Abstract
The development and maintenance of spiral ganglion neurons (SGNs) appears to be supported by both neural activity and neurotrophins. Removal of this support leads to their gradual degeneration. Here, we examine whether the exogenous delivery of the neurotrophin brain-derived neurotrophic factor (BDNF) in concert with electrical stimulation (ES) provides a greater protective effect than delivery of BDNF alone in vivo. The left cochlea of profoundly deafened guinea pigs was implanted with an electrode array and drug delivery system. BDNF or artificial perilymph (AP) was delivered continuously for 28 days. ES induced neural activity in two cohorts (BDNF/ES and AP/ES) while control animals received BDNF or AP without ES (BDNF/- and AP/-). The right cochleae of each animal served as deafened untreated controls. Electrically-evoked auditory brainstem responses (EABRs) were recorded immediately following surgery and at completion of the drug delivery period. AP/ES and AP/- cohorts showed an increase in EABR threshold over the implantation period while both BDNF cohorts exhibited a reduction in threshold (P < 0.001, t-test). Changes in neural sensitivity were complemented by significant differences in both SGN survival and soma area. BDNF cohorts demonstrated a significant trophic or survival advantage and larger soma area compared with AP-treated and deafened control cochleae; this advantage was greatest in the base of the cochlea. Importantly, ES significantly enhanced the survival effects of BDNF throughout the majority of the cochlea (P < 0.05, Bonferroni's test), while there was no evidence of trophic support provided by ES alone. Co-treatment of SGNs with BDNF and ES provide a substantial functional and trophic advantage; this treatment may have important implications for neural prostheses.
Keywords: neural degeneration, neurotrophin, deafness, electrical stimulation, cochlear implant, neural prostheses
Introduction
The development and maintenance of spiral ganglion neurons (SGNs), the primary afferent neurons of the cochlea, appears to be regulated via a complex combination of neural activity and neurotrophic support from a variety of sources (Fritzsch et al., 1999; Hansen et al., 2001; Hegarty et al., 1997). Removal of this support leads to their degeneration (Fritzsch et al., 1999; Schimmang et al., 2003; Ylikoski et al., 1993).
During development, SGNs receive neurotrophic support, including brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) from both the sensory hair cells of the organ of Corti and target neurons within the cochlear nucleus (Fritzsch et al., 1999; Lefebvre et al., 1992; Schecterson and Bothwell, 1994; Ylikoski et al., 1993). Accordingly, the high affinity receptors for these neurotrophins (Trk B and Trk C respectively) are expressed on SGNs (Schecterson and Bothwell, 1994; Ylikoski et al., 1993) in early development. Animals that fail to express these receptors show severe SGN degeneration (Kim et al., 2001).
These neurotrophins are also thought to contribute to neuronal maintenance in the adult inner ear (Qun et al., 1999; Schimmang et al., 2003; Ylikoski et al., 1993). Moreover, in the absence of hair cells, the neuroprotective effects of chronically delivered exogenous BDNF or NT-3 on SGNs in vivo has been demonstrated (Ernfors et al., 1996; Gillespie et al., 2003; Miller et al., 1997; Staecker et al., 1996).
Neural activity also plays an important role in SGN survival. In vitro studies have demonstrated that depolarization provides trophic support to SGNs during development (Hansen et al., 2001; Hegarty et al., 1997). In mature animals, the loss of hair cells results in a dramatic reduction of both driven and spontaneous neural activity in SGNs (Hartmann et al., 1984; Liberman and Kiang, 1978; Shepherd and Javel, 1997). A number of in vivo studies have shown that chronic depolarization of the auditory nerve via electrical stimulation provides a trophic influence on SGNs devoid of hair cell contact (Hartshorn et al., 1991; Kanzaki et al., 2002; Leake et al., 1991; Leake et al., 1999; Leake et al., 1992; Mitchell et al., 1997), however this is not a universal finding (Araki et al., 1998; Li et al., 1999; Shepherd et al., 1994; Shepherd et al., 2003a).
There are major practical as well as theoretical reasons for examining techniques designed to rescue SGNs. Importantly, these neurons are the target neurons for cochlear implants - a neural prosthesis designed to electrically stimulate residual SGNs in severe to profoundly deaf subjects in order to provide temporal and pitch cues necessary for speech perception (Clark, 2003; Seligman and Shepherd, 2004). Greater numbers of viable SGNs available for stimulation is likely to result in improved clinical performance among cochlear implant subjects (Gantz et al., 1993; Nadol et al., 1989). Accordingly, the aim of the present study was to investigate the putative trophic support by chronic electrical stimulation with simultaneous delivery of BDNF on SGNs in vivo. Aspects of this work have been presented in abstract form previously (Serruto et al., 2003; Shepherd et al., 2003b).
Material and Methods
Experimental subjects
Twenty-six healthy young pigmented guinea pigs (mean weight 645 g, range 332-816 g; mean age 16.9 weeks, range 7-27 weeks) were used in the present study under approval of the Royal Victorian Eye and Ear Hospital's Animal Research and Ethics Committee, and conformed to the guidelines of the National Health and Medical Research Council of Australia.
All animals had otoscopically normal tympanic membranes and normal hearing determined by the click-evoked auditory brainstem response (ABR; threshold <43 decibels peak equivalent sound pressure level [dB p.e. SPL] re 20 μPa). ABRs were recorded differentially using stainless-steel needle electrodes (vertex positive; neck negative; thorax ground). Computer generated 100 μs duration rarefaction clicks were presented to a loud speaker placed 10 cm from the pinna of the test ear. The system was calibrated so that at this distance the maximum sound level at the pinna was 98 dB p.e. SPL. Click stimuli were presented at 33 per second, and the scalp recorded responses amplified by 105 and band-pass filtered (150 Hz-3 kHz). The output of the filter was fed to a 10 bit analogue-digital converter (ADC) and sampled at 20 kHz for 12.5 ms following the stimulus onset. Five hundred responses were averaged for each recording and stored for subsequent analysis. A programmable attenuator controlled stimulus intensity; two recordings were made at each intensity. Threshold was defined as the smallest click amplitude required to evoke a peak-trough response amplitude of >0.25 μV for wave III of the ABR, i.e. within a latency window of 2.25-3.25 ms following stimulus onset for both responses. Because wave III of the ABR is the most robust in this recording configuration, it was selected as the indicator of threshold. All recordings were made in a sound attenuated, electrically shielded room.
The animals were divided into four treatment groups: BDNF alone (BDNF/-; n=5); BDNF with chronic electrical stimulation (BDNF/ES; n=5); artificial perilymph alone (AP/-; n=5); and artificial perilymph with chronic electrical stimulation (AP/ES; n=5; Table 1). Two animals served as normal hearing controls. For all treatment groups, animals were systemically deafened using a single co-administration of kanamycin (400 mg/kg subcutaneously [sc]; Sigma, St. Louis, MO) and frusemide (100 mg/kg intravenously [iv]; Troy laboratories, Smithfield, NSW, Australia) (Shepherd and Xu, 2002). Only deafened animals exhibiting ABR click thresholds >93 dB p.e. SPL in both ears were used in this study. This reflects an elevation in threshold of > 50 dB (four animals were rejected based on this criteria).
Table 1.
