Abstract
A recently described avian neuropeptide, gonadotropin inhibitory hormone (GnIH), has been shown to have seasonal regulatory effects on the hypothalamic-pituitary-gonadotropin axis (HPG) in several avian species. In the bird, GnIH expression is increased during the photorefractory period and has inhibitory effects on the HPG. A recently described mammalian neuropeptide, RF-amide-related peptide-3 (RFRP-3), may be genetically related and functionally similar to this avian neuropeptide. The purposes of this study were to first see if rat RFRP-3 is expressed in the male rat brain and second to determine if ICV injections of RFRP-3 will have effects on feeding and sex behaviors, as well as hormone release from the anterior pituitary. Results confirm other studies in that immunoreactive cell bodies and fibers are observable in areas of the male rat brain known to control the HPG and feeding and sex behaviors. RFRP-3 fibers are also observed in close proximity to GnRH immunoreactive cell bodies. Behavioral tests indicate that high but not low ICV RFRP-3 (500 vs. 100 ng, respectively) significantly (p < 0.05) suppressed all facets of male sex behavior while not having any observable effects on their ability to ambulate. Sex behavior was later exhibited when those same male rats received the ICV vehicle. While suppressing sex behavior, ICV RFRP-3 significantly (p < 0.05) increased food intake compared to controls. ICV RFRP-3 also significantly reduced plasma levels of luteinizing hormone but increased growth hormone regardless of the time of day; however, at no time did RFRP-3 alter plasma levels of FSH, thyroid hormone, or cortisol. These results indicate that although RFRP-3 has similar effects on LH as observed with GnIH in avian species, in the rat RFRP-3 has additional roles in regulating feeding and growth.
Keywords: GnRH, GnIH, food intake, reproductive homeostasis
Introduction
By regulating the secretion of gonadotropin-releasing hormone (GnRH), central neuropeptides control the hypothalamic-pituitary-gonadal axis (HPG). Although many neuropeptides have a central role in stimulating the HPG and sexual behaviors (e.g. galanin like peptide or GALP (Cunningham, 2004)), few—if any—hypothalamic neuropeptides have a primary role in the inhibition of the HPG and sexual behaviors. Recent studies have suggested that stimulatory neuropeptide systems, such as galanin-like peptide (GALP), are critical for maintaining reproductive physiology and behavior, but a loss of GALP expression or activity is insufficient to account for the complete loss of reproductive function in diabetic rats; these observations suggests that other inhibitory factors may be involved (Stoyanovitch, Johnson, Clifton, Steiner, and Fraley, 2005a). A recently discovered RFamide peptide family has proven to contain prime candidates to fill this role as an endogenous inhibitory factor to reproduction (Kriegsfeld, Mei, Bentley, Ubuka, Mason, Inoue, Ukena, Tsutsui, and Silver, 2006; Tsutsui, Saigoh, Ukena, Teranishi, Fujisawa, Kikuchi, Ishii, and Sharp, 2000; Ukena and Tsutsui, 2004; Ukena, Ubuka, and Tsutsui, 2003).
Over the past ten years it has been shown that vertebrate brains produce a number of RFamide peptides. The neuropeptide FMRFamide was first described in the venus clam and RFamide peptides were later found in many vertebrate species (for review of the RFamide peptides see: (Tsutsui and Ukena, 2005; Ukena and Tsutsui, 2005)). Avian GnIH is an endogenous neuropeptide that elicits strong inhibitory actions on gonadotropin secretion-- central administration of GnIH in birds is known to elicit a dramatic decrease in GnRH-mediated LH secretion and inhibit sex behaviors (Ukena et al., 2003). Furthermore, GnIH peptide levels were highest at the end of the breeding season when GnRH levels were lowest, again correlating GnIH expression to GnRH release (Bentley, Perfito, Ukena, Tsutsui, and Wingfield, 2003). Using immunocytochemical analyses, cell bodies and their terminals containing GnIH were found to be isolated in the paraventricular nucleus (PVN) and in the median eminence (ME) of birds, areas of the brain known to regulate the anterior pituitary gland (Bentley et al., 2003). Furthermore, double-label immunocytochemistry (ICC) revealed a high probability that GnIH terminals were in close contact with GnRH neurons and fibers (Bentley et al., 2003). Recently, another members of this family (RFRP-1 & RFRP-3) were isolated and sequenced in the rat and found to be widely expressed in the rat hypothalamus (Hinuma, Shintani, Fukusumi, Iijima, Matsumoto, Hosoya, Fujii, Watanabe, Kikuchi, Terao, Yano, Yamamoto, Kawamata, Habata, Asada, Kitada, Kurokawa, Onda, Nishimura, Tanaka, Ibata, and Fujino, 2000; Ukena, Iwakoshi, Minakata, and Tsutsui, 2002; Yano, Iijima, Kakihara, Hinuma, Tanaka, and Ibata, 2003). Although structurally similar peptides, the gene sequence encoding avian GnIH is missing in the homologous mammalian gene (Hinuma, Habata, Fujii, Kawamata, Hosoya, Fukusumi, Kitada, Masuo, Asano, Matsumoto, Sekiguchi, Kurokawa, Nishimura, Onda, and Fujino, 1998). The biological activities of the transcripts of the mammalian gene, RFRP-1 and RFRP -3, have been not been investigated in birds and information regarding these peptides is sparse in mammals. It is known that RFRP-1 stimulates prolactin secretion in the rat (Hinuma et al., 1998); but, any physiological or behavioral effects of RFRP-3 in the rat are unknown.
