Abstract
Adolescence is characterized by behavioral traits such as emotional lability and impulsivity that are associated with increased vulnerability to affective illness and addictions. Research in rodents has found that adolescent rats and mice differ from adults on measures of anxiety-like behavior, novelty-seeking and stress-responsivity. The present study sought to extend these data by evaluating fear-, anxiety- and depression-related behaviors in male C57BL/6J mice aged four (early adolescent), six (peri-adolescent) or eight (early adult) weeks of age. Age-groups were compared on: Pavlovian fear conditioning and extinction, anxiety-like behavior and exploratory locomotion (using elevated plus-maze and novel open field), and depression-related behavior (via forced swim test). Results showed that early adolescent mice exhibited enhanced fear conditioning, but extinguished at a similar rate as adults. There were no major differences in anxiety-like behavior across age-groups, although early adolescent and peri-adolescent mice exhibited less exploratory locomotion than adults. Depression-related immobility behavior in the forced swim test was lower in early adolescents than adult mice across three test exposures. Present findings in the C57BL/6J inbred strain add to growing evidence of changes in rodent fear- and stress-related behaviors across the developmental transition from juvenility through adulthood. Understanding the neural basis of these ontogenic changes could provide insight into factors could provide insight into the pathogenesis and treatment of affective disorders that have their origins in adolescence.
Keywords: mouse, development, juvenile, ontogeny, adolescence, learning, memory, elevated plus-maze, forced swim, open field
Introduction
Emotional lability, impulsivity and risk-taking are distinguishing characteristics of human adolescence and are associated with increased vulnerability to a variety of neuropsychiatric disorders including affective illness and addiction [4, 5, 44, 49, 50, 52]. The behavioral features of adolescence are thought to reflect the incomplete maturation of neural systems regulating emotion and inhibitory behavioral control [8, 20, 32].
Previous studies in rodents have shown that adolescents exhibit differences from adult rodents on measures of fear-, anxiety- and depression-related behaviors and reactivity to stress (for recent reviews, see [3, 43]). For example, adolescent rats have been found to exhibit either lesser, greater or normal levels of anxiety-like behavior in various behavioral assays as compared to adults [9, 13, 15, 18, 25, 41, 43, 51]. In addition, a number of studies have shown that conditioned fear responses to footshock in rats emerges in the early adolescent period (reviewed in [6, 23, 24, 47]). While less research has been conducted in mice, relatively lesser anxiety-like behavior and relatively greater exploration of novel environments has been reported in some but not all studies of adolescent mice [1, 2, 29, 34, 48]. Existing data on stress-reactivity in adolescent mice is also somewhat inconsistent, with studies showing both attenuated and exaggerated neuroendocrine, neural and behavioral responses to stress relative to adults [2, 10, 43, 48, 53], and to our knowledge little work has been conducted on depression-related behaviors.
The objective of the present study was to clarify and extend these findings by comparing mice at various stages of adolescent development through early adulthood for fear-, anxiety- and depression-related behaviors. Associative fear learning and memory was assayed using a Pavlovian fear conditioning paradigm [28]. Subsequent extinction of the fear memory was also assessed, to provide a measure of inhibitory fear learning across adolescence [26, 38]. Anxiety-like behaviors were measured using two pharmacologically well-validated exploration-based tests, elevated plus-maze and novel open field [11, 42]. Depression-related behavior was also tested using a pharmacologically-validated test, the forced swim test [11]. The subjects employed for this study were C57BL/6J mice, aged four, six or eight weeks considered to approximate to human early adolescence, peri-adolescence and early adulthood, respectively [12, 30, 45]. The C57BL/6J inbred strain was chosen on the basis of its widespread use in behavioral neuroscience research and its inclusion as a ‘group A’ priority strain in the Mouse Phenome Project; an international effort to provide the biomedical research community with phenotypic data on the most commonly used mouse strains (http://aretha.jax.org/pubcgi/phenome/mpdcgi?rtn=docs/home).