Summary of treatment groups
| Treatment group | Implant duration (days) |
Chronic electrical stimulation |
Contents of osmotic pump |
|---|---|---|---|
| BDNF/ES1 | 28 | Yes | BDNF2 |
| BDNF/−3 | 28 | No | BDNF |
| AP/ES | 28 | Yes | Artificial perilymph4 |
| AP/− | 28 | No | Artificial perilymph |
Notes:
ES denotes chronic electrical stimulation
62.5 μg of BDNF/ml in 1% guinea pig albumin in 200 μl of Ringer's solution
- denotes control (i.e. electrode assembly implanted but no chronic electrical stimulation)
200 μl Ringer's solution.
Electrode array and delivery techniques
The electrode array consisted of three platinum (Pt) band electrodes on a 0.6 mm diameter silicone carrier. Each Pt electrode was connected to a stainless-steel leadwire system via a 25 μm diameter platinum/iridium (90/10) wire (Shepherd and Xu, 2002). A Pt marker was located 5 mm from the tip of the array as a guide to insertion depth. A 0.75 mm internal diameter (ID) polyvinyl chloride tube connected a mini-osmotic pump (Alzet 2004; Durect Corporation, Cupertino, CA) to a 0.124 mm ID polyimide delivery-tube located within the central core of the electrode array. The contents of the osmotic pump were delivered to the scala tympani of the cochlea through a lumen in the delivery-tube at the tip of the array (Shepherd and Xu, 2002).
Preparation of the osmotic pump
The Alzet 2004 mini-osmotic pump has a reservoir capacity of 200 μl and a flow rate of 0.25 μl/hour, providing a continuous infusion period of 28 days. Under sterile conditions pumps were loaded with recombinant human BDNF (PeproTech, Rocky Hill, NJ) containing 1% guinea pig albumin in 200 μl of Ringer's solution giving a BDNF concentration of 62.5 μg/ml. After allowing for 20% absorption by the pump and delivery cannula this effectively delivers 10 μg of BDNF per cochlea (Gillespie et al., 2003). Control pumps delivering AP were loaded with 200 μl of sterile Ringer's solution. The loaded pumps were incubated in sterile Ringer's solution for 36-48 h at 37°C prior to surgery in accordance with the manufacturers' specifications.
Surgical details
Five days following deafening each animal was anaesthetized with ketamine (60 mg/kg intramuscularly [im]; Parnell Laboratories, Alexandria, NSW, Australia) and xylazine (4 mg/kg im; Troy Laboratories), and prepared for surgery. ABRs were recorded to confirm that the hearing loss was severe to profound. Surgery was performed under sterile conditions. Supplemental doses of anesthesia were administered during surgery at a level sufficient to maintain the animal in an areflexic state. Carprofen (4 mg/kg sc; Pfizer, West Ryde, NSW, Australia) and Baytril (10 mg/kg sc; Bayer, Pymble, NSW, Australia) were administered to provide long-term analgesia and broad-spectrum antibiotic cover respectively. A dorsal approach was used to expose the left cochlea of each animal. The round window membrane was incised and the electrode array was inserted ∼ 4.5 mm into the scala tympani. The round window was sealed with muscle, the leadwire proximal to the electrode array was fixed using dental cement (Duralon; ESPE Dental AG, Seefeld, Germany), and the distal leadwire exited the skin via a small incision in the neck. The osmotic pump was connected to the delivery-tube and implanted into a subcutaneous pocket between the scapulae (Brown et al., 1993). The wounds were sutured in two layers and the wound sites sprayed with Opsite® (Smith & Nephew Pty. Ltd, Hull, England). Each animal was given 10 ml of Hartmann's solution sc. During surgery the animal's temperature was maintained at 37°C using a heating pad. No surgery was performed on the right cochlea of each animal; these cochleae served as deafened, untreated controls.
Electrically-evoked ABRs and chronic electrical stimulation
Immediately following implant surgery, electrically evoked ABRs (EABRs) were recorded differentially using stainless-steel needle electrodes (vertex positive; neck negative; thorax ground). Optically-isolated biphasic current pulses (100 μs per phase; 10 μs interphase gap) were generated under computer control and delivered to the intracochlear electrode array. The current pulses were presented at 33 per second and the scalp recorded response amplified by 105, the electrical artefact was removed using a sample-and-hold circuit (Black et al., 1983) and the signal bandpass filtered (150Hz-3kHz). The output of the filter was digitised using a 10-bit ADC sampling at 20 kHz for 12.5 ms following the stimulus onset. Two hundred and fifty presentations were averaged for each recording. Two sets of recordings were made at each current level and current amplitude was reduced to levels below threshold. Threshold was defined as the smallest current level required to evoke a peak-trough response amplitude of >0.25 μV for wave III of the EABR, i.e. within a latency window of 1.5-2.5 ms following stimulus onset for both responses (the EABR latency is shorter than that generated by an acoustic stimulus due to the lack of acoustic travel time and the absence of inner hair cell/auditory nerve synaptic delay). Under these recording conditions the guinea pig EABR consists of four distinct waves that occur within the first 3 ms following the electrical stimulus. These waves represent the synchronous neural activity propagating from the auditory nerve (wave I) through the ascending auditory brainstem in response to the direct electrical stimulation of SGNs. The EABR provides a quantitative measure of physiological threshold for electrical stimulation for each of the three bipolar electrode combinations. During recording the animal's temperature was maintained at 37°C. EABRs were recorded in an electrically shielded room.
Five days following implant surgery two of the four treatment groups (BDNF/ES and AP/ES) commenced a chronic electrical stimulation program using programmable current source stimulators. The output of the stimulator delivered 100 μs/phase charge balanced biphasic current pulses to a bipolar electrode pair at a stimulus rate of 1200 pulses per second (pps) and was amplitude-modulated (AM) to a depth of 50% at 30 Hertz. This stimulus waveform replicates the temporally challenging stimuli used in contemporary cochlear implant speech processing strategies. Electrode shorting and capacitive coupling were used to ensure complete charge recovery (Huang et al., 1999). The amplitude of the AM stimulus waveform was set so that the minimum current level equalled the post-operative EABR threshold (i.e. the maximum stimulus intensity was 6 dB above EABR threshold). These stimulus levels were confirmed to be acceptable in the awake animal using basic behavioural indicators. The maximum stimulus current amplitudes used in this study were in the range 0.39-1.6 mA at 100 μs/phase, developing charge densities within levels considered safe for use with platinum electrodes (Xu et al., 1997). Stimulators were carried in a harness worn by the guinea pig to enable continuous stimulation without confining the animal's daily activities. All animals in the ES groups received approximately 6 h of stimulation per day, five days per week from day 10 to day 33 post-deafening. Both stimulus current and electrode voltage waveforms were monitored twice daily. Finally, on completion of the implantation period, 33 days after deafening, EABRs were again recorded from all animals in each cohort in order to assess the functional status of the auditory pathway. Note that the animals in the two unstimulated cohorts (BDNF/- and AP/-) only received brief periods (total <120 min) of electrical stimulation during the two EABR recording sessions (day 5 & 33). The change in EABR threshold over the 28 day period was determined for each animal and compared statistically with the implanted control animals (AP/-) using a t-test.
