Abstract
Gonadectomized and surgically intact adult C57BL/6J (B6) mice of both sexes were exposed for 12 hours nightly to a high-frequency augmented acoustic environment (AAE): repetitive bursts of a half-octave noise band centered at 20 kHz, 70 dB SPL. The effects of sex, gonadectomy, and AAE treatment on genetic progressive hearing loss (exhibited by B6 mice) were evaluated by obtaining auditory brainstem response thresholds at ages 3-, 6-, and 9-months; hair cell counts (cytocochleograms) were obtained at 9 months. A sex difference in the rate of genetic progressive hearing loss in B6 mice (observed by earlier studies) was confirmed, with females exhibiting a faster rate of threshold elevations and more severe loss of hair cells at age 9 months. Gonadectomy had no consistent effects on the rate or severity of hearing loss in nonexposed mice of either sex. An unexpected finding was that the high-frequency AAE treatment caused additional ABR threshold elevations and hair cell loss. In an earlier study, the same high-frequency AAE treatment on DBA/2J mice ameliorated hearing loss. The most severe AAE-induced losses occurred in surgically intact females, suggesting a potentiating effect of ovarian hormone(s).
1. Introduction
Mice of the C57BL/6J (B6), DBA/2J (D2), and other strains exhibit genetic age-related hearing loss (for reviews see Erway et al., 1993, 2001; Johnson et al., 1997, 2000; Li and Borg, 1991; Willott, 1996; Zheng et al., 2005). A series of studies has shown this to be ameliorated by treatment with an augmented acoustic environment (AAE) consisting of 12-hour-nightly exposure to noise bursts of 70 dB SPL (re: 20μPa). Earlier studies (Turner and Willott, 1998; Willott an Turner, 1999; Willott and Bross, 2004; Willott et al., 2000; 2005) used a relatively broad noise band as the AAE, and this affected auditory responses to a correspondingly broad range of frequencies, as low as 4 kHz. In the most recent study (Willott et al., 2006), D2 mice were exposed to an AAE noise band restricted to higher frequencies (a half-octave noise band centered at 20 kHz), and this treatment lessened auditory brainstem response (ABR) threshold elevations only for frequencies of 16–32 kHz. The present study utilized the same high-frequency AAE to treat B6 mice, predicting that similar, high-frequency ameliorative effects would be observed. A related issue is that of sex differences in progressive hearing loss in B6 mice. Male and female B6 mice exhibit a similar rate and severity of hearing loss during the early stages, but in mice over 6 months of age, females exhibit more severe threshold elevations (Henry, 2002; Willott and Bross, 2004). It has been hypothesized that the emergence of this sex difference during adulthood is related to gonadal hormone activity (Willott and Bross, 2004) as it affects gene(s) that cause hearing loss in B6 mice, not general hormonal effects on the cochlea. Indeed, in CBA strains of mice that maintain good hearing as they age, older females have better hearing than males (Guimaraes et al., 2004; Henry, 2004). Thus, the present study included male and female mice that had been gonadectomized as young adults in order to disrupt gonadal hormone activity.
2. Methods
2.1 Experimental animals
C57BL/6J mice were obtained from the Jackson Laboratory, Bar Harbor, ME at age 4–6 weeks. Half of the mice received gonadectomies at age 2–3 weeks by highly experienced surgeons at the Jackson Laboratory before being shipped. Mice served in one of 8 subject groups. Mice exposed to the AAE were: 5 intact females, 8 ovariectomized (OVX) females, 5 intact males, and 9 orchidectomized (ORX) males; control (non-exposed) mice were: 7 intact females, 8 OVX females, 5 intact males, and 8 ORX males. Two additional groups of 3 males and 3 females aged 14 months (never exposed to the AAE) were also tested to further examine sex differences. The room temperature in the vivarium was maintained between 23° and 24°C and a 12-hour light/dark schedule was used. Mice were given ad libitum access to Purina rodent chow and tap water.
All procedures conformed to NIH guidelines for use of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of South Florida.
2.2. Exposure to the AAE
Mice were placed in a plastic cage (12 x 13 x 30 cm), 4 mice per cage. Here they received consecutive 12-hour nights of AAE treatment through 9 months of age.
To produce the AAE, a half-octave noise band centered at 20 kHz (rise/fall = 10 ms, duration = 200 ms, rate = 2/s) was synthesized with Tucker-Davis Technologies (TDT) equipment and downloaded to an Apple Mini-ipod. This signal was amplified with a Radio Shack MPA-200 audio amplifier and sent to Radio Shack Supertweeters placed on the filter tops above the plastic cages. The amplifier gain was set so that the mean SPL within the cages was 70 dB. The level was calibrated by inserting the microphone of a Brüel and Kjær Type 2203 Precision Sound Level Meter coupled to a Type 1613 octave band filter into holes drilled in various locations of a calibration cage and determining the appropriate amplifier settings. The signal was automatically timed to be on during the 12-hr dark cycle.
2.3. ABR thresholds
ABR thresholds were obtained at ages 3 months, 6 months, and 9 months with a range of ± 2weeks due to the large number of mice to be tested. Testing was performed at midday (noon ± 1.5 hours). The potential effect of the time of day / order of testing was evaluated during the first series of tests (3 months), and there was no evidence that ABR thresholds were affected. Nonetheless, the order of testing was balanced among the different subject groups for all three test sessions.
