Abstract
α2-Adrenoceptor (AR) agonists increase in analgesic potency and efficacy after peripheral nerve injury, and their effects are blocked by neuronal nitric oxide synthase (nNOS) inhibitors and M4 muscarinic receptor antagonists only after injury. We tested whether nNOS and M4 muscarinic receptors are co-expressed in the spinal cord, and whether destruction of a subset of sensory afferents which are essential to α2-AR analgesia would also destroy nNOS and M4 receptor expression.
Male Sprague Dawley rats underwent left L5 and L6 spinal nerve ligation. Lumbar spinal cord was removed and immunostained for M4 muscarinic receptors and nNOS alone and for co-expression. Others received intrathecal injection of saporin linked to an antibody to the neurotrophin receptor p75NTR, which eliminates cells expressing this receptor and the analgesic effects of α2-AR agonists.
nNOS staining of fibers in the superficial dorsal horn was dramatically increased after spinal nerve ligation, and this was abolished by saporin linked anti-p75NTR treatment. In contrast, nNOS staining in dorsal horn neurons was unaltered by these manipulations. M4 receptors were present on neurons in the dorsal horn, some of which co-expressed nNOS, but their pattern of expression was not altered by these manipulations.
Peripheral nerve injury increases nNOS expression in fibers in the superficial dorsal horn, some of which likely express p75NTR, and α2-AR agonists may reduce injury-induced sensitization by activation of nNOS in these fibers In contrast, changes in nNOS and M4 receptor location on spinal cord neurons are not responsible for increased analgesic potency of α 2-AR agonists after nerve injury.
Keywords: Acetylcholine, Muscarinic receptors, Spinal cord, Immunocytochemistry, Pain, Noradrenergic receptors
1. INTRODUCTION
Injury to peripheral nerves, whether from traumatic, metabolic, or oncologic processes, can result in spontaneous pain as well as pain elicited from normally innocuous stimuli. Neuropathic pain frequently responds poorly to opioid or non-steroidal anti-inflammatory drug treatment, and represents an unmet medical need. In contrast to opioids, intrathecally administered α2-adrenoceptor (AR) agonists increase in potency and efficacy after peripheral nerve injury in animals [16] and humans [8]. This coincides with a shift in circuitry activated by α2-ARs in the cord, with total dependence on activation of muscarinic cholinergic receptors and neuronal nitric oxide synthase (nNOS). The overall goal of the current study was to examine the anatomic substrates for this shift in nNOS and cholinergic circuitry.
nNOS is expressed on neurons in the dorsal horn of the spinal cord, and NO increases afferent-evoked release of excitatory neuropeptides [9], an effect which would enhance pain neurotransmission. Although the role of NO itself in generation of neuropathic pain is controversial [17], antihypersensitivity from intrathecal clonidine after nerve injury is reversed by NOS inhibitors [20], suggesting that increased expression of nNOS may play a role in the increased potency of α2-AR agonists in this setting. Interestingly, clonidine induced NO formation from spinal cord slices in vitro is inhibited by muscarinic cholinergic receptor antagonists after nerve injury [27], consistent with a direct link between muscarinic receptor and nNOS activation by α2-AR agonists, potentially in the same cell. One purpose of the current study was to determine whether nNOS and M4 receptors are co-expressed on spinal neurons.
In addition to spinal neurons, nNOS is also expressed on central terminals of afferent fibers [5], and nNOS expression is upregulated in dorsal root ganglia after nerve injury [17]. As such, we hypothesized that the enhanced potency of α2-AR agonists after nerve injury could reflect α2-AR-mediated activation of nNOS on afferent terminals in the spinal cord. To test this, we took advantage of previous observations regarding afferents which express the neurotrophin receptor, p75NTR. A subset of sensory afferents express p75NTR, and although little is known regarding the phenotype of these afferents, expression of p75NTR is upregulated following peripheral nerve injury [28]. Destruction of p75NTR expressing afferents by intrathecal injection of an anti-p75NTR antibody linked to the ribosomal uncoupler, saporin, completely blocks anti-hypersensitivity from α2-AR and cholinergic agonists while having itself no effect on nerve injury induced hypersensitivity [23]. We hypothesized that α2-AR agonist efficacy after nerve injury depends on activation of nNOS in afferent terminals, and tested whether destruction of p75NTR expressing afferents, which abolishes α2-AR agonist analgesia, also would destroy nNOS fibers in the spinal cord.
