Abstract
In 3 experiments using rats as subjects, the authors varied trial spacing to investigate the conditions under which Pavlovian and differential inhibition are observed. Experiment 1 compared Pavlovian and differential inhibition with spaced training trials. Spaced trials resulted in only the Pavlovian inhibitor passing both summation and retardation tests. Conversely, Experiment 2 compared these 2 types of inhibition with massed training trials. This training resulted in only the differential inhibitor passing both tests for conditioned inhibition. Finally, in Experiment 3 all subjects experienced Pavlovian inhibition training with massed trials. Although this training by itself did not result in behavior indicative of inhibition, subjects that also experienced posttraining extinction of the training context did pass both tests for inhibition. Overall, these results are anticipated by the extended comparator hypothesis (Denniston, Savastano, & Miller, 2001) but are problematic for most contemporary associative learning theories.
Keywords: differential inhibition, extended comparator hypothesis, Pavlovian inhibition, posttraining extinction
Several different ways to capture the notion of conditioned inhibition have been proposed, each reflecting a different underlying view of behavior indicative of inhibition. The most widely accepted view is that behavior indicative of conditioned inhibition reflects an association of negative value between the target inhibitor and the unconditioned stimulus (Rescorla & Wagner, 1972; Wagner & Rescorla, 1972). Other models have described inhibition as a second type of association between the CS and the reinforcer that can coexist with an excitatory association (e.g., Pearce & Hall, 1980; Wagner, 1981). The critical distinction between these two views is that the first postulates inhibition and excitation to be mutually exclusive, whereas the second does not. Yet another view is that behavior indicative of conditioned inhibition is the result of purely excitatory associations competing at the moment of testing with the target stimulus (Denniston, Savastano, & Miller, 2001; Miller & Matzel, 1988).
Theoretical positions aside, there appears to be general agreement concerning the different operations that result in inhibitor behavior: “An inhibitor is a nonreinforced stimulus that occurs in close proximity to another stimulus that is otherwise paired with reinforcement” (Savastano, Cole, Barnet, & Miller, 1999, p. 105). This operational definition is generally consistent with the different procedures that result in behavior indicative of conditioned inhibition (e.g., Pavlovian, negative contingency, inhibition of delay, etc.). The present report focuses on Pavlovian and differential inhibition. Pavlovian conditioned inhibition treatment involves presentations of a stimulus (A) alone that are consistently reinforced (+), and these trials are interspersed with presentations of A in compound with another stimulus (X) on which no reinforcement (−) occurs (i.e., A+/AX−; hereafter, A will always denote the excitor and X the target inhibitor). In contrast, differential inhibition treatment involves presentations of the excitor and the inhibitor consistently unpaired with each other, with only the excitor followed by the reinforcer (i.e., A+/X−). Differential inhibition superficially seems to violate the previously stated operational definition of conditioned inhibition, given that the target inhibitor is never presented in compound with an overt, otherwise-reinforced stimulus; however, many studies have successfully observed inhibition with this procedure (e.g., Hearst & Franklin, 1977; Miller, Hallam, Hong, & Dufore, 1991; Wessells, 1973). Given this apparent paradox, the central goal of the present research was to examine some of the conditions under which Pavlovian and differential inhibition are observed.
Previous studies have already compared these two procedures in their effectiveness to result in behavior indicative of inhibition. In some cases, equal levels of Pavlovian and differential inhibition were observed (e.g., Rescorla & LoLordo, 1965, in an avoidance preparation; Mahoney, Kwaterski, & Moore, 1975, in a nictitating membrane preparation). In contrast, others have found Pavlovian but not differential inhibition (e.g., Hoffman & Fitzgerald, 1982, in a conditioned heart rate preparation). In a within-subjects experiment, Cunningham (1981, Experiment 4) compared responding to a Pavlovian inhibitor X and a differential inhibitor Y to which subjects experienced equal nonreinforced exposure, but for Y alone the training excitor was not present (A+/AX−/Y−). He observed less responding to Y than to X, providing evidence suggestive of a stronger differential than Pavlovian inhibition (although Cunningham did not conduct any specific tests for inhibition, consistent with his focus on the within-compound association between A and X). Rescorla (1982) also used a within-subjects approach to compare the inhibitory strength of Pavlovian and differential inhibitors and, in contrast with Cunningham, observed more evidence of Pavlovian than differential inhibition in transfer tests. In a recent series of studies conducted between subjects with honeybees, differences between the effectiveness of the two procedures were not observed (Couvillon, Ablan, & Bitterman, 1999; Couvillon, Ablan, Ferreira, & Bitterman, 2001; Couvillon, Hsiung, Cooke, & Bitterman, 2005). In a further test of the effectiveness of Pavlovian versus differential procedures for conditioned inhibition, Williams, Travis, and Overmier (1986) trained their subjects with both treatments in a within-subjects design (A+/AX−/Y−) and used both summation and retardation tests to assess inhibition. It is interesting that they consistently observed greater differential inhibition in comparison with Pavlovian inhibition. They interpreted the absence of Pavlovian inhibition as being due to a within-compound association between the Pavlovian inhibitor X and the excitor A, which could have masked any observation of inhibition in the Pavlovian group. Consistent with their interpretation, extinguishing the associative status of the excitor A allowed them to observe Pavlovian inhibition. From this brief review of the existing literature, it can be concluded that evidence favoring either procedure, regardless of whether the comparison is conducted using between-subjects (e.g., Rescorla & LoLordo, 1965) or within-subjects (e.g., Cunningham, 1981; Williams et al., 1986) designs, is mixed. This report is intended to address these discrepancies.
A common assumption of most models of learning is that conditioned inhibition emerges from a process depending on the excitatory value of another stimulus, the putative training excitor. Thus, models of associative learning, both acquisition-focused (e.g., Rescorla & Wagner, 1972, which depends for its explanatory strength on processing during training and uses a simple response rule) and expression-focused (e.g., Denniston et al., 2001, which depends for its explanatory strength on its response rule and uses a simple acquisition rule), anticipate that the observation of Pavlovian inhibition depends on the excitatory value of the training excitor A, whereas the observation of differential inhibition depends on the excitatory value of the training context.
For example, the extended comparator hypothesis (Denniston et al., 2001), a response rule for the expression of associations, assumes that behavior indicative of inhibition results from a comparison at the time of testing between two excitatory representations of the US, one directly activated by the target CS and the other indirectly activated by the target CS. Behavior indicative of inhibition presumably decreases with increases in the directly activated US representation (X–US) and increases with increases in the indirectly activated representation (X–comparator stimulus–US). Consider the case of Pavlovian conditioned inhibition (see Figure 1). The extended comparator hypothesis explains behavior indicative of Pavlovian inhibition by positing that, at the time of testing, subjects compare the representation of the US directly activated by the target stimulus (Link 1, which is null because X is never paired with the US during conditioned inhibition training) with the representation of the US indirectly activated mainly through the comparator stimulus A (the product of Links 2 and 3). Given that during training the excitor when presented alone is reinforced (Link 3) and the target inhibitor is paired with the excitor (Link 2), at the time of testing the product of Links 2 and 3 greatly exceeds that of Link 1, and consequently behavior indicative of inhibition is observed. In the framework of the comparator hypothesis, a similar mechanism explains differential conditioned inhibition. But given that in this case the target inhibitor is never trained in compound with excitor A, the extended comparator hypothesis assumes that the training context is the only first-order comparator for X.
Figure 1.
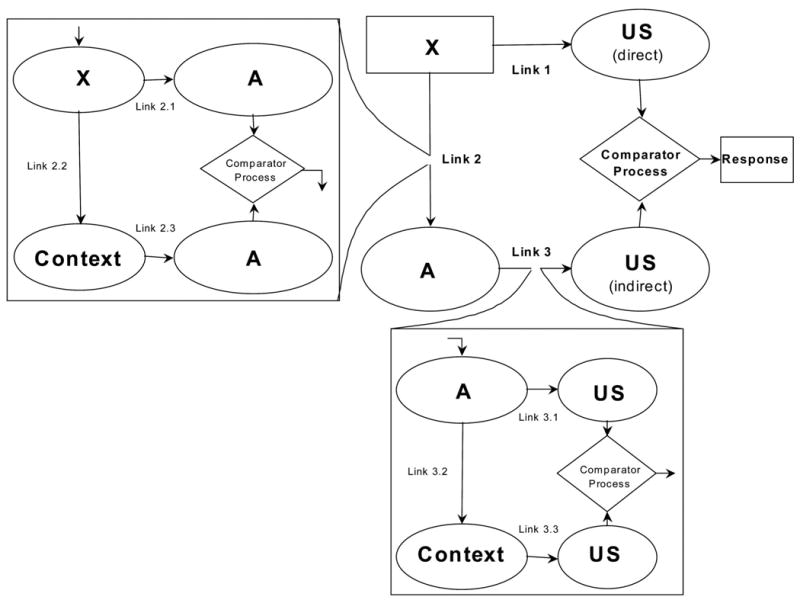
A schematic representation of the extended comparator hypothesis (Denniston et al., 2001) applied to Pavlovian conditioned inhibition. Here, the training excitor A is depicted as the target cue’s first-order comparator stimulus and the training context serves as the target cue’s second-order comparator stimulus. As it applies to differential inhibition, the context replaces A as the first-order comparator stimulus, given the absence of an X–A association, and there is no significant second-order comparator stimulus. Rectangles are physical stimuli and responses, ovals are stimulus representations, and the diamond is the actual comparator process.