The procedures have been described elsewhere (Willott et al., 2006). Stimuli were synthesized and produced using TDT System 3 hardware and software and an ESL electrostatic speaker (Tucker-Davis Technologies), which has a relatively flat frequency response between 2 kHz to 100 kHz. The speaker was mounted 20 cm in front of the mouse’s head in the free field in a foam-padded, shielded acoustic chamber. The output was calibrated by placing the Brüel & Kjær microphone in the space to be occupied by the mouse’s head and determining the appropriate setting for the desired acoustic output. A maximum SPL (re: 20 μPa) of 80 dB was employed for all stimuli. Mice were anesthetized with a cocktail of ketamine (100 mg/kg), xylazine (20 mg/kg), and acepromazine (3 mg/kg) and kept warm with a heating pad during ABR recordings. A subdermal (active) needle electrode was inserted at the vertex, and ground and reference electrodes were inserted subdermally in the loose skin beneath the pinnae of opposite ears. TDT System 3 hardware and software were used to obtain ABRs, with 500 stimulus repetitions per record. Preamplifier filters were set at 100 Hz high pass and 3000 Hz low pass.
Mice were tested with tone pips (1 ms rise/fall; 3 ms duration; 4, 8, 12, 16, 24, and 32 kHz). ABRs were obtained at each frequency by reducing the SPL in 5 dB steps beginning with a maximum of 80 dB SPL. Threshold was defined as the lowest 5 dB step at which an ABR could be recognized. Judgment of threshold was made from ABR records off-line by two independent, experimentally blind observers. The two observers obtained the same thresholds in the vast majority of cases and any discrepancies were rarely more than 5 dB. Minor between-rater discrepancies in thresholds were resolved, subject to agreement by both observers. If ABRs were not evoked by the maximum SPL of 80 dB, a nominal threshold of 85 dB was assigned.
2.4. Cytocochleograms
Cytocochleograms were prepared at the University at Buffalo under subcontract to Dr. Richard Salvi. The procedures for preparing and analyzing mouse inner ears have been described in detail in previous reports (McFadden et al., 1999a; McFadden et al., 1999b: Willott et al., 2006). Briefly, the organ of Corti was carefully microdissected out from base to apex, and specimens stained with Ehrlich’s hematoxylin solution (Ding et al., 2001). Specimens were mounted in glycerin on glass slides and examined with a light microscope with differential interference contrast optics (Zeiss Standard, 400X magnification). Hair cells were counted as present if the cell body and cuticular plate were intact. Cochleograms were constructed by plotting the percentage of missing OHCs or IHCs as a function of percent distance from the apex of the cochlea. Percentages were computed relative to laboratory norms for young CBA mice (Spongr et al., 1997).
3. Results
3.1 ABR thresholds
The ABR threshold data are presented in Figures 1 and 2. Figure 1 separates the data into three panels, one for each age, with frequency on the abscissa. In Figure 2, the same data are arranged to more clearly show the age-related changes for each frequency. For all figures, females are indicated by black symbols, males by green; filled symbols (connected by unbroken lines) are controls, unfilled symbols (connected by dashed lines) are AAE-treated; squares are gonadectomized, circles are intact.
Figure 1.
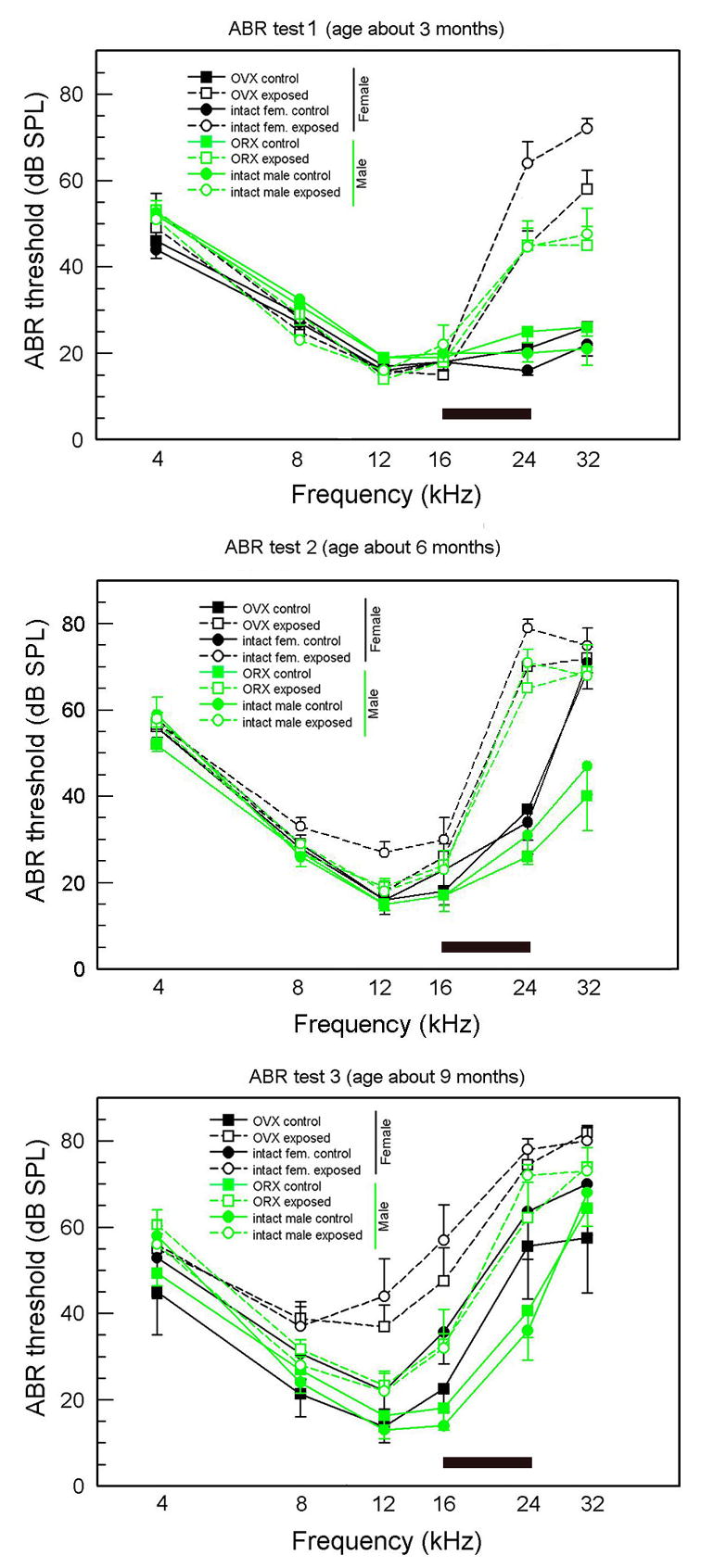
ABR thresholds for all mice at all three ages (means and standard errors). The frequency band of the AAE noise is shown by the shaded bar. Green symbols = males, black symbols = females; square symbols = gonadectomized. Round symbols = intact; unfilled symbols = AAE-exposed, filled symbols = nonexposed controls.