To investigate the effects of RFRP-3 in the rat, our laboratory utilized immunocytochemistry to determine if the RFRP-3 peptide could be visualized in the brain. We were not only able to observe GnIH immunoreactive (ir) cell bodies, but also dense RFRP-3-ir fiber networks in regions known to regulate both reproductive hormones and behavior, confirming other reports in the male rat (Hinuma et al., 1998). We therefore set out to test the hypotheses that central administration of rat RFRP-3 (Ukena et al., 2002) would have inhibitory effects on gonadotropin secretion—as seen in other species with GnIH—and on sexual behavior in the adult male rat. Our data indicates that RFRP-3 is present in the rat hypothalamus and it stimulates GH secretion but is an inhibitor of both LH secretion and sex behavior; and, RFRP-3 has no effect on FSH in the male rat.
Methods and Materials
Animals
Adult male Sprague Dawley rats (280-320 g) were obtained from Harlan Laboratories (Indianapolis, IN). The animals were housed in individual cages and fed standard rat food. Both food and water were available at all times. The lights were on a 12:12 light/dark cycle, with lights on at 0200 hours. Before the start of the experiment all rats were given three bouts of sexual experience. The Hope College Animal Care and Use Committee approved all methods used in this experiment, in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Lateral Ventricle Cannulation
The rats were anesthetized using a ketamine cocktail (100 mg/ml ketamine; 20 mg/ml xylazine; 10 mg/ml acepromazine; 5:2.5:1 ratio, respectively). They were then positioned in the stereotaxic instrument. A single incision was made on the midline of the scalp. Once the area had been prepped, a stainless steel 30- gauge (G) cannula (Plastics One, Roanoke, VA, USA) was placed in the lateral ventricle at 1.2 mm lateral to the midline, 1.0 mm posterior to the Bregma and 3.0 mm inferior of the dura mater. The cannula was kept in place on the skull by dental cement and bone screws. The animals were allowed a week to recover before beginning the ICV injections. The injections were given using a 30- gauge stainless steel cannula attached to polyethylene tubing and 10 μl Hamilton syringe (Hamilton Inc., Reno, NV). A different set of rats was used for each experiment; however, no rat was injected with RFRP-3 or vehicle more than once every 10 days.
Immunocytochemistry
Four parallel series of 40 μm coronal sections of brain tissue were cut on a sliding microtome (American Optical Company, Buffalo, NY) from the diagonal band of Broca (DBB) through the mammillary bodies, and stored in cryopreservative (0.9% NaCl, 30% sucrose, 1% polyvinylpyrolidine mw 40,000, 30% ethylene glycol in 0.05M PB) solution at −20°C until processed.
Immunocytochemistry for RFRP-3 was performed on one set of hypothalamic sections by a standard ABC (avidin/biotin complex) reaction, as previously described (Fraley and Kuenzel, 1993; Fraley, Shimada, Baumgartner, Clifton, and Steiner, 2003). Briefly, all rats were perfused transcardially with 4% paraformaldehyde in 0.1MPB, brains removed and static fixed in the same aldehyde fixative for 5 hours. Brains were then transferred to 0.1M PB with 30% sucrose for 48 hrs for cryoprotection and stored at −80°C until stained. One set of sections were washed in PB, incubated in 10 mM sodium citrate (pH = 4.3, 80°C for 30 min) followed by 0.3% H2O2 in PB (30 min @ room temp.) and pre-incubated in blocking solution (PB, 1.0% non-fat dried milk (NFM), 0.05% Triton X-100) for 3 h at room temperature. Sections were then transferred to a blocking solution containing rabbit anti-RFRP-3 polyclonal antibody raised specifically against rat RFRP-3 (1:10,000, synthesized by KT) and incubated for 48 h at 4°C with agitation. After three PB washes, sections were incubated for 3 h at RT in blocking solution with a secondary antibody (1:500, biotinylated-anti-rabbit, Vector Laboratories, Burlingame, CA). RFRP-3 immunoreactivity was visualized with the standard ABC reaction with Ni-3,3′5,5′ diaminobenzidine (DAB) as the chromagen to produce a blue-black reaction product (DAB Chromagen Kit No. PK6100, Vector Laboratories). Sections were mounted on Superfrost Plus slides (VWR Scientific, West Chester, PA), air-dried, dehydrated in graded ethanol, and cleared with Citrosolv, after which cover slips were applied.
For double-labeling experiments, rat brain sections were pre-treated as described above. Primary antibody incubations contained 1%NFM in 0.1M PB with a mouse anti-GnRH (1:3000, Chemicon Inc.) and the anti-RFRP-3 as described above. Sections incubated for 48 hr at 4°C. After through washing, the sections were subsequently incubated with an FITC conjugated secondary anti-mouse (1:500) and a Texas-red conjugated anti-rabbit antibodies in 0.1M PB with 1%NFM. The antibody cocktails showed no difference in staining from the individual antibodies and alternating the fluorochrome labels also did not effect the staining. Sections were washed, mounted with Vectashield media (Vector Labs).
Analysis of Immunocytochemistry
All sections that contained RFRP-3 immunoreactive neurons were analyzed under bright-field illumination (Leica Microsystems DM5100, Wetzlar, Germany). All RFRP-3 neurons were counted bilaterally at 20x magnification. Single-labeled RFRP-3 neurons were counted if blue-black neurons were observed with clear nuclei lacking dark staining. Fiber distribution was mapped qualitatively. Fluorescent ICC was analyzed under fluorescent microscopy. Images were captured using a Leica DFC480 digital camera attached to the microscope utilizing Leica FireCam software (v1.5). Brightfield photomicrographs were rendered to grayscale using Adobe Photoshop. Fluorescent photomicrographs (Fig. 3) were captured as two images using the appropriate filters, input into Photoshop (Adobe, Inc. v8.0) where the two fields were overlayed into a single image.