Materials and methods
Subjects
Male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at either 3, 5, or 7 weeks of age, with equal numbers of each age-group delivered together in a single shipment to control for potential effects of the stress of shipping. On arrival, mice were group-housed (2–4/cage) by age-group in a temperature- (72 ± 5°C) and humidity- (45 ± 15%) controlled vivarium under a 12 hr light/dark cycle (lights on 0600 h). Testing was conducted ~1 week later when mice were 4, 6 or 8 weeks of age, using separate mice for each experiment. Mice were acclimated to the test room for 1 hr prior to testing, with the exception of mice tested for conditioning/extinction, which were acclimated in an adjacent room to avoid exposure to the conditioned stimulus. Testing was conducted in an order counterbalanced for age. Between subjects, apparatuses were cleaned with 70% ethanol solution unless otherwise stated. All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and followed the National Institute of Health guidelines outlined in ‘Using Animals in Intramural Research.’
Pavlovian fear conditioning and extinction
Pavlovian fear conditioning and extinction was assessed based on methods previously described [26, 28, 39]. Fear conditioning was conducted in a 27 × 27 × 11 cm chamber with transparent walls and a metal rod floor, cleaned with a 79.5% water/19.5% ethanol/1% vanilla-extract solution to provide a distinctive olfactory cue. Following a 180 sec acclimation period, mice received 4 pairings (60–120 sec variable inter-pairing interval) between a 30 sec, 80 dB, 3 kHz tone (conditioned stimulus, CS) and a 2 sec, 0.6 mA scrambled footshock (unconditioned stimulus, US), in which the US was presented during the last 2 sec of the CS. There was a 120 sec no-stimulus consolidation period following the final US-CS pairing before mice were returned to the home cage. Stimulus presentation was controlled by the San Diego Instruments Freeze Monitor system (San Diego Instruments Inc., San Diego, CA).
Twenty-four hr later, recall or the CS and extinction of the response to the CS were tested. Mice were placed in a novel context (black/white-checkered walls and a solid-Plexiglas, opaque floor, cleaned with a 50% ethanol/50% water solution) housed in a novel room. Following an initial 180 sec acclimation period, the mouse received 50 × 30-sec presentations of the CS (5 sec no-stimulus interval).
Freezing (no visible movement except that required for respiration) was manually scored every 5 sec and converted to a percentage ([number of freezing observations/total number of observations] × 100). Freezing during extinction trials were averaged into 10 × 5-trial blocks for analysis. In a separate set of mice (8/age), reactivity to 3 × 2 sec, 0.6 mA scrambled footshocks (60–120 sec variable interval) was scored using a visual assessment scale: 0= no reaction, 1= running around the floor of chamber, 2= running and jumping in the air, 3= wild running and jumping.
Elevated plus-maze
The elevated plus-maze test was conducted as previously described [7, 19, 33]. The apparatus consisted of 2 open arms (30 × 5 cm; 90 lux) and 2 closed arms (30 × 5 × 15 cm; 20 lux) extending from a 5 × 5 cm central area and elevated 20 cm from the ground (San Diego Instruments, San Diego, CA). The walls were made from black ABS plastic and the floor from white ABS plastic. A 0.5 cm raised lip around the perimeter of the open arms prevented mice from falling off the maze. The mouse was placed in the center facing an open arm and allowed to explore the apparatus for 5 min. Time spent in the open arms [(open arm time/total session duration)*100], and entries into the open and closed arms was measured by the Ethovision videotracking system on the basis of the X-Y coordinates of the center of mass of the mouse image compared against a template of the dimensions of open and closed arms (Noldus Information Technology Inc., Leesburg, VA). Percent entries into the open arms relative to total entries [(open arm entries/total arm entries)*100] was calculated from these measures.
Novel open field
The novel open field test was conducted as previously described [7]. The apparatus was a 40 × 40 × 35 cm square arena (50 lux) constructed of white Plexiglas. The mouse was placed in the perimeter and allowed to explore the apparatus for 60 min. Total distance traveled in the whole arena and time spent in the center (20 × 20 cm) was measured by the Ethovision videotracking system on the basis of the X-Y coordinates of the center of mass of the mouse image compared against a template of the dimensions of center and perimeter (Noldus Information Technology Inc., Leesburg, VA). For both the elevated plus-maze and novel open field tests, the experimenter retreated to a corner of the room and remained quiet and still during testing.