Figure 2.
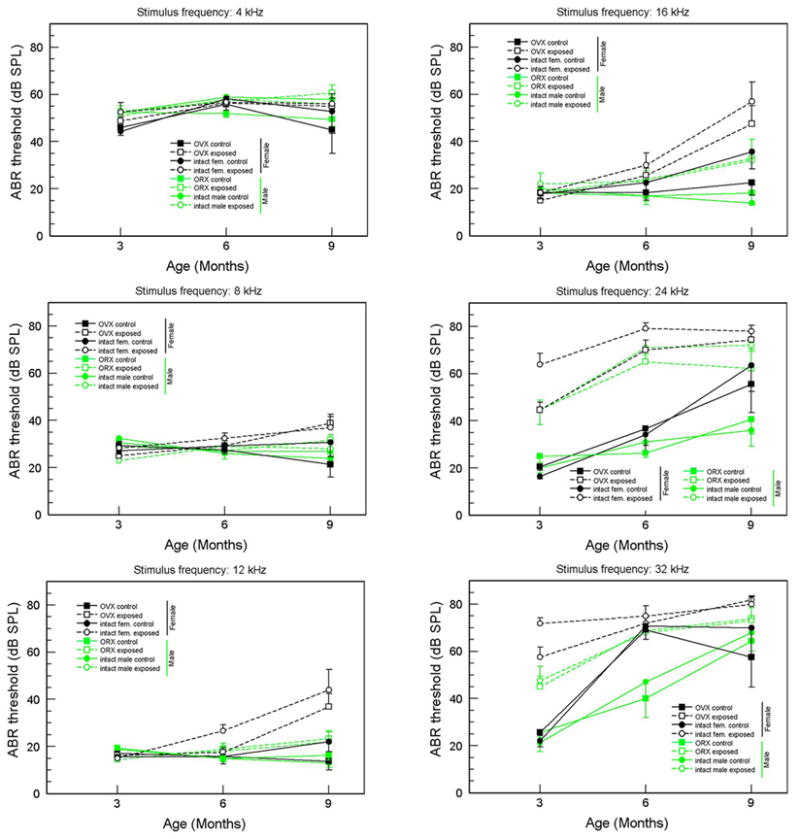
ABR thresholds (means and standard errors) as a function of age for each stimulus frequency. Green symbols = males, black symbols = females; square symbols = gonadectomized. Round symbols = intact; unfilled symbols = AAE-exposed, filled symbols = nonexposed controls.
3.1.1 Statistical analysis
The initial a 3-way analysis of variance (ANOVA) was performed using all data: Age (3) X Group (8) X Frequency(6), with Frequency as a repeated factor. Age was not treated as a repeated measure because several mice were lost or used for other procedures after the initial test session (age-related changes in individual mice were consistent with those observed in its respective group). This ANOVA was followed up with 2-way ANOVAs (Group X Age) for each frequency, and then by Fisher’s LSD (protected t) tests (p< 0.05) for comparisons among individual groups at each age or differences in a group as a function of age.
The initial 3-way ANOVA obtained a number of significant effects at p < 0.0001. These included main effects of Age [F(2,140) = 54.91], subject Group [F(7,140) = 19.42], and Frequency [F(5,700) = 687.51], as well as interactions for Age X Frequency [F(10,700) = 36.21), Group X Frequency [F(35,700) = 13.38], and Age X Group X Frequency [F(70,700) = 4.35). The only interaction that was not significant was Age X Group.
Results of the 2-way ANOVAs at each frequency are presented in Table 1. The following conclusions are supported by this analysis and the Fisher’s LSD follow-up tests.
Table 1.
