The number of SGCs with a clear nucleus within Rosenthal's canal was counted and SGC density was calculated (cells/mm2) on both sides and compared statistically.
2.2. Study 2 - Safety and efficiency of cochlear implantation in the rat
Three normal hearing adult hooded Wister rats were deafened and acutely implanted with our custom-made rat electrode assembly using the surgical techniques described above. The effectiveness of cochlear implantation in the rat was evaluated by recording electrically evoked ABRs (EABRs; see 2.2.3 below), while the safety of the procedure was assessed by histologically examining the implanted cochleae for evidence of electrode insertion trauma.
2.2.1. Deafening technique
Animals were anaesthetized with ketamine and xylazine as described above, and received an intravenous injection (via a tail vein) of the loop diuretic frusemide (175mg/kg) followed immediately by gentamicin (350mg/kg) subcutaneously (McGuinness and Shepherd, 2005).
2.2.2. Rat electrode assembly
The electrode assembly used in the rat was modified from our custom-made animal electrode assembly for cat and guinea pig (Xu et al., 1997; Shepherd and Xu, 2002). It consisted of an intra-cochlear electrode array, a connector and lead wires (Fig. 3). The electrode array was fabricated using injection-moulding techniques. Three platinum (Pt) ring electrodes, each 0.3 mm in width, with an inter-separation of 0.45 mm, were located near the tip of a silicone carrier (Dow Corning Medical Grade Elastomer MDX4-4210; Factor II, AZ USA). As the rat scala tympani is much smaller than that of cat and guinea pig, the size of the intra-cochlear electrodes was reduced to a diameter of 0.31 mm at the tip of the array, tapering to 0.35 mm (the 3rd ring from tip). Teflon-insulated Pt/Ir (90/10) wire (25 μm in diameter, MEDWIRE Sigmund Cohn Corp. NY, USA) was welded to each Pt ring. Three ‘dummy’ Pt rings were added to the distal region of the array to increase the stiffness for ease of insertion. Each Pt wire from the electrode array was connected to a Teflon-insulated multi-stranded stainless steel leadwire (Cooner Wire, Inc., CA, USA) encapsulated by silicone rubber (MDX4-4210 filled Dow Corning Silastic© Laboratory Tubing). The leadwire system provides external access to the electrodes for stimulation.
Fig. 3.
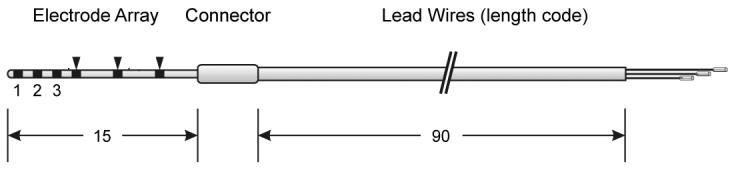
Diagram illustrating the rat electrode assembly. All dimensions are in mm. Three active electrodes (1, 2, 3) located near the tip of a silicone carrier. The array taped from a diameter of 0.31 mm at the tip to 0.35 mm at the third ring. Dummy Pt rings are illustrated by arrowhead.
The surgical technique followed that outlined in section 2.1.1. After cauterizing the SA, a cochleostomy was performed with a hand drill incorporating an implant quality stainless steel trocar Kirschner Wire (d=0.8mm) with a three-flanged sharp tip (Serial No. 232273; E. H. Stoerk Instrumente, Emmingen-Liptingen, Germany) at the edge of the bony canal of the SA over the round window promontory (Fig. 2). Bone chips were removed where possible, and the electrode array was then carefully inserted into the scala tympani (Fig 4). The opening of the cochleostomy was sealed with muscle. For chronic applications, the connector is fixed in the bulla using bone cement (Durelon®, ESPE Dental AG, Germany) and the leadwire assembly fixed to the skull using polyethylene mesh (Lars Mesh, Meadox Medicals, NJ, USA).
Fig. 4.