Fig. 3.

Fluorescent double-labeling of RFRP-3 fiber (indicated by arrows) overlying a GnRH cell body in the preoptic area. Bar = 10 μm.
Radioimmunoassay
Plasma LH, FSH, growth hormone (GH), thyroid hormone (TH), and corticosterone concentrations were measured at Northwestern University (Evanston, IL), with reagents from the NIH. For LH, the antiserum was anti-rLH-S11, the standard was rLH-RP3, the assay sensitivity was 0.1 ng/ml, and the intra-assay coefficient of variation was 2.2%. The GH was performed with a double antibody RIA kit (Linco Research, St. Charles, MO) with an intra-assay variance of 4.34%. The TH (T3) assay was performed with a solid phase RIA kit (MP Biomedicals, Orangeburg, NY) with an intra-assay variance pool of 13.0%. Plasma corticosterone levels were measured with a double antibody RIA kit (MP Biomedicals) with an intra-assay variance of 6.34%.
Experiment #1: Dose response curve for RFRP-3 on plasma LH levels
To determine the best dose for experiments based upon GnIH effects in other species, we utilized a dose response paradigm where rats were injected with 0, 10, 100, 250, and 500 ng RFRP-3 (Genosys, Inc.). Twenty minutes after injection, rats were killed by decapitation and blood collected for LH. This time point was chosen due to effects of the related avian peptide, GnIH (Osugi, Ukena, Bentley, O'Brien, Moore, Wingfield, and Tsutsui, 2004). At the end of the study, RFRP-3 was also observed to alter plasma GH levels. Thus, a post hoc dose-response test was performed for RFRP-3 effects on plasma GH levels on alternate aliquots of the same samples as the LH assay.
Experiment 2: Immunocytochemistry for RFRP-3
Brains were collected from adult, sexually experienced male rats. The brains were immediately frozen and stored at −80°C until sectioned. Coronal sections (40 μm) were processed using ICC techniques to stain for RFRP-3-ir. Areas of the hypothalamus that contained neuronal cell bodies that stained positive for RFRP-3 protein were mapped out, along with regions that had concentrated areas of immunoreactive RFRP-3 fibers.
Fluorescent double label ICC techniques were performed on coronal sections of the male rat brain for GnRH containing cell bodies and RFRP-3-ir fibers. The percentage of GnRH immunoreactive neurons that were in close proximity to RFRP-3 immunoreactive fibers were determined.
Experiment 3: Effects of ICV RFRP-3 and anti-RFRP-3 on Sex Behavior
Cannulated, sexually experienced male rats were injected with 500 ng/3 μL RFRP-3, 100 ng/3 μL RFRP-3 or an aCSF vehicle (3 μl total volume, n = 16 per group). Within 5 min following the injection, the rats were placed into a testing arena (polyethylene housing cage) with a sexually-experienced steroid-primed female rat (10 ug estradiol benzoate in 0.1 ml safflower oil injected subcutaneously (sc) 48 h prior to testing and 500 μg progesterone in 0.1 ml safflower oil injected sc 2 h prior to testing). The males were scored for the number of mounts, intromissions, and ejaculations and the latency to these behaviors in a 30 min test. A mount was recorded if the male approached the female from the rear and placed both forelegs firmly on her flanks and locked his hips (pelvic thrust alone). An intromission was recorded if the foregoing behavior was noted with the addition of a clear pelvic thrust and penile insertion into the vagina, followed by genital grooming. The behaviors were live scored and also taped for detailed analysis by at least two individuals. The sex tests were performed in a cross over design so that all animals received both the control injection and the RFRP-3 injection at both time periods.
The immunosuppression studies on reproductive physiology and behavior were carried out as described by our lab in previous studies (Stoyanovitch, Johnson, Clifton, Steiner, and Fraley, 2005b). Briefly, sexually experienced male rats (n = 12) were given 3 μl ICV injections of either affinity-purified anti-RFRP-3 (n = 12) or artificial cerebral spinal fluid (n = 12). Five minutes after the injection, the same compound was injected again into the same rat. As above, the injection paradigm was carried out in a cross-over design to eliminate order-dependant effects. Injections were given at least 72 hours apart. All sex behavior tests were done at 0900 hr (photophase) and 1500 hr (scotophase).
Within five minutes of the second injection, the males were placed with steroid-primed receptive females (10μg estradiol benzoate in 0.1 ml of safflower oil 48 hours prior and 500 μg progesterone in 0.1 ml of safflower oil two hours prior to test). The number of mounts, intromissions, ejaculations and the latencies to each of these behaviors was recorded during a 30-minute period as described above.
Experiment 4: Effects of ICV RFRP-3 on Food Intake
A preliminary weight was established for all rats and recorded. Half of the rats (n= 8) received ICV injections of RFRP-3 and half were injected with aCSF during the photophase at 0900. After injection, the rats were placed back in the cages with a known weight of rodent chow. Food weights were measured and recorded after one and two hours had elapsed. Body weights were measured again 24 hours later.
Experiment 5: Effects of ICV RFRP-3 and anti-RFRP-3 on Plasma Hormone Levels
At least 10 days following the last sex behavior test, rats were injected with either RFRP-3 or vehicle as before, decapitated, and the trunk blood collected at 0900 hours (n = 4 per group). The remaining eight were similarly sacrificed at 1500 hours (n = 4 per group). The blood plasma was stored for GH, TH, LH, FSH and corticosterone assays.
Statistical Design
All data are expressed as a mean ± SEM for each treatment. Sex behaviors were analyzed between groups using repeated measures ANOVA. Hormone and food intake data were analyzed using t- tests. A significance level of p < 0.05 was considered significant.