Forced swim test
The forced swim test was conducted as previously described [7, 40]. The apparatus was a transparent Plexiglas cylinder 25 cm high, 20 cm diameter) filled halfway with water (24 ± 1°C) such that the mouse could not touch the bottom with its tail. The mouse was gently lowered into the water and manually observed for the presence/absence of immobility (cessation of limb movements except minor involuntary movements of the hind limbs) every 5 sec during the last 8 min of a 10 min test session every 24 hr for 3 consecutive days.
Statistical analysis
The effect of age was analyzed using analysis of variance (ANOVA) and Newman-Keuls post-hoc comparisons where appropriate. The effect of trial on freezing during extinction was analyzed using repeated measures ANOVA. Change in freezing from the first to the last trial-block during extinction and from first extinction trial-block to the first extinction-recall trial-block in each age was analyzed using paired t-tests. Statistical significance was set at P<.05.
Results
Fear conditioning and extinction across adolescence
During conditioning there was a significant interaction between age and CS-trial for freezing (F8,132=2.72, P<.01). Four-week old mice froze more than older mice during the second CS-trial and more than 8-week olds only on the fourth CS-trial (Figure 1A). Assessment of the average reaction to footshock during conditioning in a separate cohort of mice revealed a significant effect of age (F2,21=3.83, P<.05), with 4-week olds (= 1.04 ± 0.17) showing a significant lesser reaction as compared to 6-week olds (= 1.63 ± 0.16) and a trend for a lesser reaction as compared to 8-week olds (= 1.46 ± 0.13).
Figure 1.
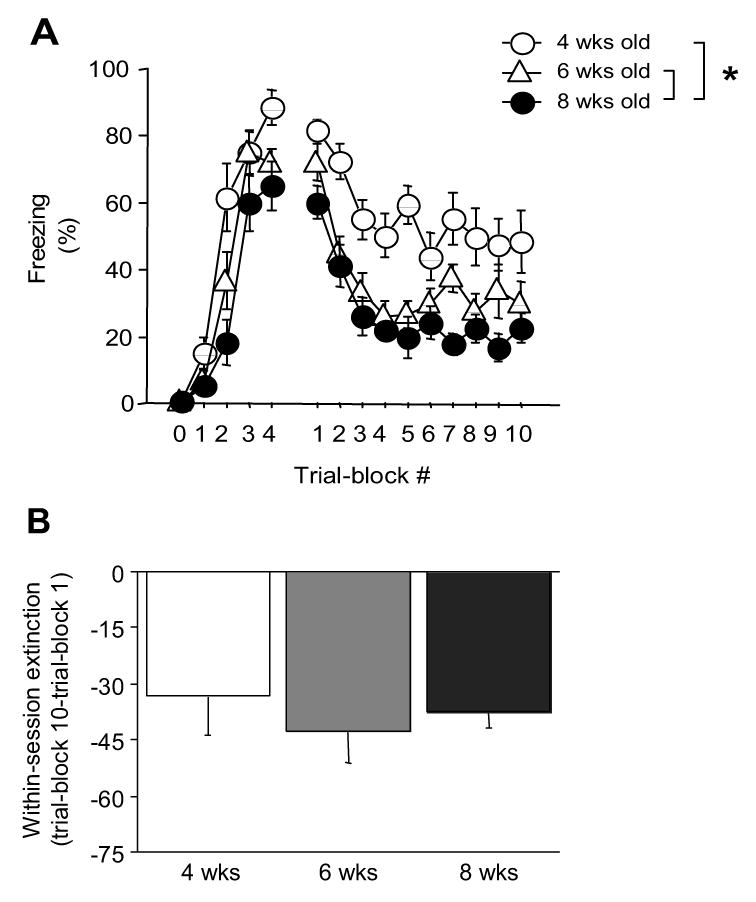
Pavlovian fear conditioning and fear extinction across adolescent development. Early adolescent (4-week old) mice showed significantly more freezing during conditioning than adults (8-week old), and both early adolescent and peri-adolescent (6-week old) mice generally froze more during extinction learning than adults (A). Age-groups showed an equivalent decrease in freezing during extinction training (B). n=12/age-group. *P<.05. Data in Figures 1–4 are Means ±SEM.