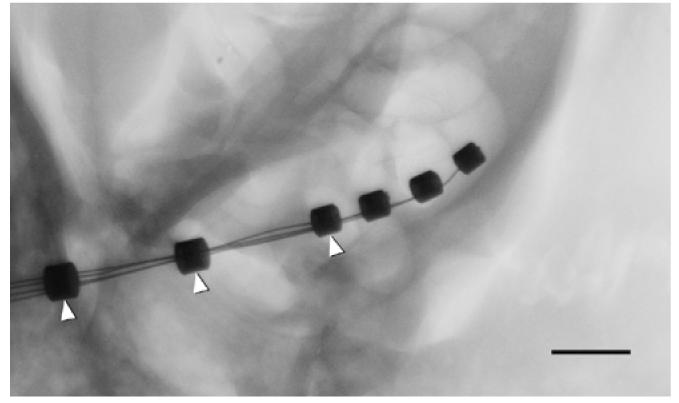
Micro-focus radiograph showing an electrode array (three active rings) inserted into basal turn of rat cochlea. Dummy rings, added to strengthen the distal part of the electrode array, are indicated by arrowheads. Scale bar = 1 mm.
2.2.3. Electrically evoked ABRs (EABRs)
Immediately following implant surgery, EABRs were recorded in response to bipolar electrical stimulation as described in detail previously (Xu et al. 1997, Hardie & Shepherd, 1999). Briefly, EABRs were recorded differentially using stainless-steel needle electrodes (vertex positive; neck negative; thorax ground). Optically-isolated biphasic current pulses (50 or 100 µs per phase; 10 μs interphase gap) were generated under computer control and delivered to the intracochlear electrode array. The recording conditions were identical to that used with ABRs with the addition of a sample-and-hold amplifier placed before the filter in order to remove stimulus artefact. Threshold was defined as the smallest current level required to evoke a peak-trough response amplitude of >0.25 μV for wave III of the EABR, i.e. within a latency window of 1.5-2.5 ms following stimulus onset for both responses. During recording the animal's temperature was maintained at 37.0 ± 1°C. EABRs were recorded in an electrically shielded room. Following EABR recording, the animals were sacrificed and the cochleae were harvested for histological evaluation as described above in order to assess the degree of trauma caused by the surgical procedure.
The care and use of animals reported in this study were approved by the Royal Victorian Eye and Ear Hospital's Animal Research Ethics Committee (project number 03/097A). All experiments were conducted in accordance with the Australian National Health and Medical Research Council's animal experimental guidelines.
3. Results
3.1. The effect of cauterizing of the stapedial artery (SA) on hearing status
3.1.1. General health status
While all animals lost body weight following anaesthetics and surgery, their weight increased to pre-surgical levels over the three-week recovery period. There were no abnormal signs observed in feeding, balance or general behaviour observed following surgery.
3.1.2. Hearing status following cauterization of the SA
All cochleae exhibited normal click and tone pip evoked ABR waveform morphology both pre- and post-cauterizing surgery. Post-operative ABR thresholds were maintained at the pre-operative level for all frequencies tested (Fig 5). There was no statistically significant difference in ABR threshold between pre- and post- cauterization (p>0.05).
Fig. 5.
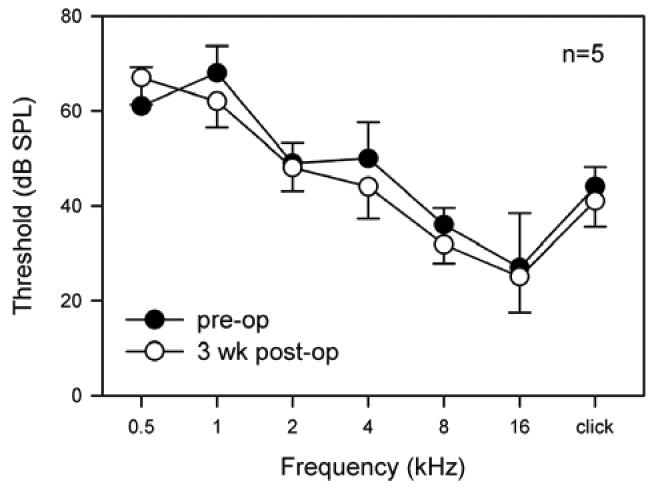
Mean pre-operative (solid circles) and 3 weeks post-operative (open circles) ABR thresholds (±SEM) plotted against stimulus frequency (n=5).