Results
Experiment #1: Dose response curve for RFRP-3 on plasma LH levels
Only the 500 ng dose of RFRP-3 gave the maximal reduction in plasma LH levels (Figure 1A). However, the 100 ng dose gave the maximal response to increase GH secretion (Figure 1B). The dose response curve for GH levels demonstrated that GH secretion is more sensitive to RFRP-3 in that a lower dose of RFRP-3 (100 ng) elicited a robust and profound increase in plasma GH levels. At higher doses there was either no effect or a reduction in plasma GH levels. The differences in the observed hormone responses could be accounted for by a difference in the time-course of responsiveness, although this was not determined by these experiments.
Fig. 1.

A) Dose response curve for RFRP-3 effects on plasma LH levels. B) Dose response effects of RFRP-3 on plasma GH levels. * = p < 0.05 compared to controls, ** = p < 0.01, *** = p < 0.001 compared to controls.
Experiment 2: Immunocytochemistry for RFRP-3
Positive staining for RFRP-3-ir cell bodies and fibers was observed throughout the diencephalon and the medial amygdala. Figure 2 illustrates representative RFRP-3-ir. Large numbers of cell bodies containing RFRP-3-ir were found in both the dorsal and ventral components of the dorsomedial hypothalamic nucleus (DMH, essentially surrounding the VMN) and the dorsal tuberomammillary nucleus (TMN). This distribution of RFRP-3 is similar to that reported previously (Yano, Iijima, Hinuma, Tanaka, and Ibata, 2004; Yano et al., 2003). Yano et al. (Yano et al., 2004; Yano et al., 2003) suggested that both RFRP-1 (quail RFRP-3) and RFRP-3 (rat RFRP-3) were found in the rat hypothalamus, however no specificity data was presented for either of the two antisera used in their experiments–given the high degree of similarity in tertiary structure between these two small peptides (12 AA vs 16 AA, respectively) it is possible a great deal of cross-reactivity occurred in that study. We are aware that our antisera for rat RFRP-3 does indeed cross react with quail RFRP-3 (RFRP-1, (Ukena et al., 2002)). We also show a greater distribution of RFRP-3-ir cell bodies than described in other species, possibly due to our antigen retrieval protocol (an identical distribution of cell bodies was observed without the addition of this protocol). Pre-absorption studies with an excess of rat RFRP-3 did prevent all cell body staining both with and without the antigen retrieval protocol—althoguh very occasional fibers were still observed. However, preabsorption of antibody with other RF-amide proteins (eg. kisspeptin) had no effect on staining.
Fig. 2.
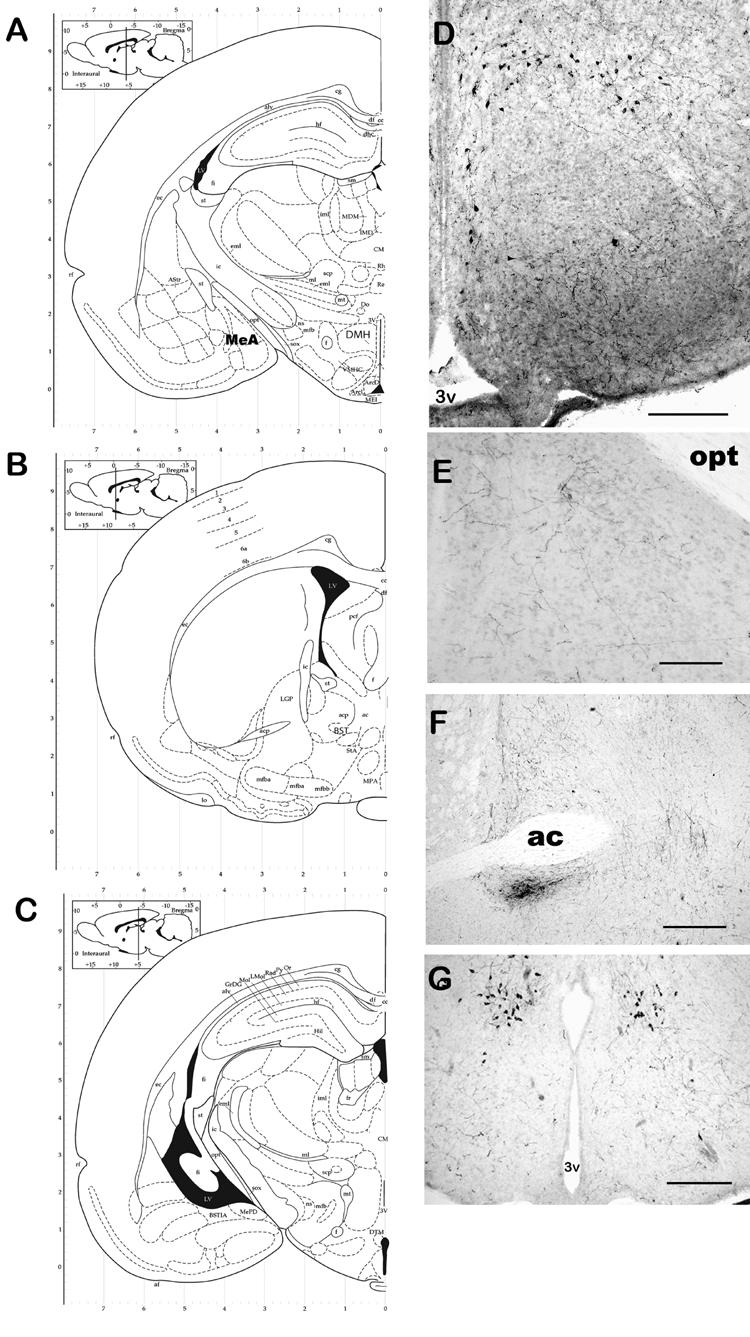

Representative photomicrographs of RFRP-3 ICC. Panels A, B & C are atlas plates illustrating anatomical localization of photomicrographs. Cell bodies containing GnIH-ir were observed in the DMH (A & D) and DTM (C & G). RFRP-3-ir fibers were observed in several areas including areas known to be involved in male sexual behavior including the BST (B & E) and MeAD (A & F) and PVT (H). RFRP-3 immunoreactivity was blocked by preabsorption of sections with full-length RFRP-3 (I) but not with another RFamide, kisspeptin (J). Bars = 40 μm (D, G, & H) and 10 μm (E, F, I, & J). opt = optic tract, 3v = third ventricle, ac = anterior commisure.