Finally, intrathecal injection of α2-AR agonists stimulates acetylcholine release in animals [4] and humans [3]. Acetylcholine release is essential to analgesia from α2-AR agonists after nerve injury, since their effects are completely antagonized by co-administration of atropine [21] or by destruction of spinal cholinergic neurons [24]. Spinally released acetylcholine produces analgesia primarily by actions on M2 and M4 receptors [7]. We previously showed that analgesia from intrathecal injection of the α2-AR agonist, clonidine after nerve injury was partially blocked by a highly selective toxin to M4 receptors [12]. A final purpose of the current study was to determine whether nerve injury altered the expression of M4 receptors in the spinal cord and whether blockade of α2-AR agonist analgesia by destruction of p75NTR expressing cells altered M4 receptor expression.
2. RESULTS
All animals recovered from spinal nerve ligation and intrathecal catheterization without neurologic deficits. Withdrawal threshold was reduced from >22.3 g prior to spinal nerve ligation to < 4 g after spinal nerve ligation in all animals. Withdrawal threshold did not differ in spinal nerve ligated animals before and one week after intrathecal anti-p75NTR saporin treatment (data not shown).
M4 muscarinic receptor immunoreactivity (IR) was diffusely localized across superficial and deep dorsal horn of the spinal cord, with primarily nuclear and peri-nuclear cellular staining but minimal fiber labeling (Figure 1). This pattern did not differ between normal and spinal nerve ligated animals or, in spinal nerve ligated animals, between the side ipsilateral and contralateral to injury (Figure 1A,B). Quantification revealed no differences among normal spinal cord, or ipsilateral or contralateral spinal nerve ligated spinal cord sections, either in number of objects, in total number of pixels above threshold, or in intensity of pixels above threshold. The number of pixels above threshold were 4.7 ± 0.32 × 103 contralateral to injury and 4.2 ± 0.87 × 103 ipsilateral to injury. The pattern of M4 muscarinic receptor-IR was not altered one week after anti-p75NTR saporin treatment, either in ipsilateral or contralateral dorsal horn (Figure 1C,D). Quantification revealed no effect of anti-p75NTR saporin treatment on M4-IR. The number of pixels above threshold were 4.4 ± 0.69 × 103 and 4.6 ± 0.48 × 103 contra- and ipsilateral to injury in anti-p75NTR saporin treated animals, respectively.
Figure 1.

M4-IR in the dorsal horn of SNL rats with i.t. injection of saline and p75-saporin. M4-IR cells were distributed throughout all laminae of the dorsal horn. A–D shows M4-IR cells in LI to LIII. There was no apparent difference in the number and distribution of M4-IR cells between ipsilateral (B and D) and contralateral (A and C) dorsal horn or between i.t. saline (A and B) and p75-saporin (C and D). Scale bar = 50μm.
Cellular nNOS-IR was located in small neurons, primarily in lamina II, and in larger cells in deeper laminae (Figure 2). Numerous long processes were observed from cells in deeper laminae extending into superficial regions (Figure 2). This pattern of cell profile-IR did not differ between tissue ipsilateral and contralateral to spinal nerve ligation (Figure 2A,B), nor did it differ between tissue from normal or spinal nerve ligated animals (data not shown). The pattern of cellular-IR for nNOS was not altered by anti-p75NTR saporin treatment (Figure 2C,D). Quantification confirmed no difference in the number of cell profiles with nNOS-IR, either in superficial or deep dorsal horn, between tissue ipsilateral or contralateral to spinal nerve ligation, or in animals with or without anti-p75NTR saporin treatment (Figure 3A).
Figure 2.

nNOS-IR in the dorsal horn of SNL rats with i.t. injection of saline and p75-saporin. nNOS-IR cells were distributed throughout all laminae of the dorsal horn while nNOS-IR axons were primarily localized in the lamina I and II (A–D). nNOS-IR axons were evidently increased in the ipsilateral dorsal horn (B) compared to the contralateral dorsal horn (A) in saline injected SNL rats. However, nNOS-IR axons in the ipsilateral dorsal horn of p75-saporin injected SNL (D) rats were dramatically reduced compared to the ipsilateral dorsal horn of saline injected rats (B). Scale bar=50μm.