3.1.3. Cochlear histopathology following cauterization of the SA
There was no evidence of histological change to cochlear structures when comparing the right cochleae (SA cauterized) with the left control cochleae (Fig. 6). Furthermore, SGCs in the cochleae showed no evidence of degeneration (e.g. shrunken somata, hyperpigmented nuclei and the presence of large vacuoles). Finally, there was no statistically significant difference in mean spiral ganglion cell densities across the five rats between the SA cauterized side and the control side (p>0.05) when examined three weeks following surgery (Fig. 7).
Fig. 6.
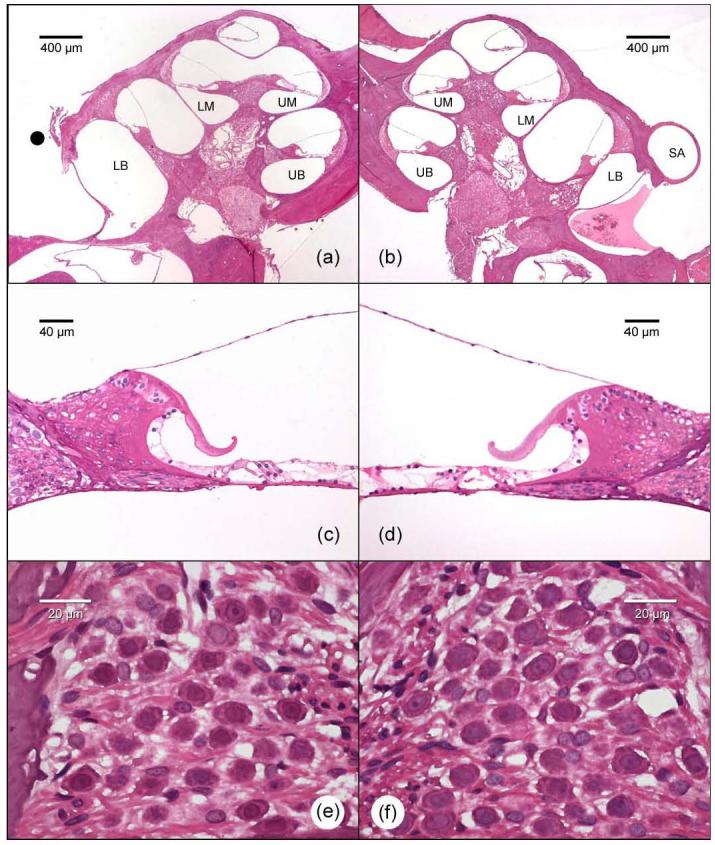
Representative low power histological images of the rat cochleae showing the normal intra-cochlear structures on a right cochlea where the SA has been cauterized (a) and its left control cochlea (b). Photomicrographs of the upper basal turns illustrating the presence of hair cells in both the SA cauterized (c) and control cochlea (d). Higher power photomicrographs illustrating well preserved SGCs in Rosenthal's canal of the upper basal turn on the SA cauterized side (e) and its contralateral control side (f). The black circle represents the site where the SA has been cauterized. LB – lower basal turn; UB – upper basal turn; LM – lower middle turn; UM – upper middle turn; SA – stapedial artery.
Fig. 7.