According to the comparator hypothesis, as well as other theories of associative learning (e.g., Rescorla & Wagner, 1972; Wagner, 1981), differential inhibition should be observed when training is conducted with massed trials, which are known to strengthen the X–context (Link 2) and context–US associations (Link 3). When training is conducted with massed trials, the context becomes a good predictor of the US because it is not presented alone for longer periods of time in the absence of reinforcement (i.e., extinction). In order to capture the effect of trial spacing across many conditioned inhibition studies, we have calculated the total time subjects have spent during training in the training context and divided it by the total time the punctate stimulus was presented, either reinforced or nonreinforced. Although this is not exactly the way timing models (e.g., Gibbon & Balsam, 1981) estimate the so-called C/T ratio, this ratio allows comparisons among studies that used different parameters and serves as an index of the relationship between trial duration and overall session duration, which is known to determine the trial spacing effect in excitatory conditioning (i.e., superiority of behavioral control with spaced training trials). Note that a smaller ratio indicates relatively massed trials whereas a greater ratio indicates relatively spaced trials. Following this approach, it is readily observed that studies demonstrating strong differential inhibition have employed relatively massed training trials. For example, Williams et al. (1986) compared Pavlovian and differential inhibition in a within-subjects design. Specifically, they administered during training 16 trials each day (60-s CS on each trial) within a 118-min session. Thus, the ratio for this training situation is 7080/960, or 7.37. Consistent with most theories of learning, this relatively massed training resulted in differential but not Pavlovian inhibition, as evidenced by both summation and retardation tests. Miller et al. (1991) also studied the conditions under which differential inhibition is observed and found that the target inhibitor passed both summation and retardation tests for inhibition when training was conducted with a ratio of 6.66. In contrast, most of the studies that observed reliable Pavlovian inhibition used relatively spaced training trials. For example, Friedman, Blaisdell, Escobar, and Miller (1998) used a ratio of 22.5 and observed reliable Pavlovian condition inhibition, as evidenced by both tests of conditioned inhibition (for similar results with a large ratio, see Hallam, Matzel, Sloat, & Miller, 1990).
Considering that the available evidence regarding the relative strengths of Pavlovian and differential inhibition is mixed, the first objective of the present series of experiments was to investigate the conditions under which these two types of inhibition are readily observed (Experiments 1 and 2). The second goal of these experiments was to contrast current models of conditioning that predict different results with Pavlovian inhibition training under massed and spaced trial conditions (Experiments 2 and 3). In Experiment 1, we used a between-subjects design with relatively spaced trials to compare Pavlovian and differential inhibition with the same number of reinforced and nonreinforced training trials. Based on both acquisition and expression-focused models of learning, it was anticipated that when training trials are spaced, Pavlovian inhibition would be stronger than differential inhibition.
Experiment 1
Experiment 1 used a factorial design in which separate groups received either Pavlovian conditioned inhibition training (A+/ AX−) or differential inhibition training (A+/X−) with spaced training trials. Two groups experienced each treatment followed by transfer excitor training (B+; see Table 1). Subsequently, one of the groups from each condition was tested for summation (BX), while the remaining group served as a control (B compounded with an irrelevant stimulus Y). After this first test of inhibition, the group that served as a control for the summation test experienced reinforced presentations of the target inhibitor (X+; retardation training) and was tested for retardation (X), while the group that was the target group for the summation test served as a control group for the retardation test (Y+, then test on Y). Given that the number of reinforced and nonreinforced trials during training was the same for Pavlovian and differential inhibition, this approach allowed a direct comparison of the two treatments as evidenced by the two-test strategy proposed by Hearst (1972) and Rescorla (1969).
Table 1.
Design of Experiments 1 and 2
| Group | Preexposure | Phase 1 inhibition training | Phase 2 transfer training | Summation test | Retardation training | Retardation test |
|---|---|---|---|---|---|---|
| Pav-Ret | 4 X−/4 Y− | 36 A→US/60AX− | 4 B→US | BY | 4 X→US | X |
| Pav-Sum | 4 X−/4 Y− | 36 A→US/60AX− | 4 B→US | BX | 4 Y→US | Y |
| Dif-Ret | 4 X−/4 Y− | 36 A→US/60X− | 4 B→US | BY | 4 X→US | X |
| Dif-Sum | 4 X−/4 Y− | 36 A→US/60X− | 4 B→US | BX | 4 Y→US | Y |
Note. X and Y = white noise and tone, counterbalanced; A = flashing light; B = clicks; US = footshock. → denotes followed by. − denotes no reinforcement. Pav = Pavlovian inhibition training; Dif = differential inhibition training; Ret = retardation; Sum = summation. Numbers next to the pairings indicate total number of each type of trial during training. Note that the same experimental design as well as total number of trials was used in Experiments 1 and 2, with the only differences being the intertrial interval (and total session length) during inhibition training, which was 14.5 min in Experiment 1 and 117 s in Experiment 2.
Method
Subjects
Subjects were 48 female (172–244 g) and 48 male (216–356 g) experimentally naïve, Sprague–Dawley-descended young adult rats (N = 96) bred in our colony. Subjects were individually housed and maintained on a 16-hr light/8-hr dark cycle with experimental sessions occurring roughly midway through the light portion. All subjects were handled for 30 s three times per week from weaning until the initiation of the study. Subjects had free access to food in their home cages. One week prior to initiation of the experiment, water availability was progressively reduced to 20 min per day, provided approximately two hours after any scheduled treatment. The experiment was conducted in two completely counterbalanced replications.
Apparatus
Six instances of each of two contexts (Chambers V and R) were used in this experiment. Chamber V was the shape of a vertical truncated V, 27 cm long, 29.5 cm high, 21.5 cm wide at the top, and 5.5 cm wide at the bottom. The ceiling was clear Plexiglas, the front and back walls were black Plexiglas, and the sidewalls were stainless steel. The floor was comprised of two 27-cm-long stainless steel plates, 2 cm wide, with a 1.5-cm gap between the two plates. A constant-current footshock, produced by a high voltage AC circuit in series with a 1.0-MΩ resistor, could be delivered through the metal walls and floor of the chamber. Each instance of Chamber V was housed in a separate sound- and light-attenuating environmental isolation chest. The chambers were each illuminated by a 7-W (nominal at 120 VAC, but driven at 60 VAC) lightbulb, which was mounted on the inside wall of the environmental enclosure, approximately 30 cm from the center of the experimental chamber. The light entered the chamber primarily by reflection from the ceiling of the environmental chest.
Chamber R was rectangular, measuring 24.0 × 9.0 × 12.5 cm (l × w × h). The walls and ceiling of Chamber R were clear Plexiglas, and the floor was comprised of stainless steel rods measuring 0.5 cm in diameter, spaced 1.5 cm apart (center to center). The rods were connected by NE-2 bulbs, which allowed for the delivery of a constant-current footshock produced by a high voltage transformer in series with a 1.0-MΩ resistor. Each instance of Chamber R was housed in a separate light- and sound-attenuating environmental isolation chest. Each chamber was dimly illuminated by a 2-W (nominal at 120 VAC, but driven at 60 VAC) incandescent houselight mounted on an inside wall of the environmental chest located approximately 30 cm from the animal enclosure. The light intensities inside the two types of chambers were approximately equal due to the difference in opaqueness of the walls of Chambers V and R.