Figure 3.
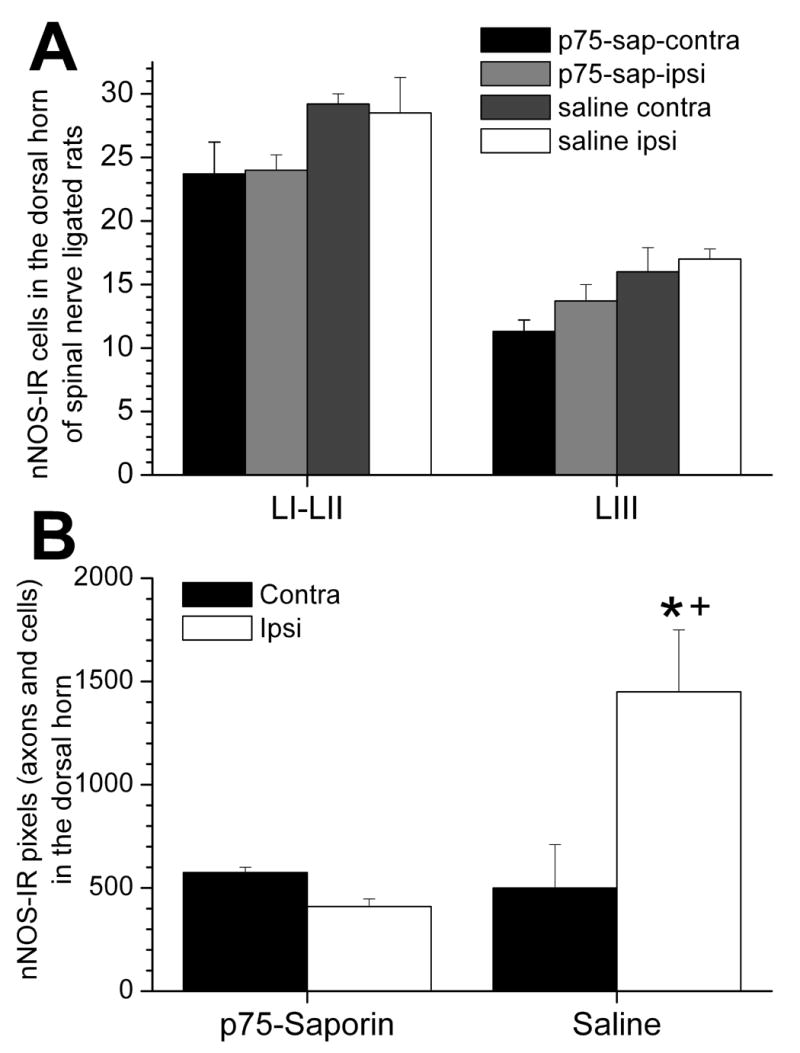
Quantitative analysis of nNOS-IR cells and axons in the dorsal horn of SNL rats with i.t. injection of saline and p75-saporin. The mean number of nNOS-IR cells in LI-II and LIII in the ipsilateral dorsal horn of SNL rats with i.t. injection of p75-saporin was not significantly different from that in the contralateral dorsal horn as well as compared to the ipsilateral dorsal horn of saline injected rats (A). In contrast, the mean pixel number of nNOS-IR axons and cells in the ipsilateral dorsal horn of SNL rats with saline injection was significantly higher than the contralateral dorsal horn (B, *, p<0.001) and than the ipsilateral dorsal horn of SNL rats with p75-saporin injection (B, +, p<0.001). There are no significant difference in the mean pixel number of nNOS-IR between the ipsi- and contralateral dorsal horn of SNL rats with p75-saporin injection. Mean±SEM, n=5, one-way ANOVA with post hoc Student-Newman-Keuls multiple comparison method.
In addition to cells, nNOS-IR was clearly observed in fibers, with a concentration in laminae II. The density of fiber nNOS immunostaining was greater in superficial, but not deep dorsal horn ipsilateral to spinal nerve ligation compared to contralateral (Figure 2,C,D). Quantification revealed a 3 fold increase in the number of pixels (fibers and cell profiles) in superficial laminae ipsilateral to spinal nerve ligation compared to contralateral tissue (Figure 3B) or to normal animals (P<0.01, data not shown).