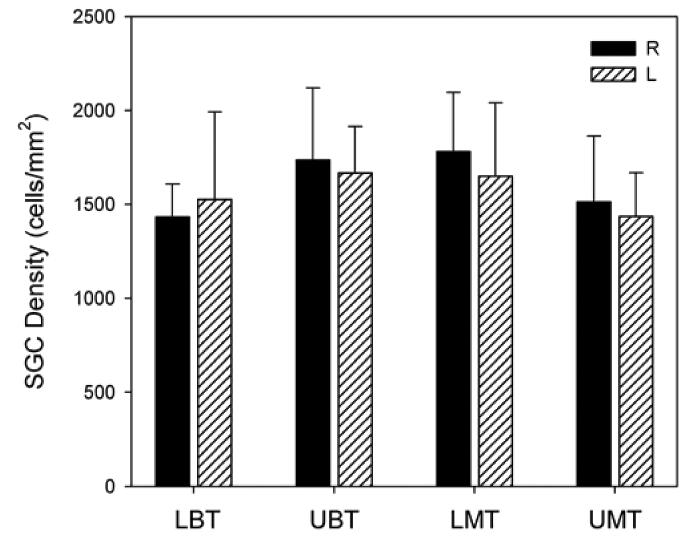
Comparison of mean spiral ganglion cell densities for all 5 rats in this study. The results illustrated that there was no overall statistically significant difference between the right side (SA cauterised, solid bar) and the left side (control side, dashed bar) in lower basal turn (LBT), upper basal turn (UBT), lower middle turn (LMT) and upper middle turn (UMT).
3.2. Safety and efficiency of cochlear implantation in the rat
EABRs could be readily recorded following implant surgery. The EABRs appeared to have normal morphology for this species (Fig. 8) and confirmed the functional status of the cochlear implant. The mean EABR threshold for bipolar pair 1/3 using 50 ms/phase biphasic current pulses was 87.5 μA (SD=17.6 μA; n=3).
Fig. 8.
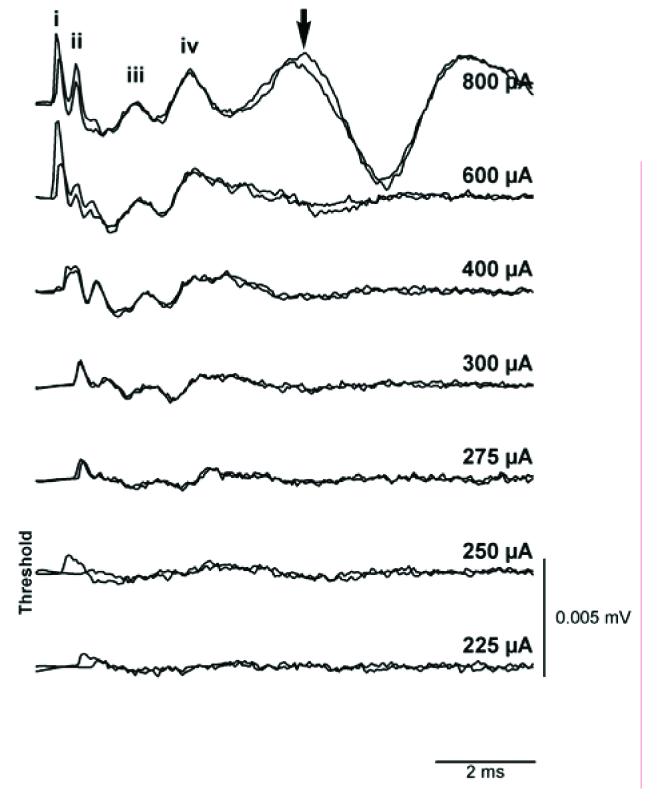
Representative EABRs evoked by 100μs biphasic current pulses delivered to bipolar scala tympani electrodes in a deafened rat. Two responses were recorded at each current level. An arrow indicates the presence of myogenic activity at a high stimulus intensity. Waves i-iv have been identified, although wave i has been partially blanked by the technique used to remove the stimulus artefact.
Intra-cochlear trauma caused by the cochleostomy and cochlear implantation was assessed by histological observation. There was no evidence of intra-cochlear structural damage in two of the three implanted cochleae (Fig. 9). One implanted cochlea exhibited a localized tear in the basilar membrane in the lower basal turn that was presumably associated with the electrode insertion.