During CS-recall and extinction, there was a significant effect of age (F2,33=15.94, P<.01), and trial-block number (F9,297=21.70, P<.01) but no interaction for freezing. Four-week old mice generally exhibited more freezing than older mice, and 6-week old mice freezing more than 8-week olds (Figure 1A). All age groups showed a decrease in freezing with trial-block. Examining the change in freezing from the first to the last trial-block, ages did not differ (ns) (Figure 1B).
Anxiety-like behavior in the elevated plus-maze across adolescence
There was no significant effect of age for open arm time (Figure 2A), open arm entries (Figure 2B), percent open arm entries (data not shown), or closed arm entries (Figure 2C). There was a significant effect of age for total distance traveled: 4-week old mice traveled a significantly shorter distance than 6- and 8-week olds (F2,21=9.89, P<.01) (Figure 2D). Differences in total distance traveled were accounted for by age-group differences in distance traveled in the closed arms (F2,21=16.92, P<.01, 4-weeks=671 ± 48, 6-weeks=902 ± 41, 8-weeks=978 ± 24 cm).
Figure 2.
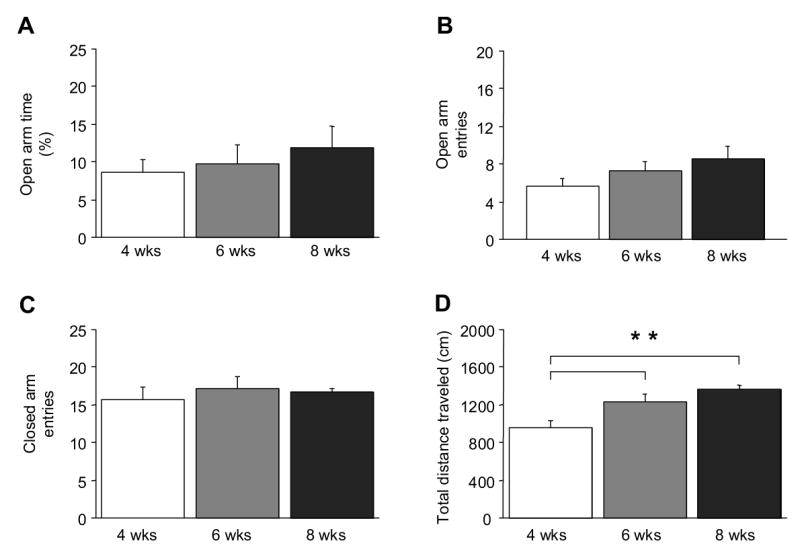
Anxiety-like behavior and locomotor activity in the elevated plus-maze across adolescent development. Age groups did not differ in percent open arm time (A), open arm entries (B) or closed arm entries (C), while early adolescents (4-week old) mice traveled a significantly shorter distance than peri-adolescent (6-week old) and adults (8-week old) (D). n=8/age-group. **P<.01.
Anxiety-like behavior and exploratory locomotion in the novel open field across adolescence
As shown in Figure 3A, there was a significant effect of age (F2,29=3.72, P<.05) and time (F11,319=36.81, P<.01) but no age × time interaction for total distance traveled. Collapsing across time, post-hoc analysis showed that 8-week old mice were generally more active than both 4- and 6-week olds. Mice of all age groups showed a progressive decrease in total distance traveled across the session. There was a significant effect of age for percent time spent in the center of the open field. As shown in Figure 3B, 6-week old mice showed less percent center time than 8-week olds (F2,29=3.50, P<.05).
Figure 3.