All chambers could be equipped with a water-filled lick tube that extended 1 cm from the rear into a 5.0-cm deep cylindrical niche, which was 4.5 cm in diameter, centered left and right on a narrow wall of the chamber, with its bottom 1.75 cm above the floor of the apparatus. There was a horizontal photobeam detector 1 cm in front of the lick tube that was broken whenever the subject licked the tube. Three 45-Ω speakers on the inside walls of the isolation chests could deliver a 3000-Hz tone, a click (6/s), or a white noise stimulus, all 8 dB (C) above background (76 dB, produced mainly by a ventilation fan). A visual stimulus that consisted of a flashing light (0.25 s on/0.25 s off) could also be presented. The light was provided by either a 25-W bulb (Chamber R) or a 100-W bulb (Chamber V), both nominal at 120 VAC but driven at 60 VAC. The bulbs were mounted on an inside wall of the environmental chest, approximately 30 cm from the center of the experimental chamber. A 1.0-mA footshock, which served as the US, could be delivered through the chamber floors of both types of chambers. The same footshock intensity was used in each phase of the experiment. In this experiment, the tone, white noise, light, and click CSs were 30 s in duration, and the footshock US was 0.5 s in duration. For all animals, the flashing light was CS A, the click train was CS B, and the white noise and tone were CS X and Y, respectively, counterbalanced within groups.
Procedure
Subjects were randomly assigned to one of four groups: Pavlovian retardation (Pav-Ret), Pavlovian summation (Pav-Sum), differential retardation (Dif-Ret), and differential summation (Dif-Sum), counterbalanced for sex (ns = 24). Phase 1 and retardation training were conducted in one (inhibition training) context (V or R), and all other treatments were conducted in the remaining (test) context in a counterbalanced manner within groups. Two contexts were used to minimize differential fear of the test context summating with fear to the CS. Additionally, Schachtman, Brown, Gordon, Catterson, and Miller (1987) found that retardation tests for inhibition are most effective if the retardation training occurs in the inhibition training context.
Acclimation and preexposure
Prior to inhibition training, two days of preexposure to the test context and to some of the stimuli used in the present design were conducted. Subjects were exposed daily to the experimental chamber during 60-min sessions and received two presentations of X and Y each day. This was conducted in order to reduce unconditioned responding to X and Y and to reduce the likelihood of X and A being configured during subsequent training. Water lick tubes were available during these two days. Stimuli X and Y were presented in an interspersed manner at 10, 25, 30, and 50 min into the 60-min session.
Phase 1: inhibition training
Prior to Phase 1, the lick tubes were removed from the inhibition training chambers. On Days 3–14, during daily 100-min sessions, subjects in Condition Pav received three A+ trials and 5 AX− trials. Subjects in Condition Dif received three A+ trials and 5 X– trials. The mean (± standard error) intertrial interval (ITI) was of 14.5 (± 6) min. On reinforced trials, the US was presented during the last 0.5 s of the CS.
Phase 2: transfer training
On Day 15, all subjects received, in the test context, training with a transfer excitor to be used on the summation test. Specifically, subjects received four reinforced trials of CS B (B+). As in previous phases, CS B duration was 30 s and the US was presented during the last 0.5 s of the CS presentation. Session duration was 60 min, and reinforced trials took place at 5, 30, 43, and 54 min.
Reacclimation
On Days 16 and 17, the lick tubes were reinserted, and subjects were allowed to drink during daily 60-min sessions in the test context. This treatment was intended to restabilize baseline levels of drinking. These sessions did not include any nominal stimulus presentations.
Summation testing
On Day 18 with the lick tubes present in the test context, all subjects were tested for suppression to the transfer excitor in the presence of the conditioned inhibitor X (Groups Pav-Sum and Dif-Sum) or an irrelevant stimulus Y (Groups Pav-Ret and Dif-Ret). This was accomplished by presenting the corresponding compound BX or BY immediately on completion of the first 5 s of cumulative licking (as measured by the total amount of time the infrared photobeam was disrupted) during the test session. Thus, all subjects were drinking at the time of CS onset. Time to complete this initial 5 s of cumulative licking (pre-CS scores) and time to complete an additional 5 s after the onset of the Test CS (CS score) were recorded. Test sessions were 16 min in duration with a ceiling score of 15 min being imposed on the time to complete 5 s of cumulative drinking in the presence of the Test CS. Once on, the CS stayed on regardless of the rat’s behavior. Consequently, all subjects received a full 15 min of exposure to BX or BY.
Retardation training
On Day 19 in the inhibition training context with the lick tubes absent, subjects in the retardation condition experienced four reinforced trials with the target stimulus (X+) and subjects in the summation condition experienced four reinforced trials with a control CS (Y+) in order to subsequently assess the capability of the putative inhibitor to become a conditioned excitor. As in all other phases of training, the CS duration was 30 s and the same reinforcer was presented during the last 0.5 s of CS presentation. Note that the groups that were tested with the target stimulus X in the summation test (Groups Pav-Sum and Dif-Sum) served as control groups for the retardation test, whereas those groups that served as control groups in the summation test (Groups Pav-Ret and Dif-Ret) were in this phase trained and tested with the target inhibitory stimulus X. This procedure has successfully been used in other studies (Williams et al., 1986). No drinking tubes were presented during this phase of the experiment. Session duration was of 30 min, and reinforced trials took place at 4, 8, 20, and 27 min. The rationale for using this trial spacing was that we planned to conduct a second study in which all conditions were the same, except for the trial spacing during inhibition training. Therefore, we chose a trial spacing for retardation training that was intermediate between the ones used in Experiments 1 and 2 during inhibition training.
Reacclimation
On Days 20 and 21, the lick tubes were reinserted and subjects were allowed to drink freely during these daily 60-min sessions in the test context. This treatment served to restabilize baseline levels of drinking.
Retardation testing
On Day 22 with the lick tubes present in the test context, subjects were tested for suppression to the target CS X (Groups Pav-Ret and Dif-Ret) or the control CS Y (Groups Pav-Sum and Dif-Sum) by presenting X or Y immediately on completion of the first 5 s of cumulative licking. Thus, all subjects were drinking at the time of CS onset. Time to complete this initial 5 s of cumulative licking (pre-CS score) and time to complete an additional 5 s after the onset of the Test CS (CS score) was recorded. Test sessions were 16 min in duration with a ceiling score of 15 min being imposed on the time to complete 5 s of cumulative drinking in the presence of the Test CS.
Data Analysis
Following the convention of our laboratory, subjects that took more than 60 s to complete their first 5 s of cumulative licking (i.e., prior to CS onset) on either test day, thereby exhibiting an unusual reluctance to drink in the test context, were eliminated from that day’s analyses. Based on this criterion, 1 subject from Group Pav-Ret and 1 from Group Dif-Ret were excluded from the analyses of the summation data. Following the same criterion, 1 subject from Group Pav-Sum, 3 from Group Dif-Ret, and 2 from Group Dif-Sum were excluded from the analyses of the retardation test data. Suppression data were transformed to log (base 10) scores to approximate a normal distribution of scores within groups and thereby facilitate the use of parametric statistics. An alpha level of .05 was adopted for all statistical tests. The transformed data were analyzed with a 2 × 2 ANOVA with type of inhibition training (Pavlovian or Differential) and target stimulus presented at the time of testing (Inhibitor or Control) as factors. These 2 × 2 ANOVAs were performed twice, once for the summation data and once for the retardation data. The study was conducted in two replications of N = 48 each, due to an experimenter error in the first replication that lost all of the retardation test data. Therefore, the present analyses for the summation test was performed on the data gathered in both replications (n = 94, following the above-mentioned criteria), and the retardation test analysis was performed on the data collected on only the second replication (n = 42).
Results and Discussion
Summation Test
As can be observed in Figure 2 (left), with spaced training trials less suppression to the compound of stimuli B and X on the summation test was observed in subjects trained with the Pavlovian inhibition procedure than with the differential inhibition procedure. Responding to B and Y in all other groups was similarly greater, indicating that differential inhibition treatment did not decrease suppression to the transfer excitor B, nor did the neutral stimulus (Y) in the control groups tested with BY. These impressions were confirmed by the following analyses.
Figure 2.
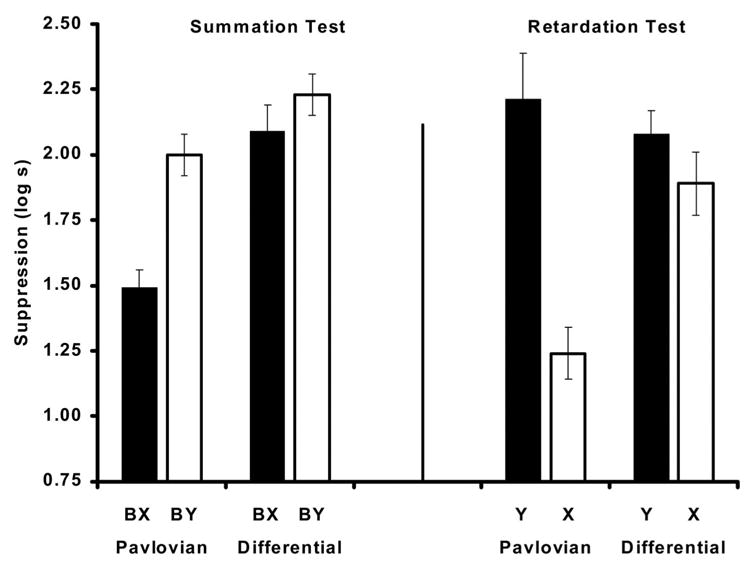
Mean times to complete 5 cumulative seconds of drinking upon presentation of the target CS in Experiment 1. Left: Results of the summation test. Right: Results of the retardation test. Note that subjects that served as controls for the summation test (Ret groups) were the target groups for the retardation test, and vice versa. The actual test stimuli are indicated within parentheses. See Table 1 for the treatments of the four groups.