Co-localization studies revealed that many cells in both superficial and deep dorsal horn expressed nNOS-IR, localized primarily in cytoplasm with confocal imaging, and M4 muscarinic receptors, localized primarily near the nucleus with confocal imaging (Figure 4). The degree of co-localization did not appear to be affected by spinal nerve ligation or by anti-p75NTR saporin treatment.
Figure 4.
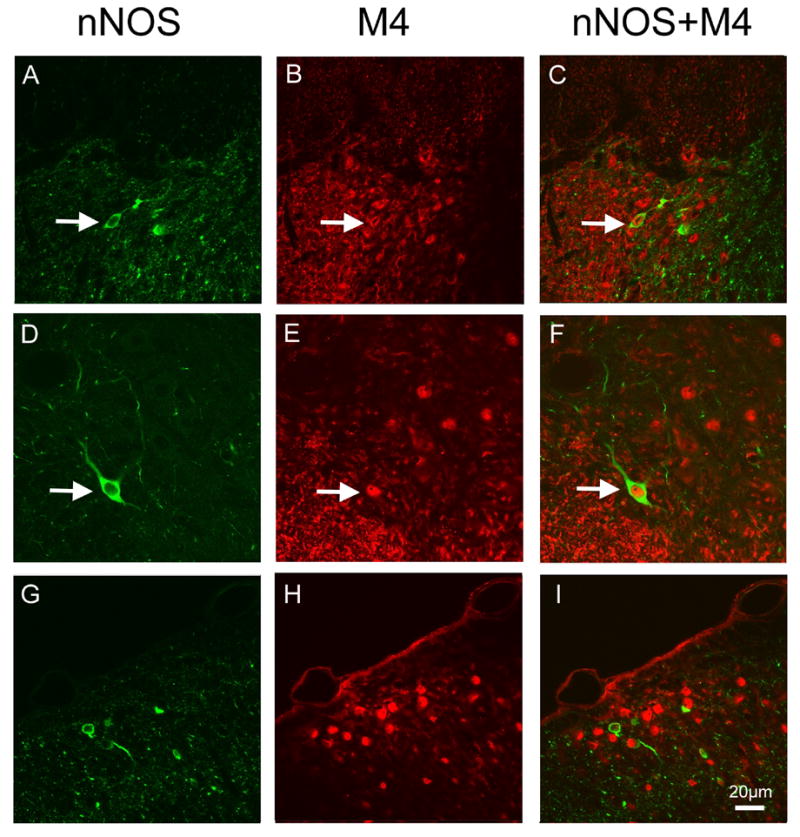
Color confocal images of double immunostaining of M4 and nNOS in the dorsal horn of SNL rats with saline and p75-saporin injection. In LI (A–C) and LIII (D–F) of the dorsal horn of saline injected SNL rat and LI (G–H) of a p75-saporin injected rat. Throughout these laminae, the majority of nNOS-IR (A and D green) were observed to co-express M4-IR (B and E, red) in the same cell profiles (C and F, yellow), as indicated by arrows. Scale bar = 20μm.
3. DISCUSSION
Peripheral nerve injury results in numerous changes to peripheral afferents, spinal cord, subcortical, and cortical structures thought to underlie ongoing pain and sensitization. But nerve injury can also alter neural substrates for analgesia, resulting in increased analgesic potency or efficacy. Intrathecal clonidine, for example, increases in potency and maximum efficacy after nerve injury in animals [16] and in the presence of neuropathic pain in humans [8]. Both cholinergic [21] and nitrergic [20] interactions are important to this increased efficacy of clonidine, and the current study provides new insights regarding the mechanisms for these nerve injury induced interactions.
The role of spinally synthesized NO in regulation of sensory neurotransmission after peripheral nerve injury is unclear, with experiments demonstrating pro-nociception, antinociception, or no effect [2,18,19]. In contrast, spinal NO synthesis is important to analgesia from opioids [14] and α2-AR agonists [20]. In the case of norepinephrine, the naturally occurring α2-AR neurotransmitter in the spinal cord, there is a feed forward mechanism, with stimulation of nNOS by α2-AR activation leading to increased norepinephrine release [2,15]. The current study demonstrates a unilateral increase in nNOS-IR in fibers in the superficial dorsal horn ipsilateral to spinal nerve ligation. Since nNOS expression is upregulated in injured primary afferent neurons [5], we speculate that these fibers are likely the central terminals of primary afferents. Additionally, since anti-p75NTR saporin treatment dramatically blocked this increased fiber immunostaining, we consider it likely that nNOS and p75NTR are co-localized in a subset of primary afferent fibers, and that these are important to the increased efficacy of intrathecal clonidine after peripheral nerve injury.