Two-way ANOVAs for ABR thresholds at each frequency
| Age [F(2,140)] | Group [F(7,140)] | Age X Group [F(14,140)] | |
|---|---|---|---|
| 4 kHz | 4.73, p = 0.01 | NS | NS |
| 8 kHz | NS | NS | 1.93, p = 0.27 |
| 12 kHz | 12.92, p < 0.0001 | 6.28, p < 0.0001 | 4.25, p < 0.0001 |
| 16 kHz | 22.12, p < 0.0001 | 5.97, p < 0.0001 | 3.27, p = 0.0002 |
| 24 kHz | 35.65, p < 0.0001 | 23.52, p < 0.0001 | NS |
| 32 kHz | 63.54, p < 0.0001 | 11.87, p < 0.0001 | 2.61, p = 0.0022 |
3.1.2. Sex differences in nonexposed control mice
Looking at untreated control mice in Figure 1 (filled symbols), it is evident that thresholds for males and females are quite similar at 3 months of age. By 6 months, however, thresholds at 32 kHz have become significantly higher for both female control groups (intact and ovariectomized) compared to males, although control males were also exhibiting less severe but significant threshold elevations. Thresholds of male and female controls for 24 kHz were beginning to separate at 6 months of age, and by 9 months, female thresholds were significantly higher than those of males at 24 kHz.
ABR thresholds for the two additional groups of 14-month-old mice revealed that the sex differences persisted as threshold elevations became severe. As seen in Figure 3, the females were almost deaf at this age, whereas males had relatively low thresholds for the middle frequencies. A 2-way mixed ANOVA obtained significant effects for Group [F(1,4) = 19.88, p = 0.011] and Group X Frequency interaction [F(5,20) = 7.20, p = 0.0005], with Fisher’s LSD tests showing significant differences at 8, 12, and 16 kHz.
Figure 3.
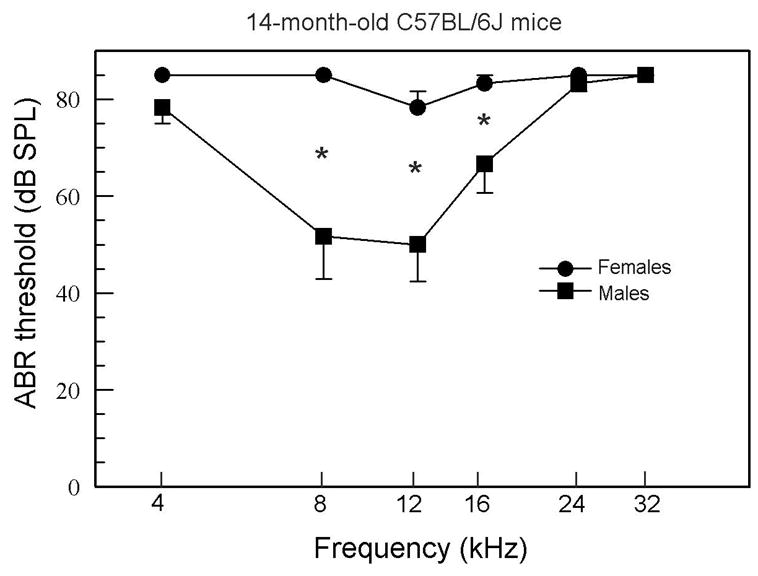
ABR thresholds for 14-month-old mice. In these older mice, females have significantly higher thresholds at 8, 12, and 16 kHz.
3.1.3. AAE effects
Contrary to the predicted ameliorative effect of AAE treatment, ABR thresholds of treated mice at higher frequencies became higher than those of control mice. This was true for both sexes and surgical conditions. At 3 months of age, thresholds for all four AAE-treated groups (unfilled symbols) were significantly elevated for 24 and 32 kHz compared to their respective control groups (filled symbols), and these became significantly higher by 6 months.
3.1.3.1. Sex differences in exposed mice
Whereas both male and female exposed mice had higher thresholds then their respective control groups, female exposed mice had higher thresholds than male exposed mice for many data points. This appeared first at the higher frequencies. At age 3 months, 24 and 32 kHz thresholds of female exposed mice were significantly higher than those of the male exposed groups, although by 6 and 9 months the males’ threshold elevations had “caught up,” and the sex differences were no longer significant. For lower frequencies, gradually rising thresholds of AAE-treated males and females did not differ significantly until age 9 months. At this age, thresholds for 12 and 16 kHz of female exposed mice were higher than those of male counterparts.
3.1.4. Effects of surgical condition
As indicated already, surgery had little effect when gonadectomized groups (square symbols) were compared with their intact counterparts (circle symbols). There was, however, evidence that intact females had more severe threshold elevations than OVX females (and, of course males). Among controls, by 9 months of age, intact females had generally higher thresholds than OVX females at the higher frequencies and this was significant for 16 kHz. Comparing AAE-treated females, intact mice generally had higher thresholds than OVX mice. This was especially clear at age 3 months, where thresholds of intact female exposed mice were significantly higher than those of OVX exposed mice at 24 and 32 kHz.
3.2. Cytocochleograms
Figure 4 shows the mean cochleograms for OHCs and IHCs. ANOVAs (8 Groups X 10 cochlear segments) showed significant main effects of both Group and Segment (p’s < 0.0001) and, more importantly, significant Group X Segment interactions for IHCs [F(63,216) = 3.68, p < 0.0001] and OHCs [F(63,216) = 3.35, p < 0.0001 ].
Figure 4.