A two-way ANOVA with type of inhibition training (Pavlovian vs. Differential) and test (BX vs. BY, in which the summation groups were tested on BX and the retardation groups were tested on BY) performed on the transformed pre-CS scores revealed no main effects or interactions (all ps > .07), indicating that subjects did not differ appreciably in their initial fear to the context before testing.
A similar ANOVA performed on the subjects’ suppression scores during the presentation of the compound revealed a main effect of inhibition training, F(1, 90) = 19.87, p < .001, MSE = 0.20; a main effect of test F(1, 90) = 12.41, p < .001, MSE = 0.20; and an interaction between these factors F(1, 90) = 4.02, p < .05, MSE = 0.20. A second analysis including replication as a factor showed that replication did not interact with any of the variables under consideration, nor did it alter the main results. Planned comparisons using the overall error term from the 2 × 2 ANOVA demonstrated that subjects in Group Pav-Sum did not suppress as much to BX as subjects in Group Pav-Ret suppressed to BY, indicating the effectiveness of our intended inhibitor to diminish responding when presented in compound with an excitor, F(1, 90) = 15.27, p < .001. As anticipated, the procedure did not lead to the development of inhibition in the differential group; these subjects (Group Dif-Sum) did not suppress more or less to BX than their respective controls (Dif-Ret) did to BY, F(1, 90) = 1.15, p < .28. An additional comparison indicated that suppression to BX was greater in Group Dif-Sum than in Group Pav-Sum, confirming our presumptions regarding the inhibitory potential of the Pavlovian and differential inhibitors trained in a spaced manner, F(1, 90) = 21.33, p < .001. The results of the summation test, as inferred from statistical analyses, showed that behavior indicative of inhibition developed only with the Pavlovian procedure.
Retardation Test
As can be observed in Figure 2 (right), subjects in the Pavlovian group tested for retardation (Pav-Ret) showed less suppression to the target stimulus X than subjects in the differential inhibition condition (Dif-Ret), which experienced the same number of reinforced trials with X. Thus, with spaced training trials, Pavlovian inhibition training resulted in more inhibition than differential inhibition training. These impressions were confirmed by the following analyses.
Analyses of the pre-CS scores from the retardation test were conducted with a 2 × 2 ANOVA with type of inhibition training (Pavlovian vs. Differential) and test (X vs. Y) as factors. This analysis did not reveal any main effects or interaction (all ps > .05). A similar analysis conducted on the suppression latencies indicated a main effect of test, F(1, 38) = 18.74, p < .001, MSE = 0.18, and an interaction between the factors under consideration, F(1, 38) = 8.35, p < .01, MSE = 0.18. A priori comparisons using the overall error term from the ANOVA found that subjects in Group Pav-Ret suppressed less than subjects in Group Pav-Sum, demonstrating the retarded emergence of behavioral control by the putative inhibitor X when compared with the acquisition of a neutral cue Y, F(1, 38) = 28.82, p < .001. Also, training with spaced trials did not lead to the development of inhibition in the differential group; these subjects (Dif-Ret) did not suppress less to X than their respective controls (Dif-Sum) did to Y, F(1, 38) = 0.94, p < .33. An additional comparison indicated that suppression to X was greater in Group Diff-Ret than in Group Pav-Ret, F(1, 38) = 11.65, p < .01. Thus, the retardation test data agreed with the summation test data in finding Pavlovian inhibition training to be more effective than differential when the training trials were spaced.
As we predicted, based on our analysis of previous data, the spaced training given in this experiment yielded good evidence of Pavlovian but not differential inhibition. We expected these results given that training was conducted with spaced trials, which would give the differential inhibitor’s comparator stimulus (the training context) a weak association with the US (Link 3) as well as with the target inhibitor (Link 2). Although here we are explaining the present results in terms of the extended comparator hypothesis, these results are not at odds with other models of learning, such as the Rescorla–Wagner model (Wagner & Rescorla, 1972) or Wagner’s SOP (Wagner, 1981). Each of these accounts explains differential inhibition as a result of the context generating an expectation of the US that is not fulfilled when the target inhibitor is presented, thus endowing the target inhibitor with inhibitory properties. Given that training was conducted under relatively spaced trials, these models also anticipate differential inhibition to be minimal under the present circumstances.
Experiment 2
The results of Experiment 1 shed light on the conditions under which Pavlovian and differential inhibition are observed but did not provide information that would allow discriminating among learning theories. Nevertheless, there is a prediction of the extended comparator hypothesis that would allow us to differentiate among these theories. The comparator hypothesis explains Pavlovian inhibition by positing that at the time of testing the indirectly activated representation of the US (mediated by all other cues presented during training of the target cue, in this case A and the training context) is larger than the directly activated representation, which in the case of inhibition training is null because the target stimulus was never paired with the US. For differential inhibition, it is the context alone that acts as a comparator because A was never paired with the target stimulus X (A will only down-modulate the strength of Link 3 by acting as a comparator stimulus for the training context). Additionally, the extended comparator hypothesis (Denniston et al., 2001) states that the links that indirectly retrieve a representation of the US at the time of test are subject to their own comparator stimuli. These stimuli are called second-order comparator stimuli and are capable of down-modulating the effectiveness of first-order comparators (see Figure 1). Thus, the extended comparator hypothesis predicts that differential inhibition will be strongest when training trials are massed but that Pavlovian inhibition should be disrupted because the context will compete with the target excitor A. In Figure 1, after massed Pavlovian inhibition training, the context should down-modulate the effectiveness of A as a comparator for X (Links 2.2, 2.3, 3.2, and 3.3 in Figure 1), and thus little behavior indicative of inhibition should be observed. The purpose of Experiment 2 was to test this prediction.
Experiment 2 was conducted to assess behavior indicative of inhibition after massed Pavlovian and differential inhibition training, in contrast to the spaced training trials of Experiment 1. Therefore, the same experimental design as Experiment 1 was used, with the only difference being the trial spacing during inhibition training (Phase 1 in Table 1). For reasons previously explained, we expected to observe behavior indicative of inhibition in the groups given differential inhibition training but perhaps not in those groups given Pavlovian inhibition training.
Method
Subjects and Apparatus
The subjects were 24 male (291–361 g) and 24 female (204–254 g) experimentally naïve Sprague–Dawley-descended rats obtained from our breeding colony. Following the design of Experiment 1, subjects were randomly assigned to one of four groups (ns = 12), counterbalanced within groups for sex. Subjects were maintained and housed as in Experiment 1. The apparatus was the same as in Experiment 1.
Procedure
The procedure throughout all phases of the experiment was the same as in Experiment 1, with the exception of the ITI and session length during inhibition training. In contrast to Experiment 1 in which the session length was 100 min and the mean ITI was of 14.5 min, in Experiment 2 the session length was 20 min and the mean (± standard error) ITI was of 117 (± 18) s (see Table 1).
Following the convention of our laboratory, those subjects that took more than 60 s to start drinking prior to CS onset (i.e., pre-CS scores) were excluded from the respective analyses. On the basis of this criterion, no subjects were excluded from the statistical analyses of either test.
Results and Discussion
Summation Test
As can be observed in Figure 3 (left), the summation test demonstrated that subjects that received Pavlovian conditioned inhibition training with massed trials did not show a decrease in suppression when a conditioned excitor (B) was tested in the presence of the intended inhibitor (X). In contrast, subjects that also were trained under massed training but with the differential inhibition procedure did show a decrease in suppression when a conditioned excitor was tested in the presence of the inhibitor (X). In other words, with massed training the differential inhibitor, but not the Pavlovian, decreased suppression to a transfer excitor when they were presented in compound. These observations were confirmed by the following statistical analyses.
Figure 3.