Fig. 9.
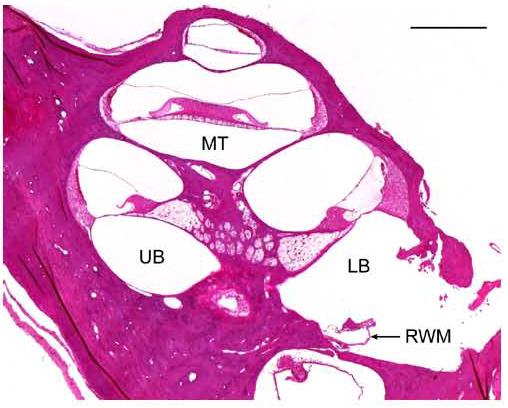
Low power histological image of one implanted rat cochlea showing intact intra-cochlear structures. RWM was ruptured for electrode insertion. Note the loss of SGCs associated with the gentamicin/ frusemide deafening procedure. RWM – round window membrane, LB – lower basal turn; UB – upper basal turn; MT – middle turn, Scale bar = 0.5 mm.
4. Discussion
The revised surgical approach presented in this paper presents a safe and effective technique for cochlear implantation successfully in a small animal model such as the rat. The key technique in our surgical approach is cauterizing the SA and then performing a cochleostomy in order to obtain reasonable surgical access to the scala tympani. We have successfully used this approach for both acute and chronic cochlear implantation in the rat.
What are the pathological implications of cauterizing the SA? In humans, the SA is in most cases obliterated as soon as the internal carotid artery is developed; in rare cases the SA remains persistent (Govaerts, et al., 1993; Araujo, et al., 2002; Yamamoto et al., 2003). A clinical study has demonstrated that a persistent stapedial artery in the postnatal human no longer forms the main blood supply to any important structure in the head, and injury to the artery is not likely to have any consequences except bleeding, which can be adequately dealt with (Govaerts et al., 1993). In contrast to humans, the SA persists in a number of animals, particularly rodents such as the rat, mouse, and gerbil. It rises from the internal carotid artery (Hellstrom, 1998), and enters the tympanic cavity ventrally, runs along the base of the cochlea and through the crura of the stapes (Albiin et al., 1985; Govaerts et al, 1993; Hebel and Stromberg, 1986). Many authors have stated that the artery must be preserved in order to preserve hearing or even survival of the animal (Praetorius et al., 2001; Pinilla et al., 2001; Yamamoto et al., 2003). In our investigation, the animals not only survived cauterizing the SA, but cochlear structures ipsilateral to the cauterized SA, appeared well preserved (Fig. 6). The hearing status of these animals showed no statistically significant change in ABR threshold following cauterization in the frequency range from 0.5 to 16 kHz (Fig. 5). Due to equipment limitations, we were unable to test the hearing at higher frequencies (the frequency range of normal hearing in rat is 1,000 - 50,000 Hz, Sohmer and Friedman, 1992), and therefore cannot dismiss the possibility of the procedure resulting in a high frequency hearing loss. However, Emadi and colleagues (Emadi et al., 2004) reported no significant hearing loss in gerbils using compound action potential thresholds over a frequency range of 1.6-50 kHz following removal of the SA. Importantly for cochlear implant research, the SGC density remained unchanged in all cochlear turns, including the lower basal turn, in the SA cauterised side compared with the contralateral control side.
In the rat, the blood supply to the orbit comes almost exclusively via the SA, which gives rise to the ophthalmic and the lacrimal arteries (Hebel and Stromberg, 1986). Therefore, cauterising the SA may raise a concern regarding the function of the eye ipsilateral to the procedure. In our studies, there was no evidence of a visual deficit in any treated animal although we did not examine this organ histologically.