Cochlear histology
Immediately following the final EABR recordings each animal was killed with an overdose of anaesthetic (150 mg/kg sodium pentobarbital intraperitoneal; Virbac Pty. Ltd., Peakhurst, NSW, Australia) and systemically perfused with heparinized normal saline at 37°C followed by phosphate buffered (pH=7.4) 4% paraformaldehyde at 4°C. The insertion depth of the electrode array was determined, the drug delivery system was checked for its patency, the presence of leaks and an intact connection to the osmotic pump, and the pump was examined for evidence of residual fluid.
Both cochleae were removed for histology. The cochleae were decalcified, dehydrated, embedded in resin and serially sectioned at 2 μm. Sections every 126 μm were stained with haematoxylin and eosin. SGN densities for each of the four cochlear turns (t1-t4) were measured from five randomly selected mid-modiolar sections containing all four turns. Each cochlear turn was identified and the cross-sectional area of Rosenthal's canal within each turn was measured using NIH Image. All neurons with a clear nucleus were then counted in each turn and SGN density (cells/mm2) determined. All neuron counts were corrected for variation in SGN size across both cohorts and cochlear turns using the method of Abercrombie (Coggeshall and Lekan, 1996). Briefly, for each cochlear turn and treatment group, the nucleus diameters of 50 randomly selected SGNs were measured. Only neurons containing a clear nucleolus were included. The mean nucleus diameter was then calculated and the corrected neuron number determined using N=(n × D)/(D+T); where N is the corrected neuron count; n is the actual count; D is the mean nucleus diameter; and T is the section thickness. Nuclear diameters were measured using NIH Image.
Spiral ganglion neuron soma area measurements were recorded from the same five sections as those used for the density measurements. Only the soma area of cells clearly exhibiting a nucleolus were measured using NIH Image. SGN density and soma area measurements were made from both the treated (left) and the deafened untreated (right) cochlea of each animal, as well as the normal controls. A single observer performed all area measurements blind. Results were expressed as mean ± standard error of the mean (SEM) and statistical analysis was performed using a one-way ANOVA for normally distributed data, or Kruskal-Wallis one-way ANOVA on Ranks for non-parametric data. A difference was considered statistically significant at p<0.05. All photomicrographs were taken using a Zeiss Axioplan 2 microscope fitted with an AxioCam MRm digital camera and stored as TIFF images. Photomicrographs were edited for publication using Adobe Photoshop 6.0.
Results
At post mortem examination all electrode arrays were found to be located within the scala tympani. The consistent location of the Pt marker noted at both surgery and post mortem indicated that no array had moved during the implantation period. The connection between the delivery-tube and osmotic pump was confirmed in all animals, and the delivery-tubes were shown to be functioning normally. Finally, no residual fluid was detected in any osmotic pump.
Chronic BDNF delivery evokes functional changes in SGNs
Representative EABRs from an AP/ES and BDNF/ES animal, recorded immediately post-operatively (day 5) and at completion of the implantation period (day 33), are illustrated in Figures 1A and 1B respectively. All AP/ES and AP/- animals exhibited an increase in EABR threshold over the 28-day implantation period. In contrast, both cohorts that received chronic intracochlear infusion of BDNF exhibited a reduction in EABR threshold over the same time period. The mean change in EABR threshold over the 28-day implantation period is illustrated in Figure 2. The reduction in EABR threshold associated with the two BDNF cohorts was highly statistically significant when compared to the increases in threshold associated with the two AP treated cohorts (P < 0.001, t-test). There was no statistically significant difference between the stimulated and unstimulated animals within the BDNF or the AP treated animals, indicating that exposure to chronic depolarization did not affect EABR threshold.
Figure 1.
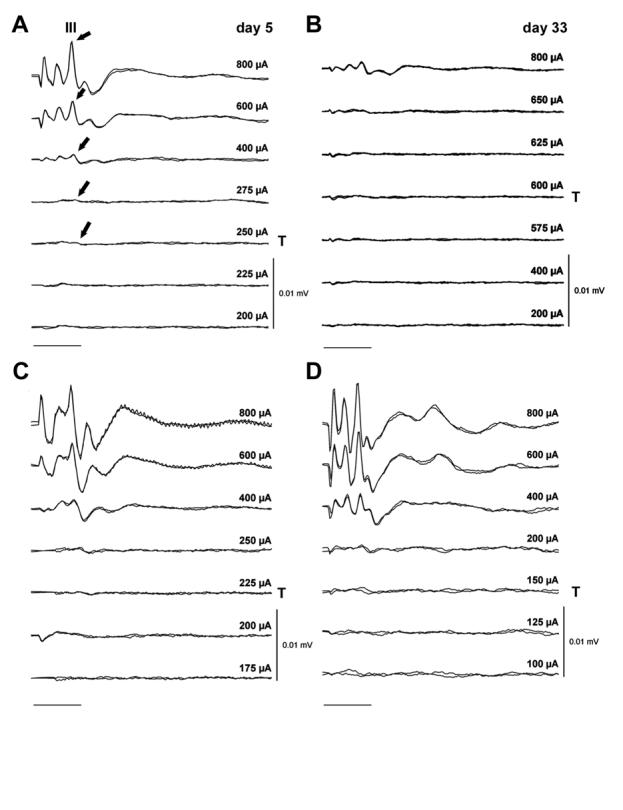
Representative EABRs recorded from an animal in the AP/ES cohort (A and B) and the BDNF/ES cohort (C and D) immediately following surgery (day 5; A and C), and at completion of the 28-day implantation program (day 33; B and D). All EABRs were evoked using a 100 μs/phase biphasic current pulse delivered to bipolar electrode pair 1/2. Two responses are recorded at each current level; each response is averaged from 250 presentations. Wave III of the EABR, from which the threshold data are obtained, is illustrated over its dynamic range (arrows) in the AP/ES responses recorded at day 5 (A). Note the reduction in threshold and maintenance of response amplitude over the implant period for the BDNF/ES treated animal. Peak stimulus intensity (in μA) and threshold (T) is indicated for each set of recordings. For clarity responses from both animals are plotted using the same gain. Scale bar = 2 ms.
Figure 2.
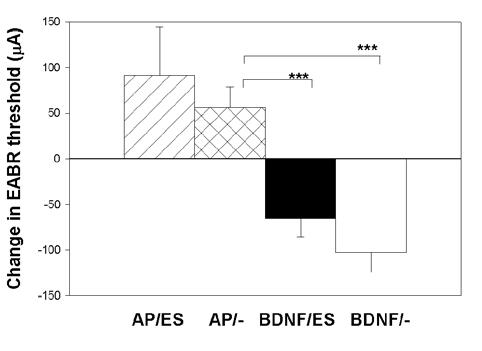
Mean change in EABR threshold over the 28-day implantation period for each of the four cohorts in this study. While there was no statistically significant difference between the stimulated versus unstimulated animals in either the BDNF or AP treatment groups, the reduction in threshold over time for the two BDNF cohorts was highly statistically significant when compared to the increase in threshold over the same period for the two AP cohorts (p<0.001; t-test). Error bar = standard error of the mean.