Nitric oxide is classically activated by muscarinic receptor signaling, and it is conceivable that nitric oxide could be synthesized in cholinergic neurons in response to autoreceptor feedback on inhibitory M2 or M4 receptors. nNOS and M4 receptors were co-localized in many cells in the superficial and deep dorsal horn in the current study, and we speculate that some of the deep dorsal horn neurons may be cholinergic, in that others have observed a high degree of co-localization of nNOS and choline acetyltransferase as a marker for spinal cholinergic neurons [25]. These cells are unlikely substrates for the α2-AR interaction after nerve injury, however, since neither the number or pattern of nNOS cells nor their degree of co-localization were altered by spinal nerve ligation, and neither were altered after anti-p75NTR saporin treatment, which reduces clonidine efficacy. Failure to observe a change in nNOS expressing cells after nerve injury is unlikely to reflect a lack of sensitivity of our method, since we previously showed an increased in cells expressing phosphorylated cAMP response element binding protein [22] and the α5 nicotinic receptor subunit [26] following nerve injury using this method.
Muscarinic receptors, especially inhibitory G protein coupled ones (M2 and M4), reduce nociceptive transmission at the spinal level. There is strong evidence that M2 receptors are localized on primary afferents, and reduce excitatory neurotransmitter release [6]. M2-IR increases in uninjured (L4 level) and injured (L5 and L6 level) primary afferent neuronal cell bodies after spinal nerve ligation, as does the ability of the muscarinic agonist, bethanechol, to inhibit excitation of these cells [10]. Other studies support a role for M4 muscarinic receptors in analgesia from cholinergic agonists [7], and M4 receptor blockade antagonizes clonidine efficacy after peripheral nerve injury [12]. The current study adds to radioligand binding [11] and Western blot [12] studies by demonstrating a lack of change in location, as well as expression level, of M4 receptors in spinal cord after nerve injury. We conclude, therefore, that the novel dependency of α2-AR analgesia on cholinergic mechanisms after nerve injury does not reflect a change in location or expression of M4 receptors.
In summary, nNOS-IR increases in fibers in the superficial dorsal horn ipsilateral to spinal nerve ligation and is reduced by anti-p75NTR saporin, suggesting co-localization of these proteins in a subset of sensory afferents following nerve injury. M4 muscarinic and nNOS-IR in cells of the spinal cord dorsal horn were unaffected by spinal nerve ligation or by anti-p75NTR saporin treatment. These results are not consistent with the hypothesis that the cholinergic and NO dependency of α2-AR mediated analgesia after nerve injury reflects increased co-expression of M4 receptors and nNOS on spinal cord neurons. They suggest, rather, that α2-AR activation in the spinal cord after nerve injury indirectly stimulates nNOS on a subset of primary afferent fibers, and that this mechanism is essential for analgesia from α2-AR agonists in this setting.
4. Experimental Procedure
Sixteen Sprague-Dawley rats (200–300 g; Harlan) were used for this study. All animals were housed in pairs and had free access to food and water. All experiments were performed in accordance with the regulations of Wake Forest University School of Medicine Animal Care and Use Committee.
Surgical preparation and behavioral testing
Animals underwent spinal nerve ligation as previously described [13] or no surgery. In brief, animals were anesthetized with halothane, 1–3% in oxygen, with spontaneous ventilation. The left lower lumbar and upper sacral dorsal and lateral laminae were visualized, a partial lateral laminectomy performed, and the L5 and L6 spinal nerves identified and tightly ligated with 6–0 silk suture. The wound was closed in layers and the animals allowed to recover. In animals for intrathecal anti-p75 saporin treatment, a polyethylene catheter was inserted through the cisternal membrane into the spinal intrathecal space and advanced 8.5 cm such that its tip was near the lumbar enlargement. Animals were allowed to recover 2 weeks after surgery prior to testing.