RFRP-3-ir fibers could be observed widely distributed throughout the diencephalon and the amygdala. Dense networks of RFRP-3-ir fibers were particularly noted in the bed nucleus of the stria terminalis (BnST), throughout the medial preoptic area (MPOA), the medial and lateral septal areas (MS and LS, respectively), and the paraventricular thalamic nucleus (PVT). In addition, smaller numbers of fibers were observed in the vertical and horizontal limbs of the diagonal band of Broca (DBB), and the dorsal medial hypothalamic nucleus (DMH), and in the medial amygdala (MeA, Figure 1). Interestingly, the ventromedial hypothalamic nucleus (VMN) and external zone of the median median eminence were particularly notable regarding their lack of RFRP-3-ir fibers (Fig. 1). Fluorescent double-label staining for GnRH cell bodies and RFRP-3-ir fibers showed RFRP-3-ir fibers lying in close proximity to approximately 75 ± 3.2% of GnRH cell bodies within the preoptic area (Fig. 3). It is our hypothesis that RFRP-3 can inhibit GnRH secretion by direct actions on GnRH neurons—although RFRP-3 action on the anterior pituitary is also a possibility.
Experiment 3: Effects of RFRP-3 and anti-RFRP-3 on sex behavior
To determine the effects of RFRP-3 injections on male typical sex behavior, male rats were given ICV injections of RFRP-3 (n=8) or the vehicle (n=8) in both photophase and scotophase. Sex behavior tests performed during the photophase (0900 hrs) showed a significant reduction in the number of mounts, intromissions and ejaculations in animals that received the higher, but not lower dose of RFRP-3 as compared to controls (p < 0.01, Table 1). The numbers of anogenital investigations were similar in both treatment groups, illustrating that RFRP-3 elicited no ill effects on the animals' ability to ambulate (data not shown). No significant difference was observed in the latency to each behavior, also suggesting no general motor deficits, but rather a specific reduction in sexual behavior.
Table 1.
Effects of ICV GnIH on Photophase Male Sex Behavior (0900hrs)
| Treatment | Mounts | Mount Latency (secs.) |
Intromissions | Introm. Latency (secs) |
Ejaculations | Ejaculation Latency (secs) |
|---|---|---|---|---|---|---|
| Control | 10.9 ± 1.36 | 831.2 ± 196.1 | 9.9 ± 3.54 | 1093±160.8 | 0.6 ± 0.25 | 1693±58.32 |
| RFRP-3 (500 ng) |
2.1 ± 0.83a | 1260 ± 183.9 | 1.7 ± 0.81 a | 1484±149.5 | 0.1 ± 0.08 a | 1726±73.79 |
| RFRP-3 (100 ng) |
9.5 ± 1.03 | 910 ± 83.1 | 8.5 ± 0.1.33 | 1142 ± 33.1 | 0.5 ± 0.42 | 1733± 121.3 |
| Anti-RFRP-3 | 17.5 ± 1.13 a | 411.1 ± 57.8 a | 15.1 ± 4.32 a | 787±121.8 a | 0.8 ± 0.02 | 1577±122.11 |
Letter indicates statistical difference from Control group (p < 0.01).
Data presented as means ± SEM.
Immunosuppression of RFRP-3 during the photophase elicited a significant increase in mounts and intromissions compared to vehicle controls (p < 0.05). Similarly, RFRP-3 immunosuppression caused a slight decrease in the latency to these behaviors compared to controls (p < 0.05, Table 1). There was no effect of RFRP-3 immunosuppression on the number or latency to ejaculations.
Sex tests performed during scotophase (1800 hrs) showed a non-significant decrease in sex behaviors and latencies between those animals that received ICV injections of RFRP-3 and those who received the vehicle control (Table 2). It is our opinion that a higher dose of RFRP-3, a direct injection of RFRP-3 into sex-regulating nuclei (eg BNST, mAg, mPOA), or testing at a different time during the scotophase may have elicited significant reduction in sex behavior. Similarly to RFRP-3, ICV anti-RFRP-3 had no effects on sex behavior during the scotophase.
Table 2.
Effects of ICV GnIH on Scotophase Male Sex Behavior (1500hrs)
| Treatment | Mounts | Mount Latency (secs.) |
Intromissions | Introm. Latency (secs) |
Ejaculations | Ejaculation Latency (secs) |
|---|---|---|---|---|---|---|
| Control | 10.5 ± 2.10 | 554.8 ± 199.6 | 18.0 ± 4.30 | 552.3 ± 170 | 1.5 ± 0.31 | 875.6 ± 167.5 |
| RFRP-3 (500 ng) |
10.6 ± 2.26 | 649.2 ± 187.4 | 18.5 ± 3.72 | 288 ± 61.31 | 1.6 ± 0.28 | 973.8 ± 186.0 |
| RFRP-3 (100 ng) |
9.8 ± 3.1 | 721 ± 98.3 | 17.1 ± 2.88 | 571 ± 83.11 | 1.3 ± 0.7 | 1011.1 ± 211.3 |
No significant differences were observed between treatment groups (p > 0.05).