Chronic depolarization enhances the trophic effects of BDNF in rescuing SGNs following deafness
Low power micrographs of the first three turns of a BDNF/ES treated cochlea compared with its deafened untreated contralateral control is illustrated in Figure 3. There are a number of histological features of note. First, both cochleae exhibit a complete absence of the organ of Corti in the basal turns 1 and 2, while turn 3 shows evidence of collapsed support cells presumably in the process of degeneration. Second, SGNs within Rosenthal's canal, even at this low magnification, show a far greater level of survival in the BDNF/ES treated cochlea (left panels), compared to the unimplanted, deafened control (right panels). Third, there was no evidence of extensive mechanical disruption to the treated cochlea following chronic BDNF infusion at a rate of 0.25 μl/hour. Finally, a fine fibrous tissue capsule (ft; Fig. 3) was evident in the basal turn of the treated cochlea, illustrating the location of the electrode array. Typically, this mild tissue response was observed in BDNF treated cochleae but was not evident in AP treated cochleae (e.g. see Fig. 5). The presence and extent of the tissue response was independent of ES. Figure 4 contains higher power micrographs illustrating SGNs in the first three turns of a second BDNF/ES treated cochlea with its deafened untreated contralateral control cochlea for comparison. The normal SGN packing density in all three turns of the BDNF/ES treated cochlea (left panels), contrasts with the extensive degeneration apparent in the untreated, deafened control cochlea (right panels). Importantly, both BDNF cohorts (BDNF/ES and BDNF/-) exhibited significantly greater SGN survival compared with the clear degeneration evident in both AP/ES and AP/- cohorts (Fig. 5, Table 2).
Figure 3.
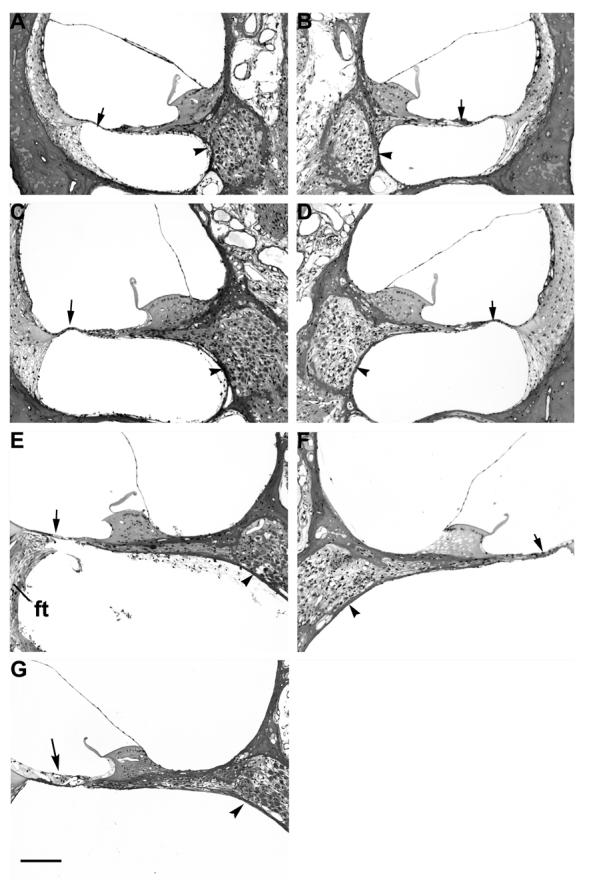
Photomicrographs of the third (A and B), second (C and D) and basal (E and F) turns of a deafened BDNF/ES treated cochlea (A, C and E) and its contralateral deafened, untreated control cochlea (B, D and F). The basal turn of a normal control cochlea has been included for comparison (G). A mild fibrous tissue (ft) reaction to the implanted electrode array is localized to the basal turn scala tympani of the BDNF/ES treated cochlea (E). The SGN population in Rosenthal's canal of the BDNF/ES cochlea contrasts with the reduced neural population in the contralateral deafened, untreated control cochlea (arrowheads). Note the absence of hair cells in all turns (arrow), with complete loss of the support cells of the organ of Corti in both the basal (E and F) and second turns (C and D) of both cochleae. Scale bar = 100 μm.
Figure 5.

Photomicrographs of Rosenthal's canal illustrating SGN survival from the basal turn of a representative cochlea from each of the four cohorts in this study (A, BDNF/ES; B, BDNF/-; C, AP/ES; D, AP/-). There is clear evidence of greater SGN survival in BDNF treated cochleae (A and B) compared to cochleae treated with AP (C and D). There was also an increased incidence of fibrous tissue (ft) reaction within the scala tympani of the BDNF treated cochleae. Scale bar = 40 μm
Figure 4.
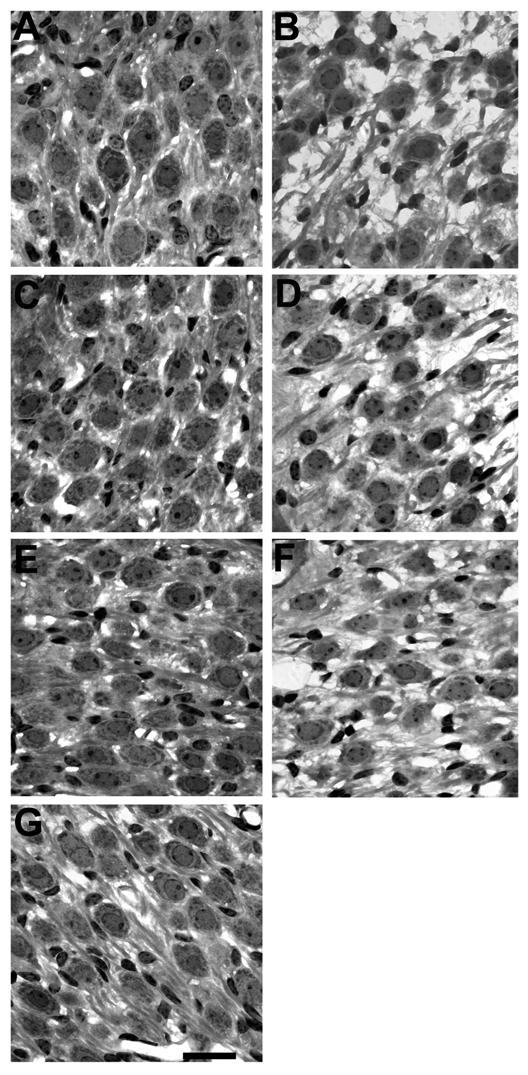
Higher power photomicrographs showing SGNs in the third (A and B), second (C and D) and basal (E and F) turns of a deafened BDNF/ES treated cochlea (A, C and E) and its contralateral deafened, untreated control cochlea (B, D and F). Again, the basal turn of a normal control cochlea has been included for comparison (G). The normal SGN packing density in all three turns of the BDNF/ES treated cochlea (A, turn 3; C, turn 2; E, basal turn), contrasts with the degeneration apparent in the contralateral deafened, untreated control cochlea (B, turn 3; D, turn 2; F, basal turn). Scale bar = 20 μm.
Table 2.