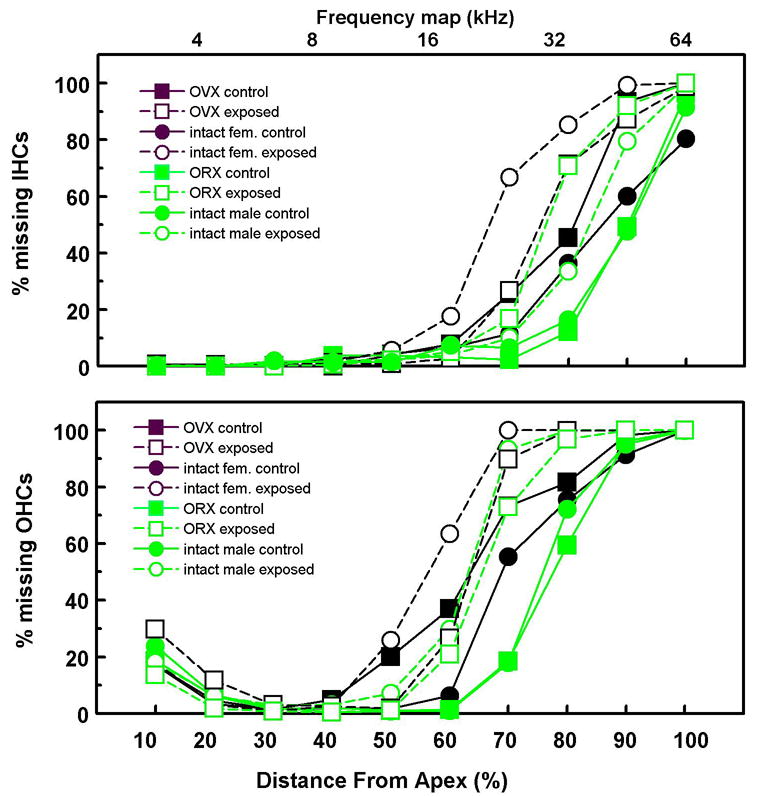
Cytocochleograms (% missing hair cells) for all groups.
The order of severity of hair cell loss comports well with that of ABR thresholds. The two male control groups (solid green symbols) had the lowest ABR thresholds and the most surviving hair cells. Conversely, the two female exposed groups (unfilled black symbols) had the highest ABR thresholds and the most severe loss of hair cells. One-way ANOVAs were performed on individual segments to confirm these observations. For IHCs, group differences were significant for the 70th, 80th, and 90th percent distance from the apex [F’s (7,24) > 3.73, p’s < 0.0072). For OHCs, group differences were significant for the 60th and 70th percent distance from the apex [F’s (7,24) > 4.18, p’s < 0.004). Fisher’s tests confirmed that male controls had less severe loss than any other group and that AAE treatment was associated with more severe hair cell loss in females as well. The AAE-induced loss of hair cells was at and above tonotopic regions of the cochlea that would respond to the AAE spectrum. As was the case for ABR thresholds, hair cell loss was especially severe in intact AAE-treated females. They had more severe losses of IHCs at the 70th percentile than any other group. Whereas the ABR thresholds of female (nonexposed) controls suggested more severe loss in intact females versus OVX females (above), this was not evidenced in cytochleograms of these groups.
4. Discussion
The present study provides further insights into the nature of sex differences in progressive hearing loss of B6 mice, while also obtaining a surprising finding: the high-frequency AAE treatment that had ameliorative effects on hearing loss in D2 mice (Willott et al., 2006) exacerbated ABR threshold elevations in B6 mice. We had predicted the opposite outcome.
4.1. Sex differences in control (non-exposed) mice and the effects of gonadectomy
Previously demonstrated sex differences in progressive hearing loss for B6 mice were clearly observed in the present study. ABR thresholds at the higher frequencies became more severely elevated in control females compared to control males (i.e., mice that were not exposed to the AAE). The sex difference persisted in 14-month-olds (Fig. 3).
A hypothesis previously offered to account for the emerging sex differences in B6 mice proposed that, during young adulthood, estrogen has a protective effect on the female cochlea (Willott and Bross, 2004; see also Guimaraes et al., 2004). As estrogen function changes during middle age, the protective effect wanes, and cochlear degeneration becomes more severe Because males never “depend” on estrogen for protection, they do not suffer a similar acceleration of hearing loss; furthermore, androgen activity persists well into the second year of life in B6 mice (Nelson et al., 1975), so any protection that might be provided by male hormones would not wane (Zhang et al., 2004). The present findings do not support this hypothesis. First, the sex differences were significant in control mice at 6 months of age (earlier than was observed in earlier studies), and AAE-exposed females already had higher thresholds than AAE-exposed males at 3 months of age (see below). During the first 6 months of life, the female reproductive system of B6 mice is still functioning at a high level (Nelson et al., 1981, 1982, 1992, 1995), so a decline in estrogen activity is unlikely at this age. Second, ovariectomized females did not have higher ABR thresholds or more severe hair cell loss than intact females, as would be predicted if estrogen were having a protective effect. Finally, orchidectomy had no effect on hair cell loss or ABR thresholds in males, suggesting that androgens are not playing a role either.
All this suggests that gonadal hormones during adulthood in B6 mice do not provide a sufficient mechanism to account for the sex difference in progressive hearing loss that occurs in B6 mice (although there may be a role in AAE effects, discussed below). It is, however, important to note that the present experiments only speak to adult levels of hormones, because the gonadectomized mice were intact prior to surgery at 2–3 weeks of age. Thus, it is possible that early hormonal effects on hearing occurred at least in part prior to surgery. At this point we cannot rule out the possibility that hormonal mechanisms, mostly acting at an early age, could be both necessary and sufficient to produce the sex differences. But, a strong adult activational effect appears unlikely.