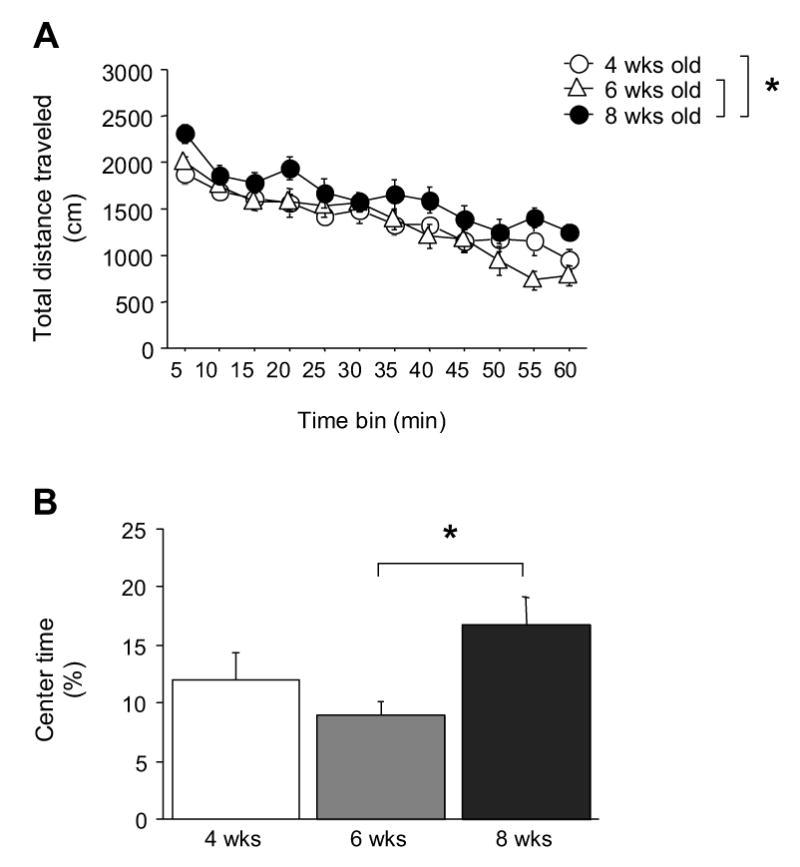
Anxiety-like behavior and locomotor activity in the novel open field across adolescent development. Early adolescent (4-week old) and peri-adolescent (6-week old) mice traveled a significantly shorter distance than adults (8-week old) (A). Peri-adolescents spent significantly less time the center of the open field than adults (B). n=10–11/age-group. *P<.05.
Depression-related behavior across adolescence
There was a significant effect of age (F2,27=13.97, P<.01), but not day, and no age × day interaction for percent immobility in the forced swim test. As shown in Figure 4, 4-week old mice showed significantly less immobility than older mice, regardless of age.
Figure 4.
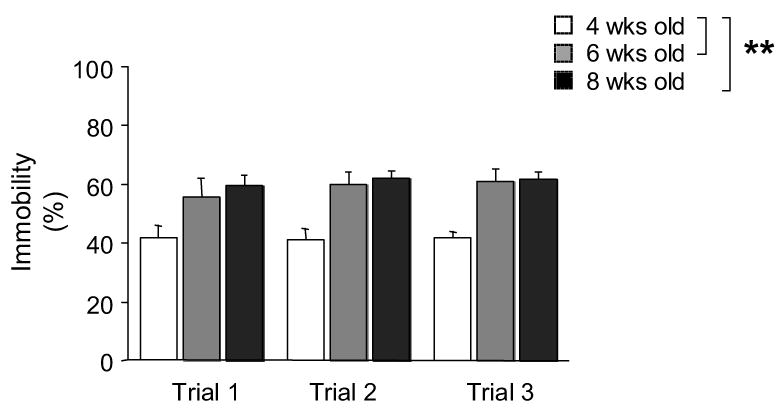
Behavior in the forced swim test for depression-related behavior across adolescent development. Early adolescent (4-week old) mice showed significantly less immobility than peri-adolescents (6-week old) and adults (8-week old) across 3 daily swim test trials. n=9–11/age-group. **P<.01.