Withdrawal threshold to tactile stimulation was determined on the left hindpaw using application of calibrated von Frey filaments. Animals were placed in a Plexiglas container with a mesh floor and allowed to acclimate for a minimum of 30 min. A filament was applied at a right angle to the surface of the left hind paw with adequate pressure to bend the filament for 5 sec. Brisk withdrawal of the paw was considered a positive response. The force required to achieve a 50% likelihood of withdrawal was determined using a sequential up-down method previously described [1].
Anti-p75-saporin treatment
At least 10 days following surgery, an anti-p75 antibody conjugated to saporin (Advanced Targeting Systems, San Diego, CA), 0.6 μg was administered as a single intrathecal injection in a volume of 10 μl followed by flush with saline, 10 μl. Animals were allowed one week after this treatment prior to study, a time when p75-expressing elements are essentially missing from the treated spinal cord [23].
Perfusion
Twelve to fourteen days post-surgery, rats were deeply anesthetized with pentobarbital and perfused pericardially with buffer (0.01M phosphate buffered saline + 1% sodium nitrite, 100 ml) followed by 4% paraformaldehyde (400 ml) at room temperature. The L4 to L6 portion of the spinal cord was extracted and post-fixed in 4% paraformaldehyde for 2–3 hours followed by cryoprotection in 30% sucrose for 48–72 hours at 4°C. Tissue was imbedded in Tissue-Tek O.C.T. Compound (Sakura Finetek, USA) and cut transversely into 40μm sections on a Leica CM3000 cryostat.
Immunocytochemistry
Immunocytochemistry was performed on free floating sections using standard biotin-streptavidin techniques. Sections were washed 2–4 times with 0.01M phosphate buffered saline + 0.15% Triton 100X (PBS+T) in between each step except following the application of blocking serum. The sections were incubated in 0.3% hydrogen peroxidase for 15 min, incubated with 50% alcohol (45 min), and blocked with 1.5% normal goat serum for 1 hour at room temperature. An anti-muscarinic M4 receptor antibody (rabbit polyclonal, 1:1000; Research and Diagnostic Antibodies, Benicia CA) and a mouse monoclonal antiserum raised against nNOS (1:1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were applied to the sections for 24 hours at 4° C. Sections were incubated with biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) for 1 hour at room temperature followed by the application of streptavidin-conjugated horseradish peroxidase (ABC Elite, Vector Laboratories) for 1 hour at room temperature. Antibodies were visualized using the enhanced glucose-nickel-diaminobenzidine method. Images were captured on a Leica Axioplan2 light microscope and digitized with QIMAGING. Positively labeled cells were identified for automated counting using SigmaScan Pro 5 at a preset intensity threshold. IR cells were counted as individual objects (defined as contiguous pixels). Labeling was examined in laminae I–II with 6-10 slices examined per animal. Absorption controls for M4 immunostaining revealed no staining.
Double immunostaining of M4 and nNOS was done on spinal cord sections of SNL rats with saline and p75-saporin injection. Following pre-treatment with 10%NGS, sections were incubated in rabbit anti-M4 antiserum (1:500, source as above) and mouse monoclonal nNOS antiserum (1:500, source as above) diluted in 10% NGS in PBS+0.3% Triton X 100 overnight at 40C. Sections were then incubated in biotinylated goat anti-rabbit IgG (1:200, Vector Lab.) for 1 hour and then in a mixture of StrepAvidin conjugated with Alexa Fluor 488 (1:200, Molecular Probes Inc.) and goat anti-mouse IgG conjugated with Alexa Fluor 568 (1:200, Molecular Probes Inc.) for another hour at room temperature. Sections were cover slipped with anti-fading mounting medium (Vector Lab.) and examined under a confocal microscope (LSM510, Carl Zeiss Microscopy, Jena, Germany).
Quantification of M4-IR and nNOS-IR in the dorsal horn
SNL rats with saline (n=5) and p75-saporin (n=5) injection were used in the quantification of M4-IR and nNOS-IR in the dorsal horn. More than 10 images from each rat were used in the counting of nNOS-IR in the dorsal horn. These images were taken under light microscope at a magnification allowing the images to cover the entire LI-LIII in the depth and one-third in breadth of the ipsilateral or contralateral dorsal horn. Since nNOS-IR cells were easily discernable, all cell profiles were counted directly. The mean number of nNOS-IR cells from each group were determined and statistically compared using one-way ANOVA with post hoc Student-Newman-Keuls multiple comparison method, saline vs. p75-saporin, ipsilateral vs. contralateral. A significance level was set at p < 0.05.