The diameter of the electrode array used in the present study was considerably smaller than our previous designs in order to accommodate the rat scala tympani (0.31 mm at tip; Fig 3). We are able to insert three active electrodes into the scala tympani of the basal turn in rats (Fig 4) thereby allowing bipolar stimulation of the auditory nerve. While the rat electrode array was functionally viable as evidenced by the recording of EABRs, at elevated current levels non-auditory myogenic activity was evident. Excitation of non-auditory structures is likely to be a far more serious problem using monopolar electrodes, as they are known to readily evoked myogenic activity in larger animal models (e.g. cat; Xu et al, 1997), limiting the useful dynamic range. Hsu et al. (2001) reported that they inserted a single platinum-iridium wire of 0.1mm diameter to a depth of 2-3 mm through a small perforation (<0.5mm) drilled into the otic capsule at the cochlear apex or base in order to achieve monopolar stimulation. This procedure may increase the risk of inserting the electrode into the scala media or scala vestibuli, and would only be viable for acute experiments.
There are a number of other important points that must be considered when developing this form of surgery in the rat. First, the facial nerve in the middle ear of the rat is in a more superficial, anterior-rostral position and is less protected when compared with the cat and guinea pig. Care to avoid injury during surgery is important because paralysed facial muscle may adversely affect the animal's general health. Second, when initially developing the surgery, bleeding of SA was our major concern. SA bleeding could be avoided by: (1) repeating the cauterizing procedure to ensure that the SA was completely occluded and the underlying bony canal was clearly exposed; (2) avoiding the use of suction near the SA; (3) the use of fine surgical instruments for cauterizing the SA and performing the cochleostomy. Moreover, the use of a three-flanged hand drill manufactured from quality stainless steel wire, was an effective way to make a small cochleostomy safely (see 2.2.2.). Finally, localized damage to the basilar membrane in the lower basal turn can occur during cochleostomy near the RW. It is important to make the cochleostomy as posterior as possible to reduce the risk of intracochlear trauma.
In conclusion, the surgical technique presented in this paper presents a safe and effective procedure for acute or chronic cochlear implantation in the rat model. Moreover, this technique will have relevance to other small rodents that posses a SA and have limited surgical access to the cochlea including the gerbil and mouse.
Acknowledgements
This work was funded by a grant from The United States National Institutes of Health (contract NIH-N01-DC-0-2109) and China Scholarship Council (CSC No. 22841039). We are grateful to Stephanie Epp, Anne Coco and Jenny Hardman for their expert research assistance, Helen Feng for design and fabricating rat electrode, Maria Clarke and Prudence Nielsen for histological support, Dr. Sue Pierce and Elisa Borg for veterinary advice and animal maintenance, Rodney Millard for engineering assistance, David Lawrence for photographic assistance, Benjamin Wei MBBS for surgical advice, and Cochlear Limited for supporting electrode manufacturing.
Footnotes
Supporting grants: This work was funded by a grant from The United States National Institutes of Health (contract NIH-NO1-DC-3-1005). Dr. Wei Lu was sponsored by the China Scholarship Council (CSC No. 22841039).