Discussion
The results of the present study demonstrate developmental alterations in various measures of fear conditioning, anxiety-like behavior and depression-related behavior across the juvenile-to-adult period in C57BL/6J mice.
Four-week old, early adolescent mice showed more rapid and more robust acquisition of a conditioned association between an auditory stimulus (conditioned stimulus, CS) and footshock as compared to older mice. These young mice showed higher freezing than adults by the second presentation of the CS, demonstrative of augmented one-trial learning.
Enhanced fear conditioning in early adolescent mice was further evidenced by higher freezing during the last of four CS-shock presentations. Importantly, these differences did not appear to be due to increased sensitivity to the pain of the footshocks in the early adolescents, as reactions to footshock was actually lesser in these mice as compared to the older groups. Enhanced fear acquisition during conditioning was paralleled by relatively greater recall and expression of the fear response when early adolescent mice were again presented with the CS twenty-four hours later in a novel environment. Moreover, while mice of all age groups showed a significant reduction in freezing to the CS with repeated tone presentations (i.e., within-session extinction), freezing was generally higher throughout the session in the early adolescents. Thus, age-related differences in fear conditioning were not associated with differences in rates of within-session extinction learning: the magnitude of the reduction in freezing from start to the end of the 50-trial extinction session was no different between the adolescents and adults. Although peri-adolescent mice exhibited normal freezing during conditioning, overall freezing during the recall and extinction session was modestly but statistically higher in this age-group than in adults. Taken together, these data suggest that adolescent mice, particularly during early adolescence, have a greater propensity to acquire and/or express conditioned fear responses than adults, and extend previous work showing that various conditioned fear responses, including freezing, develop in early adolescence in rats (reviewed in [6, 23, 24, 47]). More generally, these findings potentially speak to view that human adolescence is a period of vulnerability to anxiety disorders characterized by abnormalities in emotional memory, such as posttraumatic memory (PTSD) [4, 5, 44, 49]. Although beyond the scope of the current study, elucidating the neural substrates of enhanced fear conditioning in adolescent mice could provide insight into the pathophysiology of adolescent PTSD [8, 32]. For example, examining the development of the amygdala and prefrontal cortex across mouse adolescence would be of significant interest [26, 31, 37].
Interestingly, ontogenic shifts in emotional learning and memory were dissociated from changes in unconditioned anxiety-like behaviors across adolescence. Exploration of the aversive, open arms of the elevated plus-maze was similar in adolescents and adults; while exploration of the aversive center of the novel open field was lower in peri- but not early adolescents than adults. These data concur with the finding that 4-week old Swiss Webster mice were no different from 8-week olds on the elevated plus-maze at baseline [48] (see also [21]), but not with another report that 7-week, but not 5-week old CD-1 mice showed lesser anxiety-like behavior in this test than adults [34], or with data variously reporting either relatively increased or decreased anxiety-like behavior in adolescents rats in both the elevated plus-maze, light/dark exploration test and the social interaction test [9, 13, 15, 18, 25, 41, 43, 51]. Such differences may be due to methodological differences between studies. Another potentially critical variable influencing these behaviors and other is genetic background of mice tested. Mouse strains are well known to differ on various measures anxiety- and depression-related behavior and stress reactivity (for recent review, see [22]). Recent findings also demonstrate that adolescent mice of different strains may vary in their anxiety-related responses to certain environmental manipulations. For example, A/J mice housed with C57BL/6J mice during adolescence subsequently exhibited lower levels of anxiety-like behavior in adulthood as compared to A/J mice housed with conspecifics from the same strain while, in contrast, anxiety-like behaviors in C57BL/6J mice were unaffected by social housing conditions [22]. These data suggest that the ontogeny of anxiety- and possibly other ‘emotion-related’ behaviors may vary across genetic backgrounds, and that a comparison of adolescents versus adults in different mouse strains, both inbred and outbred, may yield valuable insights.