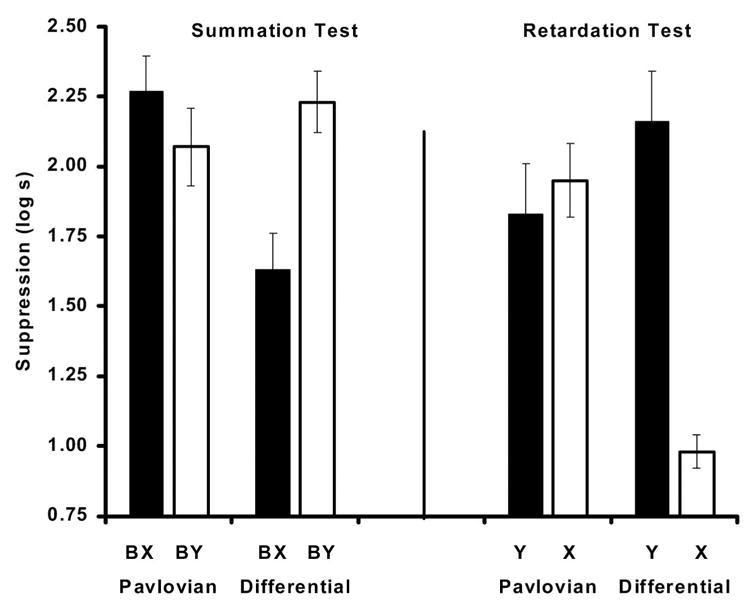
Mean times to complete 5 cumulative seconds of drinking upon presentation of the target CS in Experiment 2. Left: Results of the summation test. Right: Results of the retardation test. Note that subjects that served as controls for the summation test (Ret groups) were the target groups for the retardation test, and vice versa. See Table 1 for the treatments of the four groups.
A 2 × 2 ANOVA with main factors of type of inhibition training (Pavlovian vs. Differential) and test condition (BX vs. BY) was used to analyze pre-CS scores. This analysis did not show any main effects or interaction (all ps > .50). A similar ANOVA conducted on the transformed latencies to complete 5 s of consecutive licking in the presence of the compound demonstrated a marginal main effect of type of inhibition training, F(1, 44) = 3.73, p < .06, MSE = 0.19, and an interaction between the factors under consideration, F(1, 44) = 9.89, p < .01, MSE = 0.19. Planned comparisons using the overall error term from the ANOVA demonstrated that subjects trained with differential conditioned inhibition suppressed less when the transfer excitor was tested in the presence of the target inhibitor (BX; Group Dif-Sum) than when the excitor was presented in compound with a relatively neutral stimulus (BY; Dif-Ret), F(1, 44) = 11.05, p < .01. This same comparison among the subjects trained with Pavlovian conditioned inhibition did not reveal any differences, F(1, 44) = 1.26, p < .26. A final comparison among the two groups that were tested with the intended inhibitors demonstrated that subjects trained with the differential procedure (Group Dif-Sum) suppressed less to BX than subjects trained with the Pavlovian procedure (Pav-Sum), F(1, 44) = 12.89, p < .001. In other words, as evidenced by the summation test, we observed behavior indicative of conditioned inhibition in the subjects that experienced differential inhibition training but not in those that experienced Pavlovian inhibition training.
Retardation Test
As can be observed in Figure 3 (right), retarded emergence of conditioned responding to the target inhibitor was observed in the group that experienced differential inhibition training but not in the group that experienced Pavlovian inhibition training. This observation was confirmed by the following statistical analyses.
A 2 × 2 ANOVA with type of inhibition training (Pavlovian vs. Differential) and test condition (X vs. Y) as main factors was performed on baseline scores to detect any differences in fear to the test context. This analysis did not indicate any main effect nor interactions (all ps > .80). A similar ANOVA conducted on the transformed latencies demonstrated a main effect of type of inhibition training, F(1, 44) = 4.46, p < .05, MSE = 0.27; a main effect of test stimulus, F(1, 44) = 12.21, p < .01, MSE = 0.27; and a significant interaction between the factors under consideration, F(1, 44) = 18.31, p < .001, MSE = 0.27. Planned comparisons using the overall error term from the 2 × 2 ANOVA determined that the animals trained with the differential inhibition procedure (Dif-Ret) suppressed less in the presence of the putative inhibitor X than in the presence of another stimulus Y that was equally reinforced during the retardation-test training phase (Dif-Sum), F(1, 44) = 30.22, p < .001. Similarly, the group trained with the differential inhibition procedure (Dif-Ret) suppressed less in the presence of the inhibitor X than did subjects for which the inhibitor was trained with the Pavlovian procedure (Pav-Ret), F(1, 44) = 20.43, p < .001. Groups trained with the Pavlovian inhibition procedure did not exhibit the same pattern; subjects responded equally to the putative inhibitor X and to the control stimulus Y that was equally reinforced during the retardation training phase, F(1, 44) = 0.30, p = .58.
In sum, the present experiment demonstrated behavior indicative of inhibition, as evidenced by both summation and retardation tests, in subjects that were trained with the differential inhibition procedure but not in subjects trained with the Pavlovian inhibition procedure. In the framework of the extended comparator hypothesis, behavior indicative of inhibition is a performance effect that occurs as a result of a comparison between the independent excitatory associative strengths of the CS and comparator stimuli. The comparator stimuli for a given CS are presumably those cues that are temporally and spatially proximate to the target CS during training. Therefore, the context should serve as a comparator stimulus for CS X in a differential inhibition situation, and the excitor (A) primarily should serve as the comparator stimulus in a Pavlovian conditioned inhibition situation. In the present study, because training was conducted with massed trials, we expected the context to gain associative strength and facilitate observation of behavior indicative of inhibition in the differential inhibition procedure, but not in the Pavlovian procedure. In the latter case, both A and the training context are presumably first-order and second-order comparator stimuli for the target (X), as well as comparators for each other. Thus, the context is assumed to down-modulate A’s role as a first-order comparator stimulus, and A is assumed to down-modulate the context’s role as a first-order comparator stimulus, with these two processes conjointly preventing any observation of inhibition. The present study confirmed these predictions. Notably, this account anticipates weak responding (as distinct from weak learning) to A following massed training trials due to the comparator process; however, we did not Test CS A in this study.
The present results are problematic for acquisition-focused theories of learning because these models predict that, under the present conditions, the target CS should acquire as much inhibitory status in the Pavlovian inhibition situation as in the differential inhibition situation, rather than less. This assertion relies on the fact that most acquisition-focused models view inhibition as the result of a discrepancy between the expected US and the absence of the US. As applied to the case of Pavlovian inhibition in the present experiment, both the excitor A and the context were good predictors of the US due to the massed training. Because the expectancy of the US activated by the context and A in the Pavlovian case should have been at least equal to that by the context alone in the differential case, the discrepancy between the expected US and the absence of the US should always be the same (if not greater during early training) in Pavlovian inhibition than in differential inhibition. Therefore, behavior indicative of inhibition should have been observed in the Pavlovian groups as it was observed in the differential groups, for which only the context was a predictor of the US. Contrary to this prediction, behavior indicative of inhibition was observed only in the groups trained with the differential procedure.
The results of Experiment 2 are interesting for two reasons. First, they discriminate between the predictions of different theories of learning. Second, they suggest a further prediction based on the extended comparator hypothesis. In Experiment 2, we did not observe behavior indicative of inhibition when Pavlovian conditioned inhibition training occurred with massed trials. Based on the extended comparator hypothesis, this outcome was due to the context (acting as a second-order comparator) down-modulating A (the training excitor), which is presumably the primary basis for the observation of behavior indicative of inhibition when X is presented at test. If this is correct, then extinguishing the training context following training should allow the observation of Pavlovian inhibition after massed training trials because the context would no longer down-modulate the effectiveness of A as a comparator stimulus for X. Testing this prediction of the extended comparator hypothesis was the aim of Experiment 3.
Experiment 3
Experiment 3 was conducted to assess the effect of extinguishing the inhibition training context after Pavlovian inhibition training with massed trials. If the absence of inhibition in the Pavlovian groups of Experiment 2 was due to the context interfering with the indirect retrieval of the representation of A, then contextual extinction should counteract this interference. We used a factorial design in which four groups of rats were given massed Pavlovian inhibition training. On the four days immediately following training, half of the subjects were massively exposed to the training context. As in previous studies in this series, both summation and retardation tests of inhibition were conducted. The experimental design is presented in Table 2.
Table 2.