Summary of 1 way ANOVA of SGN density
| Cochlear turn | DF | F | P |
|---|---|---|---|
| t1 | 3 | 56.5 | <0.001 |
| t2 | 3 | 43.9 | <0.001 |
| t3 | 3 | 26.9 | <0.001 |
| t4 | 3 | 10.5 | <0.001 |
Figure 6 illustrates the mean SGN density compared across treatments in each cochlear turn. These data were corrected using the Abercrombie technique described in Materials and Methods. There was a highly significant difference across treatments within each cochlear turn (Table 2). Both BDNF (BDNF/ES and BDNF/-) cohorts exhibited SGN densities greater than normal hearing controls in the basal turn of the cochlea (t1). More apically, the BDNF/ES cohort maintained a trophic advantage over the deafened untreated controls (dashed line; Fig 6) and the AP treatment groups, while the trophic effects of the BDNF/- cohort reduced systematically with increasing distance from t1. In contrast, the AP treated cohorts (AP/ES and AP/-) in the basal turn exhibited SGN densities approximately half that of the two BDNF groups, and at a level similar to that of the deafened, untreated control cochleae. That is, there was no evidence of neurotrophic support provided by electrical stimulation (AP/ES) compared with artificial perilymph alone (AP/-), or deafened untreated control cochleae. The SGN density for both AP cohorts was similar to the deafened, untreated control cochleae across all four cochlear turns, with the exception of an increase in neuron survival in the AP/- cohort in t4. Finally, an important feature of these data was the small but significant increase in SGN density associated with the BDNF/ES treatment group over BDNF/-. These differences were statistically significant in turns 1 (P < 0.05; Bonferroni's post-hoc t-test), 2 (P < 0.01) and 3 (P < 0.01).
Figure 6.
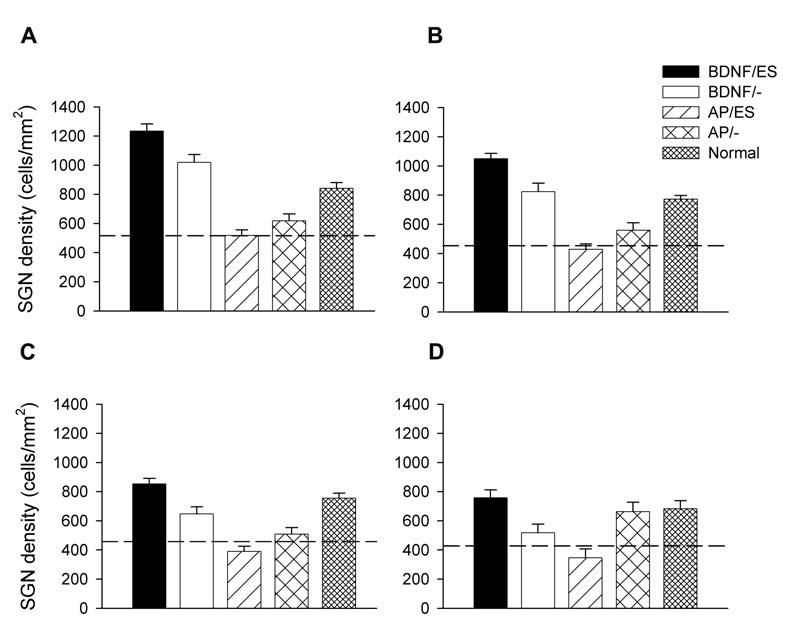
Mean SGN density of the treated cochleae for each cohort in this study. These data are illustrated from A (turn 1); B (turn 2); C (turn 3) and D (turn 4). For comparison, the mean SGN density for normal hearing cochleae are also illustrated. The mean SGN density for deafened, untreated control cochleae are illustrated by the dashed line. Error bar = standard error of the mean.
Figure 7 illustrates the mean soma area for each cohort in this study. Again, there was a highly significant difference in soma area across treatments within each cochlear turn (Table 3). Both BDNF cohorts (BDNF/ES and BDNF/-) exhibited SGN soma areas that were greater than that of normal hearing controls in t1. More apically, the BDNF/ES cohort maintained soma areas greater than the normal control data, while the BDNF/- cohort exhibited a systematic reduction with distance from t1 to levels that approached the deafened, untreated controls (dashed line; Fig. 7). In contrast, the AP treated cohorts (AP/ES and AP/-) exhibited SGN soma areas similar to that of the deafened, untreated control cochleae. This trend was similar across all cochlear turns.
Figure 7.
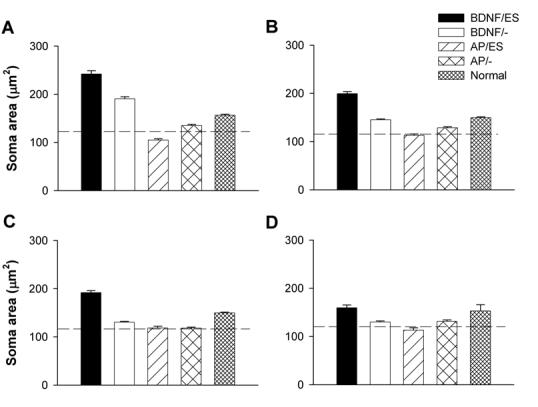
Mean SGN soma area of the treated cochleae for each cohort in this study. These data are illustrated from A (turn 1); B (turn 2); C (turn 3) and D (turn 4). The mean SGN soma area for normal hearing cochleae are also illustrated. The mean SGN soma area for deafened, untreated control cochleae are illustrated by the dashed line. Error bar = standard error of the mean.
Table 3.
Summary of Kruskal-Wallis 1 way ANOVA on Ranks of SGN soma area
| Cochlear turn | DF | H | P |
|---|---|---|---|
| t1 | 3 | 303.3 | <0.001 |
| t2 | 3 | 333.3 | <0.001 |
| t3 | 3 | 258.2 | <0.001 |
| t4 | 3 | 65.9 | <0.001 |
Discussion
This paper examines the functional and neuroanatomical response of the deafened guinea pig cochlea following chronic electrical stimulation with and without co-administration of the neurotrophin BDNF. The results show a clear and highly significant reduction in EABR threshold in animals that were chronically administered with BDNF (BDNF/ES; BDNF/-) compared with the increased EABR thresholds in control animals administered with artificial perilymph (AP/ES; AP/-) over the same period (28 days). The increased EABR thresholds associated with the AP/ES cohort suggests that chronic electrical stimulation per se did not contribute to a reduction in EABR threshold; that is, the functional changes in sensitivity were related to the chronic delivery of BDNF.
The changes observed in neural sensitivity were complemented by significant differences in both neuronal survival and soma area among the cohorts. Both SGN survival (measured as neural density) and soma size tended to be much greater in the BDNF compared with the AP cohorts.
The most marked difference in neural survival and size occurred in the basal turn of the cochlea, adjacent to the bipolar electrode array. Given the relatively localized current distribution, particularly near threshold, for this electrode geometry (Hartmann et al., 1984; van den Honert and Stypulkowski, 1987), it is not surprising that such large neuroanatomical differences also resulted in significant functional changes as measured by the EABR.
Exogenous neurotrophins and functional sensitivity
Two previous studies have also examined the effects of chronic neurotrophic factor delivery on SGN survival and neural threshold sensitivity in deafened guinea pigs. Kanzaki et al., (2002) combined ES with virally mediated glial cell line-derived neurotrophic factor (GDNF). Following treatment over a 36 day period they reported no significant difference in mean EABR thresholds across their three cohorts (ES, GDNF and GDNF/ES), with all three groups exhibiting a slight reduction in threshold over time. In contrast to the present study, Kanzaki et al., (2002) reported that ES alone resulted in a small but significant increase in SGN survival compared with deafened controls. GDNF alone produced more robust trophic support or rescue of SGNs, and this effect was enhanced in the GDNF/ES cohort. Similar to the present study, the trophic support appeared more extensive in the basal region of the cochlea compared with the apex. Given that Kanzaki et al., (2002) reported some trophic advantage in all three treatment groups, it is not surprising that they observed no difference in functional sensitivity across cohorts. In a second study, Shinohara et al., (2002) delivered BDNF with ciliary neurotrophic factor for 26 days via an osmotic pump, after unilaterally deafening the animals via intracochlear neomycin infusion. Control animals were administered with AP. Although an electrode was implanted within the cochlea to evoke EABRs, no attempt was made to chronically stimulate SGNs. Shinohara et al., (2002) reported significantly enhanced SGN survival in the neurotrophic factor-treated cochleae compared with the deafened AP treated controls. Importantly, they described changes in EABR thresholds over time that were similar to the results of the present study; neurotrophic factor-treated cochleae exhibited a significant decrease in EABR threshold over implantation time compared with the longitudinal increase in threshold associated with AP treated cochleae.