Data presented as means ± SEM.
Experiment 4: Effects of RFRP-3 on food intake
Rats who received either dose of ICV RFRP-3 during photophase showed a significant increase in food intake compared to controls (p < 0.01, Fig. 4). There were no differences in food intake after the first hour, nor were differences in body weight observed. Preliminary feeding studies during the scotophase resulted in equivocal data and were thus not included in this report. This is most likely do to the fact that rats preferentially eat more than 80% of their daily intake during the scotophase; thus, any potential stimulatory effects of RFRP-3 may be overshadowed by the rats natural predilection for nocturnal food intake.
Fig. 4.

ICV RFRP-3 significantly increased food intake during the light cycle compared to controls. ** = p < 0.01.
Experiment 5: Effects of ICV RFRP-3 on Plasma Hormone Levels
Analysis of blood plasma for LH revealed significant differences, but not FSH (Fig. 5). Rats injected with RFRP-3 showed a significant decrease in plasma LH levels (p < 0.01) whether injected during the light (0900 hrs) or dark cycle (1500 hrs). Table 3 illustrates that although RFRP-3 and anti-RFRP-3 had no effects on plasma TH and corticosterone there were significant differences observed in plasma GH levels following RFRP-3 (not anti-RFRP-3). Similar to Experiment #1, ICV RFRP-3 (100 ng but not 500 ng dose) significantly increased plasma GH levels compared to controls (p < 0.01) but anti-RFRP-3 had no effect on plasma GH levels (p > 0.05) compared to controls during both the photophase and scotophase.
Fig. 5.
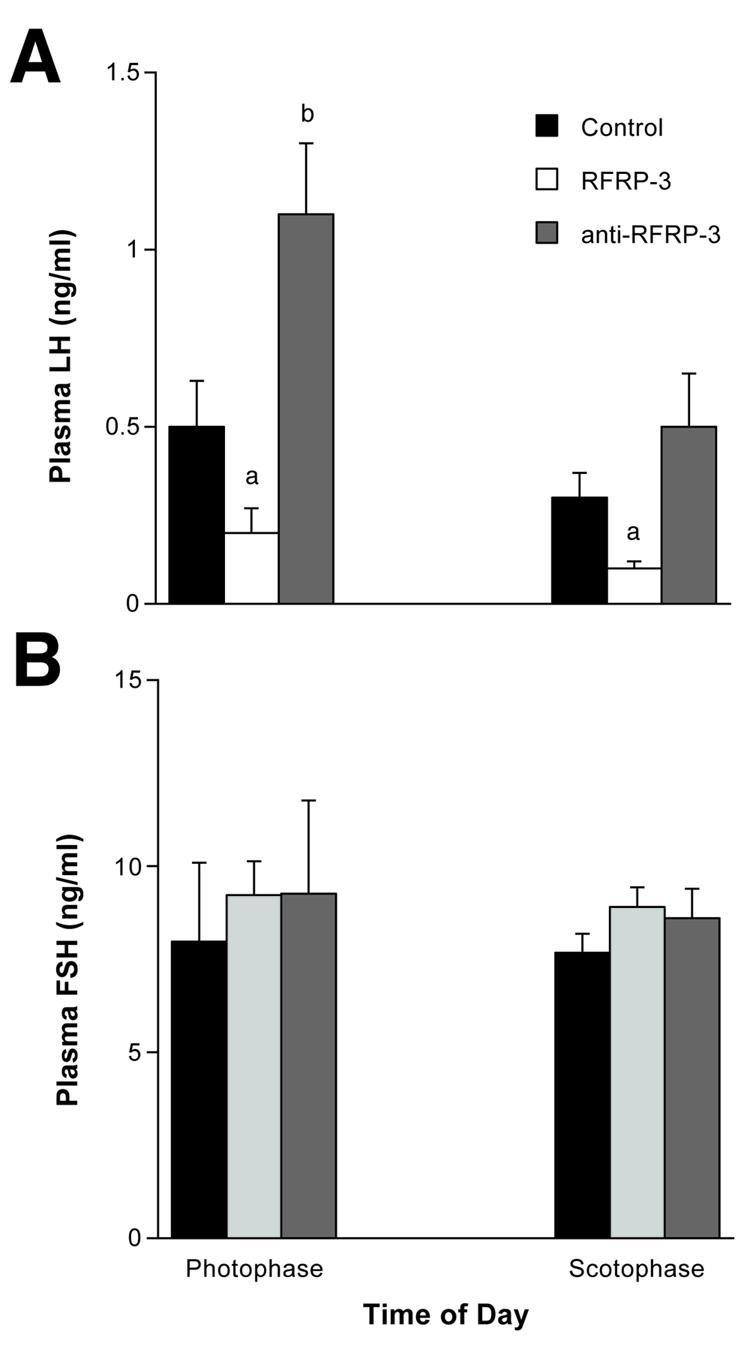
ICV RFRP-3 significantly reduced plasma LH but not FSH levels compared to controls regardless of the time of day. Letters = p < 0.01 compared to Controls.
Table 3A.