Design of Experiment 3
| Group | Preexposure | Phase 1 inhibition training | Phase 2 extinction | Phase 3 transfer training | Summation test | Retardation training | Retardation test |
|---|---|---|---|---|---|---|---|
| NExt-Ret | 4 X−/4 Y− | 36 A→US/60 AX− | ctx | 4B→US | BY | 4X→US | X |
| NExt-Sum | 4 X−/4 Y− | 36 A→US/60 AX− | ctx | 4B→US | BX | 4Y→US | Y |
| Ext-Ret | 4 X−/4 Y− | 36 A→US/60 AX− | CTX | 4B→US | BY | 4X→US | X |
| Ext-Sum | 4 X−/4 Y− | 36 A→US/60 AX− | CTX | 4B→US | BX | 4Y→US | Y |
Note. X and Y = white noise and tone, counterbalanced; A = flashing light; B = clicks; US = footshock. → denotes followed by. − denotes no reinforcement. ctx denotes brief context exposure. CTX denotes prolonged context extinction. Ret = retardation; Sum = summation; NExt = no context extinction; Ext = context extinction. Numbers next to the pairings indicate total number of each type of trial during training.
Method
Subjects and Apparatus
The subjects were 48 female (206–278 g) and 48 male (308–407 g) experimentally naïve Sprague–Dawley-descended rats obtained from our breeding colony. Subjects were randomly assigned to one of four groups (ns = 24), counterbalanced within groups for sex. We intentionally increased the number of subjects for each group in the present study relative to Experiments 1 and 2 because, although statistically significant, the effect size in previous experiments was relatively small. Subjects were maintained and housed as in Experiments 1 and 2. The apparatus was the same as in Experiments 1 and 2.
Procedure
With the exception of inhibition training and extinction treatments (Phases 1 and 2), all other treatments in this experiment were the same as in previous experiments. In other words, acclimation and preexposure, transfer training, reacclimation, summation testing, retardation training, and retardation testing were conducted as in the previous experiments.
Phase 1: inhibition training
Prior to Phase 1, lick tubes were removed from the experimental chambers. On Days 3–14, during daily sessions, all subjects experienced 3 A + trials and 5 AX– trials in the inhibition training context. The session length was 20 min in duration and the mean (± standard error) ITI was of 117 (± 18) s. On reinforced trials, the US was presented during the last 0.5 s of the CS.
Phase 2: extinction treatment
During Days 15 to 18, subjects in the context extinction condition (Groups Ext-Ret and Ext-Sum) were exposed daily to the inhibition training context for 150 min. Every 30 min the sound-attenuating enclosures were opened to ensure that the subjects were awake. Subjects in the two remaining groups (NExt-Ret and NExt-Sum) were also exposed to the training context, but for only 5 min per day. Due to this manipulation, all subjects experienced equal handling treatment. No nominal stimulus was presented during this phase.
As in previous experiments, those subjects that took more than 60 s to start drinking in the testing context prior to CS presentation were excluded from the respective analyses. On the basis of this baseline criterion, 4 subjects from Group Ext-Ret, 1 from Group NExt-Ret, and 1 from Group Ext-Sum were excluded from the analyses of the summation data. Following the same criterion, 2 subjects from Group NExt-Ret were excluded from the analyses of the retardation test.
Results and Discussion
The analyses presented below were initially conducted with replication as an independent factor, and we found that replication did not interact with any of the variables under consideration (all ps > .17). In other words, the conclusions drawn from the analyses were not confounded by replication.
Summation Test
The Figure 4 (left) shows the results from the summation test. As can be observed in the groups that resembled those of Experiment 2 (Groups NExt-Ret and NExt-Sum), no significant difference in suppression resulted when the transfer excitor was presented in compound with the target inhibitor (BX) or a neutral stimulus (BY). In other words, no behavior indicative of inhibition was observed after Pavlovian inhibition training with massed trials. In contrast, posttraining extinction of the training context caused X to serve as a conditioned inhibitor. These impressions were confirmed by the following analyses.
Figure 4.
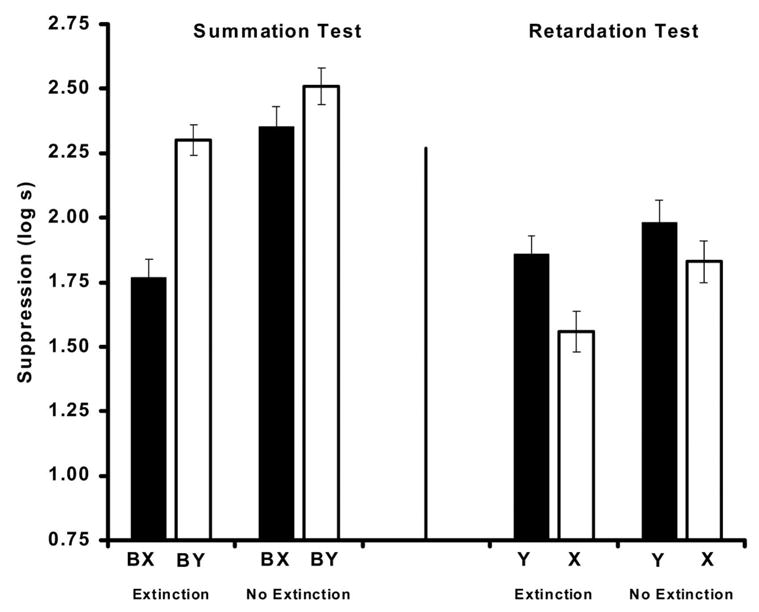
Mean times to complete 5 cumulative seconds of drinking upon presentation of the target CS in Experiment 3. Left: Results of the summation test. Right: Results of the retardation test. Note that subjects that served as controls for the summation test (Ret groups) were the target groups for the retardation test, and vice versa. All subjects received Pavlovian inhibition training. See Table 2 for treatments of the four groups.
A 2 × 2 ANOVA with posttraining context extinction (Extinction vs. No Extinction) and test (BX vs. BY) as factors conducted on pre-CS scores did not indicate any baseline differences in fear to the testing context (all ps > .08). A similar ANOVA conducted on the suppression scores during presentation of the compound of stimuli detected a main effect of posttraining context extinction, F(1, 86) = 24.31, p < .001, MSE = 0.14; a main effect of test, F(1, 86) = 18.39, p < .001, MSE = 0.14; and an interaction, F(1, 86) = 5.62, p < .05, MSE = 0.14. Subsequent planned comparisons using the overall error term from the ANOVA revealed that Group Ext-Sum suppressed less in the presence of the target compound BX than Group Ext-Ret to BY (the control group for summation testing), F(1, 86) = 21.19, p < .001, and Group NExt-Sum to BX (the control group for posttraining context extinction), F(1, 86) = 27.97, p < .001. Additionally, a comparison between the two groups that did not experience posttraining context extinction did not achieve statistical significance, F(1, 86) = 1.92, p = .17.
Retardation Test
Figure 4 (right) shows the results from the retardation test. Similar to the results of the summation test, the two groups that did not experience posttraining extinction of the training context (Groups NExt-Ret and NExt-Sum) resembled the comparable groups in Experiment 2. That is, no significant differences in suppression were observed when the putative inhibitor X was reinforced in comparison with a control stimulus Y that experienced the same number of reinforced trials. In other words, without posttraining extinction of the training context, no behavior indicative of inhibition was observed after Pavlovian inhibition with massed training trials. In contrast, extinction of the training context resulted in less responding to CS X than CS Y, as observed in the performance of Group Ext-Ret compared with Group Ext-Sum. These impressions were confirmed by the following statistical analyses.
A 2 ×2 ANOVA with posttraining context extinction (Extinction vs. No Extinction) and test (X vs. Y) as factors conducted on pre-CS scores did not indicate any baseline differences in fear to the testing context (all ps > .05). A similar ANOVA conducted on the suppression scores after presentation of the target stimulus detected a main effect of posttraining context extinction, F(1, 90) = 5.14, p < .05, MSE = 0.17, and a main effect of test stimulus, F(1, 90) = 7.20, p < .01, MSE = 0.14. The interaction between these factors was not significant. Subsequent planned comparisons using the overall error term from the ANOVA showed that Group Ext-Ret suppressed less to the presentation of X than Group Ext-Sum did to Y (the control group for testing), F(1, 90) = 6.42, p < .05, and Group NExt-Ret did to X (the control group for posttraining context extinction), F(1, 90) = 4.78, p < .05. A final comparison between the two groups that did not experience posttraining context extinction did not reach statistical significance, F(1, 86) = 1.62, p < .20. Thus, evidence of context extinction making X inhibitory was provided by the planned comparison, but not in the interaction of the ANOVA, although the nonsignificant interaction was in the same direction.
In summary, Experiment 3 replicated the results of Experiment 2, in that behavior indicative of inhibition was not observed after Pavlovian inhibition training with massed trials. Further, this experiment also demonstrated that extinction of the training context induced behavior indicative of inhibition after Pavlovian inhibition training with massed trials. Presumably, extinction of the training context restored the potential of the target A to down-modulate responding to the target inhibitor X, thereby allowing X to elicit behavior indicative of inhibition.