Since it was not possible to quantify nNOS-IR axons only in the dorsal horn, the total nNOS-IR (axons and cells) was quantified from captured images using a commercially available software package. nNOS-IR in one field which covers one-third in breadth and LI-LIII in depth of the dorsal horn was automatically quantified as pixel numbers. More than 10 images were used from each rat. The mean pixel numbers were determined in each group and statistically compared using one-way ANOVA with post hoc Student-Newman-Keuls multiple comparison method. A significance level was set at p < 0.05.
Acknowledgments
This work was supported in part by grant NS41386 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen SR, Eisenach JC, Pan HL. Intrathecal S-nitroso-N-acetylpenicillamine and L-cysteine attenuate nerve injury-induced allodynia through noradrenergic activation in rats. Neuroscience. 2000;101:759–765. doi: 10.1016/s0306-4522(00)00415-2. [DOI] [PubMed] [Google Scholar]
- 3.De Kock M, Eisenach J, Tong C, Schmitz AL, Scholtes JL. Analgesic doses of intrathecal but not intravenous clonidine increase acetylcholine in cerebrospinal fluid in humans. Anesth Analg. 1997;84:800–803. doi: 10.1097/00000539-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Detweiler DJ, Eisenach JC, Tong C, Jackson C. A cholinergic interaction in alpha2 adrenoceptor-mediated antinociception in sheep. J Pharmacol Exp Ther. 1993;265:536–542. [PubMed] [Google Scholar]
- 5.Dun NJ, Dun SL, Wu SY, Förstermann U, Schmidt HHHW, Tseng LF. Nitric oxide synthase IR in the rat, mouse, cat and squirrel monkey spinal cord. Neuroscience. 1993;54:845–857. doi: 10.1016/0306-4522(93)90579-5. [DOI] [PubMed] [Google Scholar]
- 6.Dussor GO, Helesic G, Hargreaves KM, Flores CM. Cholinergic modulation of nociceptive responses in vivo and neuropeptide release in vitro at the level of the primary sensory neuron. Pain. 2004;107:22–32. doi: 10.1016/j.pain.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Duttaroy A, Gomeza J, Gan JW, Siddiqui N, Basile AS, Harman WD, Smith PL, Felder CC, Levey AI, Wess J. Evaluation of muscarinic agonist-induced analgesia in muscarinic acetylcholine receptor knockout mice. Mol Pharmacol. 2002;62:1084–1093. doi: 10.1124/mol.62.5.1084. [DOI] [PubMed] [Google Scholar]
- 8.Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D Epidural Clonidine Study Group. Epidural clonidine analgesia for intractable cancer pain. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- 9.Garry MG, Walton LP, Davis MA. Capsaicin-evoked release of IR calcitonin gene-related peptide from the spinal cord is mediated by nitric oxide but not by cyclic GMP. Brain Res. 2000;861:208–219. doi: 10.1016/s0006-8993(99)02448-8. [DOI] [PubMed] [Google Scholar]
- 10.Hayashida K, Bynum T, Vincler M, Eisenach JC. Inhibitory M2 muscarinic receptors are upregulated in both axotomized and intact small diameter dorsal root ganglion cells after peripheral nerve injury. Neuroscience. 2006;140:259–268. doi: 10.1016/j.neuroscience.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Höglund AU, Baghdoyan HA. M2, M3 and M4, but not M1, muscarinic receptor subtypes are present in rat spinal cord. J Pharmacol Exp Ther. 1997;281:470–477. [PubMed] [Google Scholar]
- 12.Kang YJ, Eisenach JC. Intrathecal clonidine reduces hypersensitivity after nerve injury by a mechanism involving spinal m4 muscarinic receptors. Anesth Analg. 2003;96:1403–1408. doi: 10.1213/01.ANE.0000060450.80157.FF. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 14.Kolesnikov YA, Pan YX, Babey AM, Jain S, Wilson R, Pasternak GW. Functionally differentiating two neuronal nitric oxide synthase isoforms through antisense mapping: Evidence for opposing NO actions on morphine analgesia and tolerance. Proc Natl Acad Sci USA. 1997;94:8220–8225. doi: 10.1073/pnas.94.15.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XH, Rose G, Dongre N, Pan HL, Tobin JR, Eisenach JC. S-nitroso-l-cysteine releases norepinephrine in rat spinal synaptosomes. Brain Res. 2000;872:301–307. doi: 10.1016/s0006-8993(00)02551-8. [DOI] [PubMed] [Google Scholar]