Effects of ICV GnIH or anti-GnIH on Photophase Hormone Levels
Effects of ICV GnIH or anti-GnIH on Scotophase Hormone Levels
| Treatment | Growth Hormone (ng/ml) |
Thyroid Hormone (ng/ml) |
Corticosterone (ng/ml) |
|---|---|---|---|
| Control | 47.8 ± 9.91 | 38.1 ± 2.87 | 4.78 ± 2.28 |
| RFRP-3 (500 ng) | 27.4 ± 22.42 | 36.7 ± 3.69 | 4.33 ± 1.12 |
| RFRP-3 (100 ng) | 87.1 ± 8.71a | 35.2 ± 3.11 | 4.99 ± 1.81 |
| Anti-RFRP-3 | 67.3 ± 10.07 | 35.1 ± 2.02 | 4.41 ± 2.32 |
| Control | 20.7 ± 5.03 | 33.5 ± 2.39 | 3.11 ± 1.22 |
| RFRP-3 (500 ng) | 17.7 ± 3.41 | 31.1 ± 2.16 | 3.83 ± 1.07 |
| RFRP-3 (100 ng) | 48.1 ± 3.21a | 33.2 ± 1.03 | 3.43 ± 0.97 |
| Anti-RFRP-3 | 29.1 ± 4.27 | 30.7 ± 3.82 | 4.01 ± 2.12 |
Letter indicates statistical difference from Control group (p < 0.01).
Data presented as means ± SEM.
Discussion
The purposes of these studies were to first identify the location of RFRP-3 in the male rat brain and second, to test the hypothesis that ICV injections of RFRP-3 will suppress male typical sex behavior and plasma gonadotropin levels. Analysis of RFRP-3-ir showed extensive cell body and fiber staining throughout areas of the diencephalon and telencephalon—including areas known to influence reproductive physiology and behavior. Similar to reports of GnIH in avian species, in the rat ICV injections of RFRP-3 caused a significant reduction in plasma LH levels as compared to controls, however RFRP-3 had no effects on plasma FSH. Central injections of RFRP-3 nearly abolished male sex behavior during the animals' photophase, however central RFRP-3 administration had minimal effects during scotophase. Immunosuppression of RFRP-3 had the opposite effects during the photophase suggesting the specificity of the observed RFRP-3-suppressed behaviors. Interestingly, while RFRP-3 inhibited sex behavior during the photophase it concurrently increased food intake compared to controls. Surprisingly, RFRP-3 also had significant effects on GH secretion—lower doses of RFRP-3 profoundly increased plasma GH levels compared to controls. These data suggest that RFRP-3 may be an important central player in maintaining homeostasis within the reproductive system. Recent reports have suggested that rat RFRP-3 is functionally similar to the described GnIH in birds (Bentley, Jensen, Kaur, Wacker, Tsutsui, and Wingfield, 2006; Kriegsfeld et al., 2006). Although structurally related to GnIH, the mammalian homolog, RFRP-3, appears to have multiple physiologic and behavioral functions in the adult male rat.
Similarly to other reports (Kriegsfeld et al., 2006; Ukena and Tsutsui, 2004), RFRP-3 cell bodies and fibers were observed throughout the diencephalon and the amygdala in the male rat. Dense networks of fibers were found in the medial amygdala (mAg), the medial preoptic area (mPOA), the bed nucleus of the stria terminalis (BnST), and the periventricular thalamic nucleus of the thalamus (PVT)—areas of the brain that are known to regulate the HPG, male sex behavior and food intake (For review see (Cunningham, Clifton, and Steiner, 1999; Fabbri, Jannini, Gnessi, Ulisse, Moretti, and Isidori, 1989; I'Anson, Foster, Foxcroft, and Booth, 1991)). A dense network of fibers was observed in multiple regions of the rat brain known to be involved in regulating penile reflexes and sexual satiety (eg. BnST & mAg, respectively) (Parfitt and Newman, 1998; Valcourt and Sachs, 1979), male typical sex behaviors (eg. the mPOA), and the ejaculatory reflex (eg. the PVN and PVT, (Coolen, Allard, Truitt, and McKenna, 2004; Liu, Salamone, and Sachs, 1997; Veening and Coolen, 1998). The RFRP-3 fibers were also found be in close proximity to GnRH neurons and were in many cases observed to wrap around the presumed axonal hillocks. The absence of RFRP-3 fibers in the external zone of the median eminence suggests that RFRP-3 is not being released into the portal veins and thus acting directly on the anterior pituitary. Although we cannot rule out the presence RFRP-3 in the portal blood from other sources than the median eminence, our observations suggest that RFRP-3 is a direct inhibitor of GnRH neurons. Our study observed a greater distribution of cell bodies than observed by other studies in the rat (Yano et al., 2003). We confirmed the specificity of our antibody with preabsorption studies both with and without the antigen retrieval protocols; however, we cannot discount the possibility of cross reactivity with another closely related RFRP peptides. Ukena et al. (Ukena et al., 2002) have deduced that RFRP-3 is the mature, functional neuropeptide in the rat brain; thus, we are confident that our behavioral and physiological results implicate a role for endogenous actions of rat RFRP-3. The RFRP-3 distribution we observed matches our prediction for the possible behavioral and physiological effects of this neuropeptide.