Neuroanatomical changes in the deafened cochlea and the application of exogenous neurotrophins to rescue SGNs
The reduction in soma area and degeneration of SGNs following sensorineural hearing loss (SNHL) observed in the present study is a common finding in the mammalian cochlea (Elverland and Mair, 1980; Leake and Hradek, 1988; Nadol et al., 1989; Shepherd and Javel, 1997; Takeno et al., 1998; White et al., 2000). A number of studies have reported an increase in the relative proportion of the smaller Type II SGN in deafened cochleae (Leake and Hradek, 1988; Spoendlin, 1984), which may bias measurements of SGN shrinkage following deafness.
Neuronal shrinkage presumably reflects a down regulation in biosynthetic activity at the SGN perikaryon following a SNHL. Their metabolic requirements are related to the re-establishment of the resting membrane potential following the propagation of an action potential (Kadekaro et al., 1985). This would be greatly diminished as a result of the significant reduction in driven and spontaneous activity that occurs following a SNHL (Hartmann et al., 1984; Liberman and Kiang, 1978; Shepherd and Javel, 1997).
In contrast to untreated, deafened control or AP treated cochleae, the soma area of SGNs treated with exogenous BDNF were similar to or greater than normal controls. While the mechanisms underlying an increased soma area following exogenous neurotrophin delivery are unclear, this finding is not unique to auditory neurons (e.g. Gratto and Verge, 2003; Perez-Navarro et al., 1999).
The present data suggest that an increased soma area associated with exogenous BDNF may be concentration dependent, as the maximum soma area for the BDNF/- cohort was located in t1 - adjacent to the site of BDNF delivery in the scala tympani. Spiral ganglion neurons in more apical turns showed a systematic reduction in soma area that approached that of the untreated deafened controls in the most distal turn (t4). Research examining the distribution of labelled neurotrophins delivered to the cochlea via a single injection suggests that its concentration is maximum in the basal turn (Richardson et al., 2004). However, we cannot rule out the possibility that in the mature cochlea basal SGNs are more sensitive to BDNF rescue compared with neurons located in more apical regions of the cochlea.
Maintenance of Trk receptors in SNHL
In the mature cochlea, lack of TrkB (the high affinity receptor for BDNF), results in loss of SGNs (Schimmang et al., 2003). The present and previous in vivo studies investigating the trophic effects of exogenous BDNF delivery (Gillespie et al., 2003; Miller et al., 1997; Staecker et al., 1996) imply that TrkB receptors on SGNs in deafened cochleae are not significantly down-regulated and is consistent with recent immunocytochemical studies showing maintenance of TrkB receptors in SGNs of long-term deafened animals (Gillespie et al., 2004; Hurley et al., 2004).
Enhanced trophic effects of BDNF and ES
The enhancement of the trophic effects of exogenous BDNF on SGNs by chronic electrical stimulation is a novel and significant finding that may have important implications for the application of neurotrophins in a clinical environment (see Clinical Implications below). There are a number of potential mechanisms that may contribute to this effect. First, neural activity is known to up-regulate the secretion of endogenous BDNF in various neurons by regulating the transcription of the BDNF gene (Lu, 2003; Nanda and Mack, 2000; Rocamora et al., 1996). Moreover, in vitro studies have shown that patterned electrical stimulation is more effective at inducing endogenous BDNF secretion in neurons compared with more simple forms of depolarisation induced by potassium chloride (Balkowiec and Katz, 2000; Balkowiec and Katz, 2002; Gartner and Staiger, 2002; Lever et al., 2001). It is important to note, however, that BDNF synthesis in SGNs does not appear to be regulated by neural activity - at least during development (Zha et al., 2001). Second, high frequency neuronal activity induced by electrical stimulation can also up-regulate the number of TrkB receptors on the surface of activated central nervous system (CNS) neurons (Du et al., 2000; Meyer-Franke et al., 1998), although the response of peripheral neurons - including SGNs - is unknown. While both these mechanisms may result in an increase in trophic activity over the application of exogenous BDNF alone, the localized neural excitation produced by the electrical stimulus would be restricted to the basal turn of the cochlea. In contrast, we observed widespread trophic support of SGNs in the BDNF/ES cohort. In vivo studies showing trophic effects of ES alone also typically describe relative widespread trophic support of SGNs (Leake et al., 1999; Mitchell et al., 1997).
The present study demonstrated an increase in SGN density in BDNF treated cochleae compared with normal hearing controls. This effect, which was restricted to the base of the cochlea, may be associated with neural regeneration. However, our preferred explanation is a bias in the sampling of SGNs in the region of the cochlea where SGN soma were larger than normal. Clearly, future studies are required to examine for evidence of neuronal regeneration following exogenous BDNF delivery to the cochlea.
Our results show that chronic intracochlear electrical stimulation in the deafened guinea pig does not provide trophic support of SGNs, since the SGN density of AP/ES treated cochleae exhibited no evidence of increased survival compared with the deafened, unstimulated AP treated cochleae, or cochleae from deafened untreated control animals. These results are consistent with our previous data obtained from deafened, chronically stimulated cats (Araki et al., 1998; Shepherd et al., 1994; Shepherd et al., 2003a), although other groups have reported a trophic influence on SGNs using chronic ES alone (Hartshorn et al., 1991; Kanzaki et al., 2002; Leake et al., 1991; Leake et al., 1999; Leake et al., 1992; Mitchell et al., 1997). This discrepancy may reflect significant methodological differences among these studies (Miller, 2001).
The effects of electrical stimulation and neurotrophins on functional changes within the deafened cochlea
Both AP/ES and AP/- treated animals in the present study routinely exhibited a small increase in EABR threshold over the implantation period. This is consistent with previous studies that have reported increases in EABR threshold as a function of implantation time in deafened animals (Shepherd et al., 2003a; Shinohara et al., 2002). We interpret these changes as reflecting the ongoing loss of SGNs as a result of the deafening process.