While indices of anxiety-like behavior were largely similar across age-groups in our study, early adolescent mice did show lower overall levels of locomotor activity than adults in both the elevated plus-maze and novel open field, and peri-adolescents exhibited lower locomotion in the novel open field only, as compared to adults. Further analysis indicated that these differences were explained by the young mice traveling a shorter distance within the safe regions of the apparatus (i.e., closed arms of the elevated plus-maze, periphery of novel open field). Similarly, relatively lesser activity in the open field and light/dark exploration tests has been reported in, respectively, 5-week old CD-1 mice [2] and 4-week old Swiss Webster mice [21], as compared to adult counterparts. However, other studies have found relatively greater novel environment locomotor exploration in adolescent CD-1 mice [1, 29], while studies in rats report both normal and greater open field locomotion in adolescents relative to adults [14, 16, 17, 35, 36, 46]. Some authors have proposed that differences in locomotor exploration between adolescents and adults are related to the extent that novelty drives behavior in a given test situation and this factor may account for the apparent discrepancies across studies [30]. Notwithstanding, levels of locomotor activity in adolescent mice in the current study are unlikely to account for the relatively higher levels of active swimming behavior exhibited by adolescent mice in the forced swim test for depression-related behavior. Thus, early adolescents showed significantly less immobility than adults across three daily exposures to the test. In contrast to the step-wise age-dependent differences in fear extinction and locomotor activity (with peri-adolescents being intermediate), differences in the forced swim test were restricted to the early adolescent mice. Relatively lesser forced swim test immobility in early adolescents mimics the effects of antidepressant treatment typically seen in adult rats and mice [11] and as such can be interpreted as an reduced depression-like profile.
To our knowledge there are no other studies assessing depression-related behaviors in adolescent rodents. However, pertinent to present findings are data describing altered behavioral, neural and neuroendocrine responses to stress in adolescent rodents. For example, using c-fos immunoreactivity as an index of neuronal activation, Kellogg and colleagues showed that restraint stress produced less activation of certain brain regions mediating stress-reactivity, including parts of the amygdala and medial PFC, in 4-week old rats as compared to adults [27]. An attenuated stress-response in adolescent animals is consistent with the finding that corticosterone responses to forced novelty exposure were lesser in 5-week old CD-1 mice than adults [2], but not with another showing that adolescent Swiss Webster mice or various rat strains were more sensitive to social and restraint stress as measured by neuroendocrine release, body weight reductions and/or increased elevated plus-maze, light/dark exploration test or open field anxiety-like behavior [10, 43, 48, 53]. Again these data emphasize that task, species and strain factors likely play a major role in determining anxiety- and stress-related behavior in adolescent mice and highlight the need for further studies in this area of research.
In summary, the results of the present study demonstrate that the developmental transition from juvenility through adulthood in mice sees by pronounced changes in fear- and stress-related behaviors in the C57BL/6J inbred strain. Mice at the early adolescent stage acquired and expressed conditioned fear responses to a greater degree as compared to adults. In contrast, unconditioned anxiety-related behaviors in the elevated plus-maze and novel open field did not differ between adolescents and adults, while depression-related responses in the forced swim test were lower in early adolescent mice than adults. Thus, differences between adolescents and adults appear complex and specific to certain behavioral domains, at least in this strain of mice, and cannot be explained by a general ‘deficiency’ in fear/anxiety regulation and stress-reactivity in the younger animals. Rather, the distinct behavioral profile of adolescent mice likely reflects evolutionary shaped strategies for meeting the unique environmental demands of this phase of life. Elucidating the neural basis of these behaviors could shed light on the pathogenesis and improved treatment of neuropsychiatric disorders such as anxiety and depression that often have their origins in adolescence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 2.Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 3.Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 5.Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- 6.Barnet RC, Hunt PS. Trace and long-delay fear conditioning in the developing rat. Learn Behav. 2005;33:437–443. doi: 10.3758/bf03193182. [DOI] [PubMed] [Google Scholar]
