Abstract
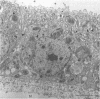
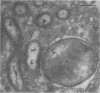
Symbioses between chemolithoautotrophic bacteria and the major macrofaunal species found at hydrothermal vents have been reported for numerous sites in the Pacific Ocean. We present microscopical and enzymatic evidence that methylotrophic bacteria occur as intracellular symbionts in a new species of mytilid mussel discovered at the Mid-Atlantic Ridge hydrothermal vents. Two distinct ultrastructural types of gram-negative procaryotic symbionts were observed within gill epithelial cells by transmission electron microscopy: small coccoid or rod-shaped cells and larger coccoid cells with stacked intracytoplasmic membranes typical of methane-utilizing bacteria. Methanol dehydrogenase, an enzyme diagnostic of methylotrophs, was detected in the mytilid gills, while tests for ribulose-1,5-bisphosphate carboxylase, the enzyme diagnostic of autotrophy via the Calvin cycle, were negative. Stable carbon isotope values (δ13C) of mytilid tissue (−32.7 and −32.5% for gill and foot tissues, respectively) fall within the range of values reported for Pacific vent symbioses but do not preclude the use of vent-derived methane reported to be isotopically heavy relative to biogenically produced methane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 2. The methanol-oxidizing enzyme of Pseudomonas sp. M 27. Biochem J. 1964 Sep;92(3):614–621. doi: 10.1042/bj0920614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks J. M., Kennicutt M. C., 2nd, Fisher C. R., Macko S. A., Cole K., Childress J. J., Bidigare R. R., Vetter R. D. Deep-sea hydrocarbon seep communities: evidence for energy and nutritional carbon sources. Science. 1987 Nov 20;238(4830):1138–1142. doi: 10.1126/science.238.4830.1138. [DOI] [PubMed] [Google Scholar]
- Childress J. J., Fisher C. R., Brooks J. M., Kennicutt M. C., 2nd, Bidigare R., Anderson A. E. A methanotrophic marine molluscan (bivalvia, mytilidae) symbiosis: mussels fueled by gas. Science. 1986 Sep 19;233(4770):1306–1308. doi: 10.1126/science.233.4770.1306. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Buchholz L. A., Remsen C. C. Effect of Copper on Methylomonas albus BG8. Appl Environ Microbiol. 1991 Apr;57(4):1261–1264. doi: 10.1128/aem.57.4.1261-1264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corliss J. B., Dymond J., Gordon L. I., Edmond J. M., von Herzen R. P., Ballard R. D., Green K., Williams D., Bainbridge A., Crane K., van Andel T. H. Submarine thermal sprirngs on the galapagos rift. Science. 1979 Mar 16;203(4385):1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- Distel D. L., Lane D. J., Olsen G. J., Giovannoni S. J., Pace B., Pace N. R., Stahl D. A., Felbeck H. Sulfur-oxidizing bacterial endosymbionts: analysis of phylogeny and specificity by 16S rRNA sequences. J Bacteriol. 1988 Jun;170(6):2506–2510. doi: 10.1128/jb.170.6.2506-2510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J. A., Smith S. W., Cavanaugh C. M. Phylogenetic relationships of chemoautotrophic bacterial symbionts of Solemya velum say (Mollusca: Bivalvia) determined by 16S rRNA gene sequence analysis. J Bacteriol. 1992 May;174(10):3416–3421. doi: 10.1128/jb.174.10.3416-3421.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E., Tsuji T., Saino T., Hattori A. A simple procedure for mass spectrometric microanalysis of 15N in particulate organic matter with special reference to 15N-tracer experiments. Anal Biochem. 1977 May 15;80(1):312–318. doi: 10.1016/0003-2697(77)90650-9. [DOI] [PubMed] [Google Scholar]