Another caveat is the possibility that there were some hormonal effects – protective or otherwise – but they were not revealed by ABR thresholds or hair cell counts. Degeneration of the auditory system in B6 mice includes other cochlear tissue, spiral ganglion cells, and central auditory neurons (Di Palma et al., 2001; Frisina and Walton, 2001; Hequembourg and Liberman, 2001; Idrizbegovic et al., 2003; Ohlemiller and Gagnon, 2004; O’Neill et al., 1997; Spongr et al., 1997; Willott, 1996; Willott and Bross, 1996; Willott et al., 1987; Zettel et al., 1997). It is conceivable that protection of some cochlear or central auditory tissue was provided by estrogen, but these effects were masked by ABR threshold elevation which was caused by diminution of hair cell integrity. Estrogen is known to affect neural systems in a variety of ways that can be neuroprotective/positive or (less commonly) neurotoxic/negative (e.g., Belisle et al., 1985; Bergman et al., 1989; Bittar et al., 2001; Easton et al., 2006; Garcia-Segura et al., 2001; Papalexi et al., 2005; Picazo et al., 2003; Toran-Allerand et al., 1999; Usui, 2006), leaving open many possibilities to be addressed by future research. Various lines of evidence suggest that ovarian hormones may affect auditory function directly (Coleman et al., 1994; Hultcrantz et al., 2006). Estrogen receptors have been observed in the mouse cochlea (Stenberg et al., 1999; but see Nathan et al., 1999), although there is no indication as to what role they might play. Estrogen may inhibit ion transport in the stria vascularis (Lee and Marcus, 2001), and cochlear blood flow may be modulated by estrogen and progesterone (Laugel et al., 1987, 1988). Perhaps hormonal modulation of cochlear damage can act via one of these routes.
The present findings certainly make feasible the possibility that the sex differences were controlled by mechanisms other than gonadal hormones. There is a good deal of evidence that sex differences in brain and behavior may be mediated by genetic mechanisms that do not involve gonadal hormones (Arnold, 1996). An influence of nonhormonal mechanism would be consistent with the minimal effects of gonadectomy.
4.2. AAE effects
The effects of high-frequency AAE treatment were unexpected and especially surprising. It was hypothesized that the high-frequency AAE treatment would ameliorate age-related ABR threshold elevations for high frequencies in B6 mice, as occurred using this AAE regimen in D2 mice. Indeed, all previous studies of AAE treatment found amelioration of progressive hearing loss in B6, D2, and other strains of mice that develop sensorineural damage (see Willott et al., 2001 for a review). Several possibilities – none of which are mutually exclusive -- may be explored to account for these findings. 1. Research has demonstrated unusually high susceptibility of B6 mice to noise-induced hearing loss (Davis, 2001; Davis et al., 2003; Vasquez et al., 2004) that can be affected by the frequency of the noise and other factors (Harding et al., 2005). Although the AAE was only 70 dB SPL, perhaps the basal half of the B6 cochlea is especially vulnerable to damage by the high- frequency noise band, so that AAE-induced insult overrode potential beneficial effects of treatment. If D2 mice were not similarly vulnerable to this noise band, this might account for the strain difference. 2. Even at an early age, D2 mice have poor sensitivity to the frequencies encompassed by the 20 kHz-centered, half-octave AAE (Willott and Turner, 1999; Willott et al., 2006). By comparison, sensitivity for those frequencies is near normal in B6 mice at the beginning of the treatment. Paradoxically, the diminished performance of the D2 cochlea may have spared it from deleterious effects of over-stimulation that may have damaged the healthier basal cochlea in B6 mice. 3. It is conceivable that the histological or physiological effects of the AAE treatment were mixed in B6 mice, and this was not detected by ABR thresholds or cytocochleograms. That is, the AAE might have protected one type of cochlear or central auditory tissue even though it damaged hair cells. The ABR thresholds may have been more heavily influenced by the “negative” effects. Histological studies (in progress) may clarify this issue. 4. Other, unknown genetic differences may have played a role in causing strain differences. These could involve genetic background (e.g., Holme and Steel, 2004) or mechanisms that modulate cochlear damage.
Possible ovarian hormone effect on AAE-induced hearing loss
The present findings do not indicate a role of hormones in the usual course of B6 progressive hearing loss, and indeed, studies on menopausal women suggest beneficial effects of hormone replacement on presbycusis (Hultcrantz et al, 2006). However, the findings do suggest that ovarian hormones may increase vulnerability to noise-induced hearing loss. AAE-treated intact females exhibited significantly higher ABR thresholds and more hair cell loss than their OVX counterparts. This observation raises the possibility that hormonally mediated female vulnerability to ambient noise might contribute to sex differences in B6 mice. In this respect it is of interest that McFadden et al. (1999, 2000) showed that female chinchillas exposed to noise exhibited more severe threshold elevations at higher frequencies than males, whereas they showed less hearing loss than males at lower frequencies. Taken together the evidence suggests that a sex-related factor might influence noise-induced damage in the basal cochlea. In B6 mice which are vulnerable to noise to begin with (see below), females could be especially affected by ambient noise, albeit of a low level in control mice.