References
- Albiin N, Hellstrom S, Salen B, Stenfors LE. The stapedial artery in the rat. A microscopical study under normal conditions and in otitis media with effusion. Acta Anat (Basel) 1983;115:134–40. [PubMed] [Google Scholar]
- Albiin N, Hellstrom S, Salen B, Stenfors LE, Wirell S. The vascular supply of the rat tympanic membrane. Anat Rec. 1985;212:17–22. doi: 10.1002/ar.1092120103. [DOI] [PubMed] [Google Scholar]
- Araujo MF, Oliveira CA, Sesana WE. Radiology quiz case 1: persistent stapedial artery, left ear. Arch Otolaryngol Head Neck Surg. 2002;128:456–458. [PubMed] [Google Scholar]
- Emadi G, Richter CP, Dallos P. Stiffness of the gerbil basilar membrane: radial and longitudinal variations. J Neurophysiol. 2004;91:474–88. doi: 10.1152/jn.00446.2003. [DOI] [PubMed] [Google Scholar]
- Govaerts PJ, Marquet TF, Cremers WR, Offeciers FE. Persistent stapedial artery: does it prevent successful surgery? Ann Otol Rhinol Laryngol. 1993;102:724–8. doi: 10.1177/000348949310200914. [DOI] [PubMed] [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–65. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. Anatomy and Embryology of the Laboratory Rat. Biomed Verlag; Wörthsee: 1986. pp. 102–5. [Google Scholar]
- Hellstrom S, Salen B, Stenfors LE. Anatomy of the rat middle ear. A study under the dissection microscope. Acta Anat (Basel) 1982;112:346–52. doi: 10.1159/000145527. [DOI] [PubMed] [Google Scholar]
- Hellstrom SO. Surgical anatomy of the rat middle ear. Otolaryngol Head Neck Surg. 1998;119:556–8. doi: 10.1016/S0194-5998(98)70127-5. [DOI] [PubMed] [Google Scholar]
- Hessel H, Ernst LS, Walger M, von Wedel H, Dybek A, Schmidt U. Meriones unguiculatus (Gerbil) as an animal model for the ontogenetic cochlear implant research. Am J Otol. 1997;18:S21. [PubMed] [Google Scholar]
- Hsu WC, Campos-Torres A, Portier F, Lecain E, Van Den Abbeele T, De Waele C, Tran Ba Huy P. Cochlear electrical stimulation: influence of age of implantation on Fos immunocytochemical reactions in inferior colliculi and dorsal cochlear nuclei of the rat. J Comp Neurol. 2001;438:226–38. doi: 10.1002/cne.1311. [DOI] [PubMed] [Google Scholar]
- Judkins RF, Li H. Surgical anatomy of the rat middle ear. Otolaryngol Head Neck Surg. 1997;117:438–47. doi: 10.1016/S0194-59989770011-1. [DOI] [PubMed] [Google Scholar]
- Kadner A, Scheich H. Trained discrimination of temporal patterns: cochlear implants in gerbils. Audiol Neurootol. 2000;5:23–30. doi: 10.1159/000013862. [DOI] [PubMed] [Google Scholar]
- Klinke R, Kral A, Heid S, Tillein J, Hartmann R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science. 1999;285:1729–33. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–62. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otology & Neurotology. doi: 10.1097/01.mao.0000185063.20081.50. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK. Characterization of wave I of the electrically evoked auditory brainstem response in the guinea pig. Hear Res. 1993;69:35–44. doi: 10.1016/0378-5955(93)90091-e. [DOI] [PubMed] [Google Scholar]
- Miller AL, Morris DJ, Pfingst BE. Effects of time after deafening and implantation on guinea pig electrical detection thresholds. Hear Res. 2000;144:175–86. doi: 10.1016/s0378-5955(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Miller JM, Duckert LG, Malone MA, Pfingst BE. Cochlear prostheses: stimulation-induced damage. Ann Otol Rhinol Laryngol. 1983;92:599–609. doi: 10.1177/000348948309200614. [DOI] [PubMed] [Google Scholar]