The rescue of SGNs in both BDNF/- and BDNF/ES cohorts was also associated with significant reductions in EABR threshold over the 28-day implant period. There are a number of potential mechanisms underlying this significant reduction in threshold; they may act in isolation or in concert. First, BDNF has a profound effect on the firing properties and potassium ion channel distribution in mammalian SGNs (Adamson et al., 2002). Moreover, BDNF is also known to depolarise CNS neurons by activation of sodium ion conduction (Blum et al., 2002; Kafitz et al., 1999). Changes in ion channel distribution and conductance could readily lead to reductions in neural threshold. Second, from a biophysical standpoint, electrical thresholds are known to be dependent on nerve fiber diameter, with larger diameter fibers exhibiting lower thresholds (McNeal, 1976; Rushton, 1951). The reductions observed in EABR thresholds may be related to the increased size of SGNs in the BDNF treated groups. Finally, neurotrophins have been shown to encourage neurite outgrowth in both in vitro (Aletsee et al., 2001; Brors et al., 2002; Gillespie et al., 2001; Lefebvre et al., 1994) and in vivo studies (Staecker et al., 1996; A. Wise, R. Richardson, J. Hardman, G. Clark and S. O'Leary, personal communication). If this growth were directed towards the electrode array (the point of highest BDNF concentration in the present study), as demonstrated by Staecker and colleagues (1996), it is likely that it would also result in a reduction in EABR thresholds.
Clinical implications
The present findings have important clinical implications for cochlear implant recipients. Significant reductions in threshold result in large reductions in power consumption (Seligman and Shepherd, 2004) and/or the development of a greater number of smaller electrode contacts. Such development would provide an opportunity to develop smaller, more efficient devices.
However, prior to any clinical application, long-term safety and efficacy studies are required to ensure that an exogenous supply of neurotrophin maintains the neuroanatomical and functional advantages observed in the present study. For example, there is some evidence to suggest that exogenous neurotrophin treatment in injured spinal cord can potentiate free radical-mediated necrosis (McDonald et al., 2002).
We observed no evidence of mechanical trauma to cochleae following long-term implantation and AP or BDNF delivery despite delivering these drugs at a rate of 0.25 μL/hour. This volume represents approximately 5% of the total volume of the guinea pig scala tympani (Thorne et al., 1999). Exogenous BDNF delivery did, however, result in an increased incidence of fibrous tissue reaction within the scala tympani (Chen, 2003; Figs. 3 & 5). Although the tissue response was relatively mild and localized to the scala tympani in the vicinity of the electrode array, such a response is undesirable, as it will result in an increase in electrode impedance (Xu et al., 1997), and associated increased power consumption. A vigorous tissue reaction may also reduce the efficacy of the neurotrophin being delivered to the SGNs, and may lead to difficulties associated with the eventual replacement of an electrode array (Shepherd et al., 1995).
Finally, these results may have application for other neural prostheses. There is considerable interest in combining knowledge from both functional electrical stimulation and neural protection/regeneration studies in order to provide improved therapeutic outcomes via neural prostheses (Grill et al., 2001).
Future studies
There are a number of additional research questions that must be carefully addressed prior to the consideration of neurotrophins for clinical application. First, the duration of neurotrophin delivery necessary to maintain a trophic advantage is a key question relating to delivery techniques, safety issues and cost benefits. Recently, Gillespie et al. (2003) reported an accelerated loss of SGNs following the withdrawal of exogenous BDNF in deafened animals. It is important to determine whether a trophic advantage can be maintained through ES alone after an initial delivery of neurotrophins. Second, the method of neurotrophin delivery must be addressed. While a drug delivery system incorporated in an electrode array is a useful experimental procedure, we do not consider it an adequate solution for clinical application. Specifically, a major limitation associated with such a design would be the risk of infection spreading from the pump/delivery system directly into the cochlea. This risk would increase greatly upon the surgical replacement of the delivery pump. Alternative techniques for the delivery of exogenous neurotrophins need to be examined for both safety and efficacy. As an example, the use of viral vectors designed to transduce cells of the inner ear to over-express neurotrophins has a number of potential advantages over the infusion of the protein via a pump (Kanzaki et al., 2002; Stover et al., 2000). Third, it is necessary to demonstrate that the neurotrophic advantage, which we and others have described in guinea pig, is also evident in other mammalian species. Finally, the effects of long-term delivery of neurotrophins on non-neural cochlear structures, as well as the CNS, must be carefully addressed in safety studies.
Conclusion
The major finding associated with the present research has been the highly significant trophic effects of exogenous BDNF combined with chronic electrical stimulation on SGNs devoid of their target hair cells. This work demonstrated both an anatomical and functional advantage that would have important implications for cochlear implant subjects.
Acknowledgements
This paper is dedicated to Dr. F. Terry Hambrecht, who appreciated before most the need to have a multidisciplinary approach in the development of neural prostheses. We are grateful to Dr James Fallon for software development, Dr Jin Xu and Ms Helen Feng for electrode manufacture, Dr Lisa Gillespie for advice on neurotrophin delivery, Mr. Rodney Millard for engineering assistance, Ms Maria Clarke for histology, Ms Jennifer Hardman and Dr. Rachael Richardson for photographic advice, Dr Sue Pierce and Ms Elisa Borg for veterinary advice and animal husbandry, and Dr Natalie Rickard and Dr Lisa Gillespie for comments on earlier versions of this manuscript. Dr Crook's present affiliation is ES Cell International Pty Ltd.
Footnotes
Supporting grants: This work was funded by the NIDCD (NO1-DC-0-2109 and NO1-DC-3-1005).
Literature cited
- Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. Journal of Neuroscience. 2002;22(4):1385–1396. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aletsee C, Beros A, Mullen L, Palacios S, Pak K, Dazert S, Ryan AF. Ras/MEK but not p38 signaling mediates NT-3-induced neurite extension from spiral ganglion neurons. J Assoc Res Otolaryngol. 2001;2(4):377–387. doi: 10.1007/s10162001000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S, Kawano A, Seldon L, Shepherd RK, Funasaka S, Clark GM. Effects of chronic electrical stimulation on spiral ganglion neuron survival and size in deafened kittens. Laryngoscope. 1998;108(5):687–695. doi: 10.1097/00005537-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. Journal of Neuroscience. 2000;20(19):7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22(23):10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RC, Clark GM, O'Leary SJ, Walters C. Intracochlear electrical stimulation of normal and deaf cats investigated using brainstem response audiometry. Acta Oto-Laryngologica Supplement. 1983;399:5–17. doi: 10.3109/00016488309105588. [DOI] [PubMed] [Google Scholar]
- Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature. 2002;419(6908):687–693. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- Brors D, Aletsee C, Schwager K, Mlynski R, Hansen S, Schafers M, Ryan AF, Dazert S. Interaction of spiral ganglion neuron processes with alloplastic materials in vitro. Hear Res. 2002;167(12):110–121. doi: 10.1016/s0378-5955(02)00355-6. [DOI] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70(2):167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- Chen LC-C. The effect of chronic neurotrophin delivery on non-sensory tissue within the cochlea. University of Melbourne; 2003. p. 86. Masters in Clinical Audiology. [Google Scholar]