4.4. Conclusions
The present study has perhaps raised more questions than it has answered. While confirming the sex difference in the rate of progressive hearing loss in B6 mice, the hypothesized role of a waning protective effect of estrogen in females was not supported. The data suggest that ovarian hormones have little effect during adulthood, and may even potentiate noise-induced hearing loss in B6 mice. Questions remain about the possibility of an early hormone effect or a non-hormonal effect causing the sex differences. These issues have potentially important implications for our understanding of sensorineural and noise-induced hearing loss, especially in women. The study also demonstrated that an AAE treatment that is ameliorative for one inbred strain (D2) can facilitate hearing loss in another (B6). Some possible explanations have been mentioned, but perhaps the general lesson is that there is much to be learned about the positive and negative aspects of an augmented acoustic environment. These must be understood before any use of AAE treatments as a therapeutic tool can be considered.
Acknowledgments
This research was supported by R01 AG07554 (JFW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold AP. Genetically triggered sexual differentiation of brain and behavior. Horm Behav. 1996;30:495–505. doi: 10.1006/hbeh.1996.0053. [DOI] [PubMed] [Google Scholar]
- Belisle S, Bellabarba D, Lehoux JG. Age-dependent, ovary-independent decrease in the nuclear binding kinetics of estrogen receptors in the brain of the C57BL/6J mouse. Amer J Obstet Gynecol. 1985;153:394–401. doi: 10.1016/0002-9378(85)90077-8. [DOI] [PubMed] [Google Scholar]
- Bergman MD, Karelus KK, felicio LS, Nelson JF. Differential effects of aging on estrogen receptor dynamics in hypothalamus, pituitary and uterus of the C57BL/6J mouse. J Steroid Biochem. 1989;33:1027–1033. doi: 10.1016/0022-4731(89)90405-6. [DOI] [PubMed] [Google Scholar]
- Bittar RS, Cruz OL, Lorenzi MC, Marone SA, Miniti A. bregm1. Morphological and functional study of the cochlea after administration of estrogen and progesterone in the guinea pig. Int Tinnitus J. 7:41–45. [PubMed] [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80:209–215. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Davis RR. Noise-induced hearing loss. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton: 2001. pp. 477–488. [Google Scholar]
- Davis RR, Kozel P, Erway LC. Genetic influences in individual susceptibility to noise: a review. Noise Health. 2003;5:19–28. [PubMed] [Google Scholar]
- Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet. 2001;27:103–107. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- Easton A, Dwyer E, Pfaff DW. Estradiol and orexin-2 saporin actions on multiple forms of behavioral arousal in female mice. Behav Neurosci. 2006;120:1–9. doi: 10.1037/0735-7044.120.1.1. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison D. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Erway LC, Zheng QY, Johnson KR. Inbred strains of mice for genetics of hearing in mammals: searching for genes for hearing loss. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton: 2001. pp. 429–440. [Google Scholar]
- Frisina RD, Walton JP. Aging of the mouse central auditory system. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton: 2001. pp. 339–380. [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim SH, Frisina RD. Sex Differences in Distortion Product Otoacoustic Emissions as a Function of Age in CBA Mice. Hear Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Vos JD. The effect of an age-related hearing loss gene (Ahl) on noise-induced hearing loss and cochlear damage from low-frequency noise. Hear Res. 2004;204:90–100. doi: 10.1016/j.heares.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Henry KR. Sex- and age-related elevation of cochlear nerve envelope response (CNER) and auditory brainstem response (ABR) thresholds in C57BL/6 mice. Hear Res. 2002;170:107–115. doi: 10.1016/s0378-5955(02)00391-x. [DOI] [PubMed] [Google Scholar]
- Henry KR. Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hear Res. 2004;190:141–148. doi: 10.1016/S0378-5955(03)00401-5. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme RH, Steel KP. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. J Assoc Res Otolaryngol. 2003;5:66–79. doi: 10.1007/s10162-003-4021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. 2006;126:10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- Idrizbegovic E, Bogdanovic N, Viberg A, Canlon B. Auditory peripheral influences on calcium binding protein immunoreactivity in the cochlear nucleus during aging in the C57BL/6J mouse. Hear Res. 2003;179:33–42. doi: 10.1016/s0378-5955(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Laugel GR, Dengerink HA, Wright JW. Ovarian steroid and vasoconstrictor effects on cochlear blood flow. Hear Res. 1987;31:245–251. doi: 10.1016/0378-5955(87)90194-8. [DOI] [PubMed] [Google Scholar]
- Laugel GR, Wright JW, Dengerink HA. Angiotensin II and progesterone effects on laser Doppler measures of cochlear blood flow. Acta Otolaryngol. 1988;106:34–39. doi: 10.3109/00016488809107368. [DOI] [PubMed] [Google Scholar]
- Lee JH, Marcus DC. Estrogen acutely inhibits ion transport by isolated stria vascularis. Hear Res. 2001;158:123–130. doi: 10.1016/s0378-5955(01)00316-1. [DOI] [PubMed] [Google Scholar]
- Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991;111:827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999a;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J Comp Neurol. 1999b;413:101–112. [PubMed] [Google Scholar]