- Nagase S, Miller JM, Dupont J, Lim HH, Sato K, Altschuler RA. Changes in cochlear electrical stimulation induced Fos expression in the rat inferior colliculus following deafness. Hear Res. 2000;147:242–50. doi: 10.1016/s0378-5955(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Paolini AG, Clark GM. Intracellular responses of the rat anteroventral cochlear nucleus to intracochlear electrical stimulation. Brain Res Bull. 1998;46:317–27. doi: 10.1016/s0361-9230(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Parkins CW. Temporal response patterns of auditory nerve fibers to electrical stimulation in deafened squirrel monkeys. Hear. Res. 1989;41:137–168. doi: 10.1016/0378-5955(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Morris DJ, Miller AL. Effects of electrode configuration on threshold functions for electrical stimulation of the cochlea. Hear Res. 1995;85:76–84. doi: 10.1016/0378-5955(95)00037-5. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Donaldson JA, Miller JM, Spelman FA. Psychophysical evaluation of cochlear prostheses in a monkey model. Ann Otol Rhinol Laryngol. 1979;88:613–25. doi: 10.1177/000348947908800505. [DOI] [PubMed] [Google Scholar]
- Pinilla M, Ramirez-Camacho R, Jorge E, Trinidad A, Vergara J. Ventral approach to the rat middle ear for otologic research. Otolaryngol Head Neck Surg. 2001;124:515–7. doi: 10.1067/mhn.2001.115370. [DOI] [PubMed] [Google Scholar]
- Praetorius M, Limberger A, Muller M, Lehner R, Schick B, Zenner HP, Plinkert P, Knipper M. A novel microperfusion system for the long-term local supply of drugs to the inner ear: implantation and function in the rat model. Audiol Neurootol. 2001;6:250–8. doi: 10.1159/000046130. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Miller JM, Wang ZX, Woolf NK. Spatial distribution of neural activity evoked by electrical stimulation of the cochlea. Hear. Res. 1990;50:57–70. doi: 10.1016/0378-5955(90)90033-l. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Clark GM, Xu SA, Pyman BC. Cochlear pathology following reimplantation of a multichannel scala tympani electrode array in the macaque. Am J Otol. 1995;16:186–199. [PubMed] [Google Scholar]
- Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172:92–8. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Roberts LA, Paolini AG. Long-term sensorineural hearing loss induces functional changes in the rat auditory nerve. Eur J Neurosci. 2004;20:3131–40. doi: 10.1111/j.1460-9568.2004.03809.x. [DOI] [PubMed] [Google Scholar]
- Shepherd R, Coco A, Epp S, Crook JM. Chronic depolarization enhances the trophic effects of BDNF in rescuing auditory neurons following a sensorineural hearing loss. J. Comp. Neurol. doi: 10.1002/cne.20564. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DW, Finley CC, van den Honert C, Olszyk VB, Konrad KE. Behavioral and electrophysiological responses to electrical stimulation in the cat. I. Absolute thresholds. Hear Res. 1994;81:1–10. doi: 10.1016/0378-5955(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Sohmer H, Friedman I. Prolonged conductive hearing loss in rat pups causes shorter brainstem transmission time. Hear Res. 1992;61:189–96. doi: 10.1016/0378-5955(92)90050-w. [DOI] [PubMed] [Google Scholar]
- Steel KP, Bock GR. Electrically-evoked responses in animals with progressive spiral ganglion degeneration. Hear Res. 1984;15:59–67. doi: 10.1016/0378-5955(84)90225-9. [DOI] [PubMed] [Google Scholar]
- Swearengen JR, Kittell CL, Davis JA, Raslear TG, Beblo DA, Colleton CA. A surgical technique for bilateral cochleotomy in the Long-Evans rat. J Invest Surg. 1993;6:431–7. doi: 10.3109/08941939309141630. [DOI] [PubMed] [Google Scholar]
- Vischer M, Haenggeli A, Zhang J, Pelizzone M, Hausler R, Rouiller EM. Effect of high-frequency electrical stimulation of the auditory nerve in an animal model of cochlear implants. Am J Otol. 1997;18:S27–9. [PubMed] [Google Scholar]
- Wu HC, Lecain E, Chiappini I, Yang TH, Tran Ba Huy P. Influence of auditory deprivation upon the tonopic organization in the inferior colliculus: a Fos immunocytochemical study in the rat. Eur J Neurosci. 2003;17:2540–52. doi: 10.1046/j.1460-9568.2003.02691.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear Res. 1997;105:1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Tominaga M, Sone M, Nakashima T. Contribution of stapedial artery to blood flow in the cochlea and its surrounding bone. Hear Res. 2003;186:69–74. doi: 10.1016/s0378-5955(03)00310-1. [DOI] [PubMed] [Google Scholar]