- Clark GM. In: Cochlear Implants: Fundamentals and Applications. Beyer RT, editor. Springer-Verlag; New York: 2003. p. 830. [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. The Journal of Comparative Neurology. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Du J, Feng L, Yang F, Lu B. Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol. 2000;150(6):1423–1434. doi: 10.1083/jcb.150.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elverland HH, Mair IW. Hereditary deafness in the cat. An electron microscopic study of the spiral ganglion. Acta Otolaryngol. 1980;90(56):360–369. doi: 10.3109/00016488009131737. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2(4):463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell & Tissue Research. 1999;295(3):369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102(12):909–916. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci U S A. 2002;99(9):6386–6391. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. LIF is more potent than BDNF in promoting neurite outgrowth of mammalian auditory neurons in vitro. Neuroreport. 2001;12(2):275–279. doi: 10.1097/00001756-200102120-00019. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to an accelerated loss of survival effects. J Neurosci Res. 2003;71(6):785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15(7):1121–1125. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- Gratto KA, Verge VM. Neurotrophin-3 down-regulates trkA mRNA, NGF high-affinity binding sites, and associated phenotype in adult DRG neurons. Eur J Neurosci. 2003;18(6):1535–1548. doi: 10.1046/j.1460-9568.2003.02881.x. [DOI] [PubMed] [Google Scholar]
- Grill WM, McDonald JW, Peckham PH, Heetderks W, Kocsis J, Weinrich M. At the interface: convergence of neural regeneration and neural prostheses for restoration of function. J Rehabil Res Dev. 2001;38(6):633–639. [PubMed] [Google Scholar]
- Hansen MR, Zha XM, Bok J, Green SH. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J Neurosci. 2001;21(7):2256–2267. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R, Topp G, Klinke R. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear Res. 1984;13:47–62. doi: 10.1016/0378-5955(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol Head Neck Surg. 1991;104(3):311–319. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17(6):1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CQ, Shepherd RK, Carter PM, Seligman PM, Tabor B. Electrical stimulation of the auditory nerve: direct current measurement in vivo. IEEE Trans Biomed Eng. 1999;46(4):461–470. doi: 10.1109/10.752943. [DOI] [PubMed] [Google Scholar]
- Hurley PA, Serruto A, Crook JM, Shepherd RK. In: TrkB receptor expression in the cochlea following sensorineural hearing loss. Martin P, editor. Melbourne: 2004. p. P180. [Google Scholar]
- Kadekaro M, Crane AM, Sokoloff L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc Natl Acad Sci U S A. 1985;82(17):6010–6013. doi: 10.1073/pnas.82.17.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401(6756):918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454(3):350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128(3):417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33(1):11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Rebscher SJ, Snyder RL. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hear Res. 1991;1991:251–271. doi: 10.1016/0378-5955(91)90120-x. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412(4):543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Chronic intracochlear electrical stimulation in neonatally deafened cats: effects of intensity and stimulating electrode location. Hear Res. 1992;64:99–117. doi: 10.1016/0378-5955(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Malgrange B, Staecker H, Moghadass M, Van de Water TR, Moonen G. Neurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitro. Neuroreport. 1994;5(8):865–868. doi: 10.1097/00001756-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Weber T, Rigo J-M, Staecker H, Moonen G, Van De Water TR. Peripheral and central target-derived trophic factor(s) effects on auditory neurons. Hear Res. 1992;58:185–192. doi: 10.1016/0378-5955(92)90127-9. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JC, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21(12):4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Parkins CW, Webster DB. Does electrical stimulation of deaf cochleae prevent spiral ganglion degeneration? Hear Res. 1999;133(12):27–39. doi: 10.1016/s0378-5955(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10(2):86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Stefovska VG, Liu XZ, Shin H, Liu S, Choi DW. Neurotrophin potentiation of iron-induced spinal cord injury. Neuroscience. 2002;115(3):931–939. doi: 10.1016/s0306-4522(02)00342-1. [DOI] [PubMed] [Google Scholar]
- McNeal DR. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng. 1976;23(4):329–337. doi: 10.1109/tbme.1976.324593. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr., Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21(4):681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL. Effects of chronic stimulation on auditory nerve survival in ototoxically deafened animals. Hear Res. 2001;151(12):1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15(45):631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105(12):30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr., Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98(6):411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nanda SA, Mack KJ. Seizures and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Brain Res Mol Brain Res. 2000;78(12):1–14. doi: 10.1016/s0169-328x(00)00054-1. [DOI] [PubMed] [Google Scholar]
- Perez-Navarro E, Alberch J, Neveu I, Arenas E. Brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5 differentially regulate the phenotype and prevent degenerative changes in striatal projection neurons after excitotoxicity in vivo. Neuroscience. 1999;91(4):1257–1264. doi: 10.1016/s0306-4522(98)00723-4. [DOI] [PubMed] [Google Scholar]
- Qun LX, Pirvola U, Saarma M, Ylikoski J. Neurotrophic factors in the auditory periphery. Ann N Y Acad Sci. 1999;884:292–304. doi: 10.1111/j.1749-6632.1999.tb08649.x. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Wise A, O'Leary SJ, Hardman J, Casley D, Clark GM. Tracing Neurotrophin-3 Diffusion and Uptake in the Guinea Pig Cochlea. Hear Res. 2004;198:25–35. doi: 10.1016/j.heares.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Welker E, Pascual M, Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton WAH. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951;115:101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73(1):92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Tan J, Muller M, Zimmermann U, Rohbock K, Kopschall I, Limberger A, Minichiello L, Knipper M. Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development. 2003;130(19):4741–4750. doi: 10.1242/dev.00676. [DOI] [PubMed] [Google Scholar]
- Seligman PM, Shepherd RK. Cochlear Implants. In: Horch KW, Dhillon G, editors. Neuroprosthetics: Theory and practice. World Scientific Publishing; Singapore: 2004. pp. 878–904. [Google Scholar]
- Serruto A, Crook J, Epp S, Shepherd R. In: Maintenance of auditory neurones following deafness. Martin P, editor. Adelaide, South Australia: Jan 28th-31st, 2003. p. 264. [Google Scholar]
- Shepherd RK, Clark GM, Xu SA, Pyman BC. Cochlear pathology following reimplantation of a multichannel scala tympani electrode array in the macaque. Am J Otol. 1995;16(2):186–199. [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II Deafened kittens. Hear Res. 1994;81:150–166. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Serruto A, Epp S, Crook JM, Hurley PA, Xu J, Fallon JB, Andrew J, Irvine DR, Millard RE. Protective Effects of Patterened Electrical Stimulation on the Deafened Auditory System. University of Melbourne; Melbourne: 2003a. pp. 1–48. Report nr 12. [Google Scholar]
- Shepherd RK, Serruto A, Epp SB, Crook JM. Protective effects of electrical stimulation and neurotrophin delivery on auditory neurons in vivo: Implications for cochlear implants. Daytona Beach, Fl.: 2003b. p. 770. [Google Scholar]
- Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172(12):92–98. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99(3):1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol Suppl. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7(4):889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Stover T, Yagi M, Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7(5):377–383. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- Takeno S, Wake M, Mount RJ, Harrison RV. Degeneration of spiral ganglion cells in the chinchilla after inner hair cell loss induced by carboplatin. Audiol Neurootol. 1998;3(5):281–290. doi: 10.1159/000013800. [DOI] [PubMed] [Google Scholar]
- Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW, Jr., Gewalt SL. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 1999;109(10):1661–1668. doi: 10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- van den Honert C, Stypulkowski PH. Single fiber mapping of spatial excitation patterns in the electrically stimulated auditory nerve. Hear Res. 1987;29:195–206. doi: 10.1016/0378-5955(87)90167-5. [DOI] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141(12):12–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear Res. 1997;105:1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65(12):69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Zha XM, Bishop JF, Hansen MR, Victoria L, Abbas PJ, Mouradian MM, Green SH. BDNF synthesis in spiral ganglion neurons is constitutive and CREB-dependent. Hear Res. 2001;156(12):53–68. doi: 10.1016/s0378-5955(01)00267-2. [DOI] [PubMed] [Google Scholar]