One alternative explanation of our data comes from demonstrations that inhibitory associations might be masked by excitatory associations so that, when the latter are extinguished, inhibition is observed after the target CS is released from its associations with excitors (the context in the present case; see Williams et al., 1986). However, in a recent report from our laboratory (Chang, Blaisdell, & Miller, 2003), it was observed that inhibition that developed after many backward pairings of the target CS with the US (i.e., US → X) was no longer observed if the training context was extinguished. In other words, in that case where only one comparator was effectively driving inhibition, extinction of that comparator stimulus did not unmask inhibition but rather deactivated inhibition. This is also consistent with the notion of inhibition as a slave process that depends on the status at test of the training excitors (Hallam et al., 1990; Lysle & Fowler, 1985). In our experiment, the putative inhibitor had two (rather than one) comparators (the excitor A and the training context) so that extinguishing the context, instead of deactivating inhibition, released A from its comparator stimulus and allowed us to observe behavior indicative of inhibition.
General Discussion
The present series of experiments was conducted to illuminate the conditions that determine the expression of Pavlovian and differential inhibition. In Experiment 1, we compared Pavlovian and differential inhibition when training trials were widely spaced (i.e., long ITIs) and observed behavior indicative of inhibition only after Pavlovian inhibition training. Experiment 2 used the same procedure as Experiment 1, but with training being conducted with massed trials (i.e., short ITIs). Contrary to the observations of Experiment 1, in Experiment 2 behavior indicative of inhibition was observed after differential inhibition training, but not Pavlovian inhibition training. As we discuss below, the Pavlovian inhibition data from Experiment 1 and the differential inhibition data from Experiment 2 are consistent with both acquisition-focused and expression-focused theories of learning. This notwithstanding, the absence of Pavlovian inhibition when training is conducted under massed trials poses a challenge to acquisition-focused theories that view inhibition as resulting from a discrepancy between the expectancy of the US and its nonoccurrence. In contrast, the results of Experiment 2 were anticipated by the extended comparator hypothesis. In Experiment 3, we observed that extinction of the training context through prolonged context exposure allowed observation of Pavlovian inhibition despite the inhibition training trials being massed. Thus, Experiment 3 suggests that the absence of Pavlovian inhibition after training with massed trials was due to the context competing with the training excitor at the time of testing, thereby preventing the training excitor from down-modulating responding to the target inhibitor X.
The extended comparator hypothesis (Denniston et al., 2001) does not differ from acquisition-focused models (e.g., Pearce & Hall, 1980; Rescorla & Wagner, 1972; Wagner, 1981) in predicting that Pavlovian and differential inhibition should be observed when training is conducted with spaced and massed trials, respectively. That is, in Pavlovian inhibition training with spaced trials, the target inhibitor X is repeatedly paired with A in the absence of reinforcement, and A when presented alone is consistently reinforced. Therefore, at the time of testing the target inhibitor X has a weak (or null) association with the US, but the representation of the US activated indirectly through mediation by A is strong, thereby resulting in behavior indicative of inhibition. Additional support for this view of Pavlovian inhibition has been reported in studies in which, after Pavlovian inhibition training with relatively spaced trials, the training excitor A was extinguished and X lost its control of behavior indicative of inhibition (e.g., Hallam et al., 1990; Lysle & Fowler, 1985). With regard to differential inhibition training, given that the target inhibitor X is never paired with A, the extended comparator hypothesis assumes that the comparator for X is the training context. That is, X is presented alone in the training context but never paired with the excitor A, so there is no within-compound association between X and A (Link 2, in the comparator framework). When training is conducted under massed trials, the context is presented alone during relatively short periods, and its association with the US does not undergo extinction during these short ITIs. At the time of testing, the indirectly activated representation of the US that allows observation of differential inhibition is mediated by the training context. Consistent with this view, most studies that observed reliable differential inhibition used relatively massed trials during training (e.g., Miller et al., 1991; Wessells, 1973). Finally, this account of differential inhibition also explains the apparent violation of the standard operational definition of conditioned inhibition highlighted in the introduction. That is, in differential inhibition it is the context, not the punctate excitatory CS, that provides the unfulfilled expectation of the outcome on the training trials in which the putative inhibitor is presented. Experiment 2 demonstrated that, if differential inhibition is trained with massed trials, the context plays the role of the otherwise-reinforced stimulus that is needed to observe behavior indicative of inhibition.
More careful reflection on the application of the extended comparator hypothesis to differential and Pavlovian conditioned inhibition provides additional insights. The prediction that differential inhibition will increase with shorter intervals between the training trials is parameter free because the context is the only first-order comparator stimulus for X. With trial massing, the context should grow more excitatory (at least until the X to US interval becomes so short as to support excitation). In contrast, the prediction for Pavlovian inhibition is more complex and must be qualified. Given any sort of Pavlovian inhibition training, A will serve as a first-order comparator stimulus for X. However, as the training trials are closer together, the context should become more excitatory. This has two consequences. First, as we have already discussed, the context, serving as a comparator stimulus to A, will down-modulate the comparator role of A, thereby decreasing the inhibitory potential of X. But second, the context will also come to serve as a first-order comparator stimulus for X, one for which its effects on X will depend on the relative strengths of the A–US and X–A associations. Critical here is the relative magnitude of these two effects. However, for the last few years the data from all experiments conducted in our laboratory to test the extended comparator hypothesis have been evaluated with respect to a single consistent set of parameters that are fully described in Stout and Miller’s (2006) mathematical implementation of this model. With these parameters, even with massed trials the context does not become a sufficiently strong first-order comparator to compensate for the degradation of A’s role as a first-order comparator stimulus in the case of Pavlovian conditioned inhibition. Thus, with this implementation of the extended comparator hypothesis, our predictions concerning the inverse effect of trial spacing in Pavlovian and differential inhibition are unambiguous.
In contrast, acquisition-focused models anticipate the observation of Pavlovian inhibition with spaced training trials and differential inhibition with massed trials, consistent with what was observed in Experiments 1 and 2. However, according to the acquisition-focused models, inhibition develops as a consequence of the US being expected but not presented. Therefore, these models predict that if Pavlovian inhibition is trained with massed trials, the excitor A and the context will conjointly create an expectation of the US occurring. Thus, the Pavlovian inhibitor should acquire as much (or perhaps even more) inhibitory strength when training is conducted with massed trials, which is the opposite of what was found in Experiments 2 and 3. Additional evidence that this prediction is incorrect can be found in the differential inhibition groups in Experiment 2. These groups demonstrated that the excitatory value of the training context was sufficient to observe differential inhibition. Therefore, according to these models, adding the excitor A to the nonreinforced trials should, if anything, enhance Pavlovian conditioned inhibition because A should summate with the context and create a greater expectation of the US and therefore increase evidence of inhibition to X (according to the Rescorla–Wagner [1972] model, at asymptote, the associative strengths of A and the context should sum to λ regardless of trial spacing). Recent revisions of the Rescorla–Wagner model (Van Hamme & Wasserman, 1994) and Wagner’s SOP (Wagner, 1981) by Dickinson and Burke (1996) that were adapted to explain learning about absent cues (i.e., retrospective revaluation) also have problems explaining the results of Experiment 3. Besides the fact that these revised models encounter the same problems as their predecessors in explaining the absence of Pavlovian inhibition after massed training trials, they anticipate that posttraining extinction of the training context should, if anything, decrease the inhibitory status of the target cue X, which is also opposite to what we observed in Experiment 3.
How does the extended comparator hypothesis anticipate the absence of Pavlovian inhibition after training with massed trials? The extended comparator hypothesis assumes, in addition to the primary comparator process, that each of the links that mediate the indirectly activated representation of the US (links 2 and 3 in Figure 1) is also subject to a comparator process (Links 2.2, 2.3, 3.2, and 3.3). Therefore, other stimuli presented during training, such as the context, can also compete to indirectly activate a representation of the US. More important, in addition to competing with the target cue presented at the time of testing, these cues compete with the primary comparator stimulus that indirectly activates a representation of the US. This competition among comparator stimuli leads, for example, to the counterintuitive prediction that the effects of two comparators that have an association between them could counteract each other rather than summate. If this mechanism is applied to Experiments 2 and 3, behavior indicative of inhibition should not be observed as a result of Pavlovian inhibition training with massed trials because the context weakens the effect of A as a comparator stimulus for X and A weakens the effect of the context as a comparator stimulus for X. Thus, in the comparator framework, both the context and A serve as comparators for X, but each attenuates the effect of the other on the inhibitory potential of X. Consistent with this view, Experiment 3 showed that extinguishing the context released A from its down modulation, thereby allowing us to observe inhibition to X. This view also explains why, in the studies by Williams et al. (1986), posttraining extinction of the training excitor A released the context from its own comparator process, thereby allowing observation of behavior indicative of inhibition in response to the target inhibitor (note that they conducted training with massed trials, resulting in a strong context–US association). In Experiment 3, we extinguished the training context rather than the excitor A, and the same outcome was observed. Thus, one might view the present Experiment 3 and Williams et al.’s data as complementary. In both studies Pavlovian conditioned inhibition training was given with massed trials, and relatively little inhibition was evident. Posttraining extinction of either the context (the present Experiment 3) or the training excitor (Williams et al.; experiment 2) resulted in increased inhibition. Presumably, extinction of the context revealed Pavlovian conditioned inhibition, and extinction of the training excitor revealed differential inhibition.