- 16.Luo L, Puke MJC, Wiesenfeld-Hallin Z. The effects of intrathecal morphine and clonidine on the prevention and reversal of spinal cord hyperexcitability following sciatic nerve section in the rat. Pain. 1994;58:245–252. doi: 10.1016/0304-3959(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 17.Luo ZD, Chaplan SR, Scott BP, Cizkova D, Calcutt NA, Yaksh TL. Neuronal nitric oxide synthase mRNA upregulation in rat sensory neurons after spinal nerve ligation: Lack of a role in allodynia development. J Neurosci. 1999;19:9201–9208. doi: 10.1523/JNEUROSCI.19-21-09201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo ZD, Chaplan SR, Scott BP, Cizkova D, Calcutt NA, Yaksh TL. Neuronal nitric oxide synthase mRNA upregulation in rat sensory neurons after spinal nerve ligation: lack of a role in allodynia development. J Neurosci. 1999;19:9201–9208. doi: 10.1523/JNEUROSCI.19-21-09201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meller ST, Pechman PS, Gebhart GF, Maves TJ. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience. 1992;50:7–10. doi: 10.1016/0306-4522(92)90377-e. [DOI] [PubMed] [Google Scholar]
- 20.Pan HL, Chen SR, Eisenach JC. Role of spinal NO in antiallodynic effect of intrathecal clonidine in neuropathic rats. Anesthesiology. 1998;89:1518–1523. doi: 10.1097/00000542-199812000-00031. [DOI] [PubMed] [Google Scholar]
- 21.Pan HL, Chen SR, Eisenach JC. Intrathecal clonidine alleviates allodynia in neuropathic rats - Interaction with spinal muscarinic and nicotinic receptors. Anesthesiology. 1999;90:509–514. doi: 10.1097/00000542-199902000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Pancaro C, Ma WY, Vincler M, Duflo F, Eisenach JC. Clonidine-induced neuronal activation in the spinal cord is altered after peripheral nerve injury. Anesthesiology. 2003;98:748–753. doi: 10.1097/00000542-200303000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Paqueron X, Li X, Eisenach JC. p75-expressing elements are necessary for anti-allodynic effects of spinal clonidine and neostigmine. Neuroscience. 2001;102:681–686. doi: 10.1016/s0306-4522(00)00528-5. [DOI] [PubMed] [Google Scholar]
- 24.Paqueron X, Li XH, Bantel C, Tobin JR, Voytko ML, Eisenach JC. An obligatory role for spinal cholinergic neurons in the antiallodynic effects of clonidine after peripheral nerve injury. Anesthesiology. 2001;94:1074–1081. doi: 10.1097/00000542-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Spike RC, Todd AJ, Johnston HM. Coexistence of NADPH diaphorase with GABA, glycine, and acetylcholine in rat spinal cord. J Comp Neurol. 1993;335:320–333. doi: 10.1002/cne.903350303. [DOI] [PubMed] [Google Scholar]
- 26.Vincler M, Eisenach JC. Plasticity of spinal nicotinic acetylcholine receptors following spinal nerve ligation. Neurosci Res. 2004;48:139–145. doi: 10.1016/j.neures.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, Chen SR, Eisenach JC, Pan HL. Role of spinal muscarinic and nicotinic receptors in clonidine-induced nitric oxide release in a rat model of neuropathic pain. Brain Res. 2000;861:390–398. doi: 10.1016/s0006-8993(00)02051-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhou XF, Rush RA, McLachlan EM. Differential expression of the p75 nerve growth factor receptor in glia and neurons of the rat dorsal root ganglia after peripheral nerve transection. J Neurosci. 1996;16:2901–2911. doi: 10.1523/JNEUROSCI.16-09-02901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