- 7.Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- 8.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheeta S, Irvine EE, Tucci S, Sandhu J, File SE. In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology. 2001;25:601–607. doi: 10.1016/S0893-133X(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 10.Choi S, Kellogg CK. Adolescent development influences functional responsiveness of noradrenergic projections to the hypothalamus in male rats. Brain Res Dev Brain Res. 1996;94:144–151. doi: 10.1016/s0165-3806(96)80005-8. [DOI] [PubMed] [Google Scholar]
- 11.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 12.Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- 13.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 14.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77:21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Adolescent and adult female rats differ in sensitivity to nicotine’s activity effects. Pharmacol Biochem Behav. 2005;80:567–575. doi: 10.1016/j.pbb.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 17.Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine’s activity effects. Pharmacol Biochem Behav. 2003;74:917–931. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 18.Genn RF, Tucci SA, Thomas A, Edwards JE, File SE. Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neurosci Biobehav Rev. 2003;27:155–161. doi: 10.1016/s0149-7634(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 19.Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- 20.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Hascoet M, Colombel MC, Bourin M. Influence of age on behavioural response in the light/dark paradigm. Physiol Behav. 1999;66:567–570. doi: 10.1016/s0031-9384(98)00333-3. [DOI] [PubMed] [Google Scholar]
- 22.Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Hunt PS, Campbell BA. In: Learning, motivation, and cognition: The functional behaviorism. Robert C, Bolles Bouton ME, Fanselow MS, editors. Americal Psychological Association; Washington, DC: 1997. pp. 5–74. [Google Scholar]
- 24.Hunt PS, Richardson R. Pharmacological dissociation of trace and long-delay fear conditioning in young rats. Neurobiol Learn Mem. 2006 doi: 10.1016/j.nlm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res. 1993;56:177–180. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 26.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellogg CK, Awatramani GB, Piekut DT. Adolescent development alters stressor-induced Fos immunoreactivity in rat brain. Neuroscience. 1998;83:681–689. doi: 10.1016/s0306-4522(97)00408-9. [DOI] [PubMed] [Google Scholar]
- 28.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 29.Laviola G, Adriani W. Evaluation of unconditioned novelty-seeking and d-amphetamine-conditioned motivation in mice. Pharmacol Biochem Behav. 1998;59:1011–1020. doi: 10.1016/s0091-3057(97)00531-5. [DOI] [PubMed] [Google Scholar]
- 30.Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 31.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 34.Macri S, Adriani W, Chiarotti F, Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Anim Behav. 2002;64:541–546. [Google Scholar]
- 35.Marin MT, Planeta CS. Maternal separation affects cocaine-induced locomotion and response to novelty in adolescent, but not in adult rats. Brain Res. 2004;1013:83–90. doi: 10.1016/j.brainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Masur J, Schutz MT, Boerngen R. Gender differences in open-field behavior as a function of age. Dev Psychobiol. 1980;13:107–110. doi: 10.1002/dev.420130202. [DOI] [PubMed] [Google Scholar]
- 37.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 38.Pavlov IP. Conditioned reflexes. Oxford University Press; London: 1927. [Google Scholar]
- 39.Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- 41.Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- 42.Rodgers RJ. Animal models of ‘anxiety’: where next? Behav Pharmacol. 1997;8:477–496. 497–504. doi: 10.1097/00008877-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Slawecki CJ. Comparison of anxiety-like behavior in adolescent and adult Sprague-Dawley rats. Behav Neurosci. 2005;119:1477–1483. doi: 10.1037/0735-7044.119.6.1477. [DOI] [PubMed] [Google Scholar]
- 44.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 45.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 46.Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Dev Psychobiol. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- 47.Stanton ME, Freeman JH, Jr, Skelton RW. Eyeblink conditioning in the developing rat. Behav Neurosci. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- 48.Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- 49.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 50.Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 52.Wallace JM, Jr, Bachman JG, O’Malley PM, Schulenberg JE, Cooper SM, Johnston LD. Gender and ethnic differences in smoking, drinking and illicit drug use among American 8th, 10th and 12th grade students, 1976–2000. Addiction. 2003;98:225–234. doi: 10.1046/j.1360-0443.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 53.Wilson JH, McKinley SA, Young BL. Prolactin levels in juvenile and adult rats following acute restraint and the open field. Physiol Behav. 2000;68:383–387. doi: 10.1016/s0031-9384(99)00194-8. [DOI] [PubMed] [Google Scholar]