- McFadden SL, Zheng XY, Ding DL. Conditioning-induced protection from impulse noise in female and male chinchillas. J Acoust Soc Amer. 2000;107:2162–2168. doi: 10.1121/1.428497. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Henselman LW, Zheng XY. Sex differences in auditory sensitivity of chinchillas before and after exposure to impulse noise. Ear Hear. 1999;20:164–174. doi: 10.1097/00003446-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Nathan CA, Kim TS, Harris JP, Koutnouyan HA, Ryan AF. Absence of mRNA encoding estrogen receptor in the rat cochlea. Acta Otolaryngol. 1999;119:853–857. doi: 10.1080/00016489950180162. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrus cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod. 1981;24:784–794. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasm estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinol. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrus cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Latham KR, Finch CE. Plasma testosterone levels in C57BL/6J male mice: Effects of age and disease. Acta Endodrinol. 1975;80:744–742. doi: 10.1530/acta.0.0800744. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Apical-to-basal gradients in age-related cochlear degeneration and their relationship to “primary” loss of cochlear neurons. J Comp Neurol. 2004;479:103–116. doi: 10.1002/cne.20326. [DOI] [PubMed] [Google Scholar]
- O'Neill WE, Zettel ML, Whittemore KR, Frisina RD. Calbindin D-28k immunoreactivity in the medial nucleus of the trapezoid body declines with age in C57B1/6J, but not CBA/CaJ mice. Hear Res. 1997;112:158–166. doi: 10.1016/s0378-5955(97)00116-0. [DOI] [PubMed] [Google Scholar]
- Papalexi E, Antoniou K, Kitraki E. Estrogens influence behavioral responses in a kainic acid model of neurotoxicity. Horm Behav. 2005;48:291–302. doi: 10.1016/j.yhbeh.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Picazo O, Azcoitia I, Garcia-Segura LM. Neuroprotective and neurotoxic effects of estrogens. Brain Res. 2003;990:20–27. doi: 10.1016/s0006-8993(03)03380-8. [DOI] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Amer. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Wang H, Sahlin L, Hultcrantz M. Mapping of estrogen receptors alpha and beta in the inner ear of mouse and rat. Hear Res. 1999;136:29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo G., Jr Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Turner JG, Willott JF. Exposure to an augmented acoustic environment alters progressive hearing loss in DBA/2J mice. Hear Res. 1998;118:101–113. doi: 10.1016/s0378-5955(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Usui T. Pharmaceutical prospects of phytoestrogens. Endocr J. 2006;53:7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- Vazquez AE, Jimenez AM, Martin GK, Luebke AE, Lonsbury-Martin BL. Evaluating cochlear function and the effects of noise exposure in the B6.CAST+Ahl mouse with distortion product otoacoustic emissions. Hear Res. 2004;194:87–96. doi: 10.1016/j.heares.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Willott JF. Aging and the auditory system. In: Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, editors. Pathobiology of the Aging Mouse, ILSI Monographs on the Pathobiology of Aging Animals. ILSI Press; Washington, DC: 1996. pp. 179–204. [Google Scholar]
- Willott JF, Bross LS. Morphological changes in the anteroventral cochlear nucleus that accompany sensorineural hearing loss in DBA/2J and C57BL/6J mice. Brain Res Dev Brain Res. 1996;91:218–226. doi: 10.1016/0165-3806(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Willott JF, Bross LS. Effects of prolonged exposure to an augmented acoustic environment on the auditory system of middle-aged C57BL/6J mice: cochlear and central histology and sex differences. J Comp Neurol. 2004;472:358–370. doi: 10.1002/cne.20065. [DOI] [PubMed] [Google Scholar]
- Willott JF, Bross LS, McFadden SL. Ameliorative effects of exposing DBA/2J mice to an augmented acoustic environment on histological changes in the cochlea and anteroventral cochlear nucleus. J Assoc Res Otolaryngol. 2005;28:1–10. doi: 10.1007/s10162-005-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Jackson LM, Hunter KP. Morphometric study of the anteroventral cochlear nucleus of two mouse models of presbycusis. J Comp Neurol. 1987;260:472–480. doi: 10.1002/cne.902600312. [DOI] [PubMed] [Google Scholar]
- Willott JF, Sundin V, Jeskey J. Effects of exposure to an augmented acoustic environment on the mouse auditory system. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton: 2001. pp. 205–214. [Google Scholar]
- Willott JF, Turner JG. Prolonged exposure to an augmented acoustic environment ameliorates age-related auditory changes in C57BL/6J and DBA/2J mice. Hear Res. 1999;135:78–88. doi: 10.1016/s0378-5955(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG, Sundin VS. Effects of exposure to an augmented acoustic environment on auditory function in mice: Roles of hearing loss and age during treatment. Hear Res. 2000;142:79–88. doi: 10.1016/s0378-5955(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Willott JF, VandenBosche J, Shimizu T, Ding D. Effects of exposing DBA/2J mice to a high-frequency augmented acoustic environment on the cochlea and anteroventral cochlear nucleus. Hearing Research. 2006 doi: 10.1016/j.heares.2006.01.010. in press. [DOI] [PubMed] [Google Scholar]
- Zettel ML, Frisina RD, Haider S, O'Neill WE. Age-related changes in the immunoreactivity of calbindin D28K and calretinin in the inferior colliculus of the CBA/J and C57/6J mouse. J Comp Neurol. 1997;386:92–110. doi: 10.1002/(sici)1096-9861(19970915)386:1<92::aid-cne9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J Neurosci. 2004;24:5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Yan D, Ouyang XM, Du LL, Yu H, Chang B, Johnson KR, Liu XZ. Digenic inheritance of deafness caused by mutations in genes encoding cadherin 23 and protocadherin 15 in mice and humans. Hum Mol Genet. 2005;14:103–111. doi: 10.1093/hmg/ddi010. [DOI] [PMC free article] [PubMed] [Google Scholar]