In summary, the present series of experiments illuminates some of the conditions necessary to observe Pavlovian and differential conditioned inhibition. As we mentioned in the introduction, comparisons between these two treatments have led to diverse results in the literature. We anticipated that the interval between trials could play a critical role in observing behavior indicative of inhibition with either procedure. Furthermore, the present results indicate that the context plays an important role in each treatment, either mediating the indirectly activated representation that makes it possible to observe differential inhibition, or interfering with the indirectly activated representation evoked by the training excitor A that makes it possible to observe Pavlovian inhibition. Such competition between competitors has also been observed in excitatory conditioning. Some treatments that typically produce a decrement in conditioned responding when administered alone counteract each other when both are administered. For example, it has recently been observed that overshadowing treatment counteracts latent inhibition treatment (Blaisdell, Bristol, Gunther, & Miller, 1998; Savastano, Arcediano, Stout, & Miller, 2003), the trial spacing effect (Stout, Chang, & Miller, 2003), the CS duration effect (Urushihara, Stout, & Miller, 2004), the outcome-alone exposure effect (Urushihara & Miller, in press), and the degraded contingency effect (Urcelay & Miller, 2006). Taken together, these observations suggest a mechanism that is anticipated by the extended comparator hypothesis, but not by current acquisition-focused models of learning.
Footnotes
Gonzalo P. Urcelay and Ralph R. Miller, Department of Psychology, State University of New York at Binghamton.
This work was supported by National Institute of Mental Health Grant 33881. We thank Jeffrey Amundson, Stephanie Cintron, Rachael Hessner, Michael Karmin, Olga Lipatova, Olga Perelmuter, Koji Urushihara, and Daniel Wheeler for their comments on an earlier version of this article. We also thank James Esposito, Jessica Fuss, and Olga Lipatova for assistance with the collection of data.
References
- Blaisdell AP, Bristol AS, Gunther LM, Miller RR. Overshadowing and latent inhibition counteract each other: Support for the comparator hypothesis. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:335–351. doi: 10.1037//0097-7403.24.3.335. [DOI] [PubMed] [Google Scholar]
- Chang RC, Blaisdell AP, Miller RR. Backward conditioning: Mediation by the context. Journal of Experimental Psychology: Animal Behavioral Processes. 2003;29:171–183. doi: 10.1037/0097-7403.29.3.171. [DOI] [PubMed] [Google Scholar]
- Couvillon PA, Ablan CD, Bitterman ME. Exploratory studies of inhibitory conditioning in honeybees (Apis mellifera) Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:103–112. [PubMed] [Google Scholar]
- Couvillon PA, Ablan CD, Ferreira TP, Bitterman ME. The role of nonreinforcement in learning of honeybees. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 2001;54B:127–144. doi: 10.1080/713932747. [DOI] [PubMed] [Google Scholar]
- Couvillon PA, Hsiung R, Cooke AM, Bitterman ME. The role of context in the inhibitory conditioning of honeybees. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 2005;58B:59–67. doi: 10.1080/02724990444000050. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Associations between the elements of a bivalent compound stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:425–436. [PubMed] [Google Scholar]
- Denniston JC, Savastano HI, Miller RR. The extended comparator hypothesis: Learning by contiguity, responding by relative strength. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Hillsdale, NJ: Erlbaum; 2001. pp. 65–117. [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgements. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 1996;49B:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Friedman BX, Blaisdell AP, Escobar M, Miller RR. Comparator mechanisms and conditioned inhibition: CS preexposure disrupts Pavlovian conditioned inhibition but not explicitly unpaired inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:453–466. [PubMed] [Google Scholar]
- Gibbon J, Balsam P. Spreading association in time. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and conditioning theory. New York: Academic Press; 1981. pp. 219–253. [Google Scholar]
- Hallam SC, Matzel LD, Sloat J, Miller RR. Excitation and inhibition as a function of posttraining extinction of the excitatory cue used in Pavlovian inhibition training. Learning and Motivation. 1990;21:59–84. [Google Scholar]
- Hearst E. Some persistent problems in the analysis of conditioned inhibition. In: Boakes RA, Halliday MS, editors. Inhibition and learning. London: Academic Press; 1972. pp. 5–39. [Google Scholar]
- Hearst E, Franklin SR. Positive and negative relations between a signal and food: Approach-withdrawal behavior to the signal. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:37–52. [Google Scholar]
- Hoffman JW, Fitzgerald RD. Bidirectional heart rate response in rats associated with excitatory and inhibitory stimuli. Animal Learning & Behavior. 1982;10:77–82. [Google Scholar]
- Lysle DT, Fowler H. Inhibition as a “slave” process: Deactivation of conditioned inhibition through extinction of conditioned excitation. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:71–94. doi: 10.1037//0097-7403.11.1.71. [DOI] [PubMed] [Google Scholar]
- Mahoney WJ, Kwaterski SE, Moore JW. Conditioned inhibition of the rabbit’s nictitating membrane response as a function of CS–US interval. Bulletin of the Psychonomic Society. 1975;5:177–179. [Google Scholar]
- Miller RR, Hallam SC, Hong JY, Dufore DS. Associative structure of differential inhibition: Implications for models of conditioned inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:141–150. doi: 10.1037//0097-7403.17.2.141. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. Orlando, FL: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not unconditioned stimuli. Psychological Review. 1980;82:532–552. [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Rescorla RA. Some consequences of associations between the excitor and the inhibitor in a conditioned inhibition paradigm. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:288–298. [Google Scholar]
- Rescorla RA, LoLordo VM. Inhibition of avoidance behavior. Journal of Comparative and Physiological Psychology. 1965;59:406–412. doi: 10.1037/h0022060. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Savastano HI, Arcediano F, Stout SC, Miller RR. Interaction between preexposure and overshadowing: Further analysis of the extended comparator hypothesis. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 2003;56B:371–395. doi: 10.1080/02724990344000006. [DOI] [PubMed] [Google Scholar]
- Savastano HI, Cole RP, Barnet RC, Miller RR. Reconsidering conditioned inhibition. Learning and Motivation. 1999;30:101–127. [Google Scholar]
- Schachtman TR, Brown AM, Gordon EL, Catterson DA, Miller RR. Mechanisms underlying retarded emergence of conditioned responding following inhibitory training: Evidence for the comparator hypothesis. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:310–322. [PubMed] [Google Scholar]
- Stout SC, Chang R, Miller RR. Trial spacing is a determinant of cue interaction. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:23–38. [PubMed] [Google Scholar]
- Stout SC, Miller RR. Sometimes competing retrieval (SOCR): A formalization of the comparator hypothesis. 2006. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Urcelay GP, Miller RR. Counteraction between overshadowing and degraded contingency treatments: Support for the extended comparator hypothesis. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:21–32. doi: 10.1037/0097-7403.32.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Miller RR. Overshadowing and the outcome-alone exposure effect counteract each other. Journal of Experimental Psychology: Animal Behavior Processes. doi: 10.1037/0097-7403.32.3.253. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Stout SC, Miller RR. The basic laws of conditioning differ for elemental cues and cues trained in compound. Psychological Science. 2004;15:268–271. doi: 10.1111/j.0956-7976.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- Van Hamme LJ, Wasserman EA. Cue competition in causality judgments: The role of nonpresentation of compound stimulus elements. Learning and Motivation. 1994;25:127–151. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Wagner AR, Rescorla RA. Inhibition in Pavlovian conditioning: Application of a theory. In: Boakes RA, Halliday MS, editors. Inhibition and learning. London: Academic Press; 1972. pp. 301–336. [Google Scholar]
- Wessells MG. Errorless discrimination, autoshaping, and conditioned inhibition. Science. 1973;182:941–943. doi: 10.1126/science.182.4115.941. [DOI] [PubMed] [Google Scholar]
- Williams DA, Travis GM, Overmier JB. Within-compound associations modulate the relative effectiveness of differential and Pavlovian conditioned inhibition procedures. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:351–362. [Google Scholar]