In all species (eg. white crowned sparrow, quail) that GnIH has been studied, central injections of GnIH have diminished HPG axis function, as seen by the reduction in plasma LH levels and sex behaviors compared to controls (Bentley et al., 2006; Bentley et al., 2003; Osugi et al., 2004; Tsutsui et al., 2000; Ubuka, Bentley, Ukena, Wingfield, and Tsutsui, 2005; Ubuka, Ueno, Ukena, and Tsutsui, 2003; Yin, Ukena, Ubuka, and Tsutsui, 2005). In the rat, we have also shown that central administration of RFRP-3 suppresses male-typical sex behavior, however, these effects appear to only occur at higher doses of RFRP-3—doses that also inhibit HPG function. This inhibitory effect was seen only during photophase but not the scotophase; however, RFRP-3 decreased LH levels regardless of the time of day, similar to reports in other species (Bentley et al., 2003; Osugi et al., 2004; Tsutsui et al., 2000; Ubuka et al., 2005; Ubuka et al., 2003; Yin et al., 2005). This study is the first to also demonstrate that ICV RFRP-3 also alters plasma GH levels. This may indicate that RFRP-3 is an endogenous neuropeptide that inhibits energetically demanding physiologic processes—like reproduction—to increase growth and maintain energy homeostasis. The effects of RFRP-3 are similar—though opposite in direction—to those described for galanin-like peptide (GALP). ICV GALP has been shown to stimulate LH secretion (Matsumoto, Noguchi, Takatsu, Horikoshi, Kumano, Ohtaki, Kitada, Itoh, Onda, Nishimura, and Fujino, 2001), sex behavior (Stoyanovitch et al., 2005b), and GH secretion (Takenoya, Hirayama, Kageyama, Funahashi, Kita, Matsumoto, Ohtaki, Katoh, Takeuchi, and Shioda, 2005) while simultaneously inhibiting food intake over a 24 hr period (for review of GALP, see (Gottsch, Clifton, and Steiner, 2004)). The disparate effects of RFRP-3 on sex behaviors between the photophase and scotophase suggest a differential regulation of RFRP-3 or its receptors by light cycles. Ubuka et al. (Ubuka et al., 2005) showed that RFRP-3 gene expression is indeed regulated by melatonin, and thus light cycles. Furthermore, at least two different receptors have been isolated and identified for the RFRP family of neuropeptides (Yin et al., 2005). Although the distribution and regulation of these receptors is not yet known in the rat, it is reasonable to assume that one or more of these receptor subtypes may be regulated by melatonin or light cycles similar to their endogenous ligand, and thus have differential regulatory properties on physiology and behavior. Our observations from ICV RFRP-3 and anti-RFRP-3 suggest that the RF-amide system is primarily effective on behavior during the photophase. The lack of effects of the lower doses of RFRP-3 on sex behaviors could be due to the lack of penetration of the peptide following ICV injections—there is certainly ample immunocytochemical evidence for direct actions of RFRP-3 on regulating sex behavior in the male rat. Alternatively, RFRP-3 fibers were also observed in areas known to contain GH- releasing hormone (GHRH) neurons. An increase in GH secretion elicits an increase in neuropeptide Y (NPY) release within the brain (Chan, Clifton, and Steiner, 1996; Chan, Steiner, and Clifton, 1996; Willesen, Kristensen, and Romer, 1999). Increased NPY activity within the hypothalamus could account for the inhibition of sexual behaviors, HPG activity and the increase in food intake (for reviews see (Bellinger and Bernardis, 2002; Kalra and Kalra, 1996)). These observations suggest that in the rat RFRP-3 has multiple physiologic functions, chief among them may be the regulation of GH secretion and somatic growth.
Our studies demonstrated that ICV RFRP-3 not only inhibits reproductive measures, but it also stimulates food intake. Many neuropeptides that regulate the reproductive system will also have concurrent—but opposite-- effects on feeding behaviors. For example, neuropeptide Y (NPY) is a potent stimulator of food intake but inhibits reproduction in male rats (Pierroz, Catzeflis, Aebi, Rivier, and Aubert, 1996; Sainsbury, Schwarzer, Couzens, Jenkins, Oakes, Ormandy, and Herzog, 2002). POMC also decreases food intake and stimulates endocrine reproductive physiology (Finn, Pau, Spies, Cunningham, Clifton, and Steiner, 2000; Kalra, Dube, Pu, Xu, Horvath, and Kalra, 1999; Thornton, Cheung, Clifton, and Steiner, 1997). Conversely, central injections of galanin-like peptide (GALP) decrease food intake and body weight (Krasnow, Fraley, Schuh, Baumgartner, Clifton, and Steiner, 2003; Seth, Stanley, Dhillo, Murphy, Ghatei, and Bloom, 2003), but stimulate sex behavior and LH secretion in male rats (Fraley, Thomas-Smith, Acohido, Steiner, and Clifton, 2004; Krasnow et al., 2003). The results of this current study show that rat RFRP-3 fits into this hypothalamic motif; RFRP-3 suppresses sex behavior while increasing food intake—similarly to that observed in the chick (Tachibana, Sato, Takahashi, Ukena, Tsutsui, and Furuse, 2005). These observations bolster our opinion that rat RFRP-3 is another important hypothalamic neuropeptide involved in maintaining homeostasis between reproduction and metabolism.
Similar to other studies of GnIH, ICV injections of RFRP-3 decreased plasma LH levels and male-typical sex behaviors; however, in the male rat RFRP-3 had no effects on plasma FSH, thyroid hormone or cortisol. In addition RFRP-3 also increases food intake, however the observed sexual and behavioral effects were dependent on the time of day and dose. RFRP-3 is another endogenous neuropeptide whose function may be to inhibit the HPG and male sex behavior in the rat. However, the observations that RFRP-3 also affects food intake and growth hormone secretion suggests that rat RFRP-3 may have an important role in the cessation of reproduction that occurs with metabolic disorders such as obesity, cachexia or metabolic wasting disease. During negative metabolic states, there may not only be a loss of stimuli (eg. GALP, (Fraley, Scarlett, Shimada, Teklemichael, Acohido, Clifton, and Steiner, 2004; Stoyanovitch et al., 2005a)) for reproduction in order to increase food intake, but also an activation of other hypothalamic factors that act to reduce reproductive physiology and behavior—again to favor refeeding to increase metabolic reserves for additional somatic growth and/or repair. RFRP-3 (designated as GnIH by others (Bentley et al., 2006)) may fulfill this latter role in the mammal.
Acknowledgements
We would like to thank Ms. Brigitte Mann of Northwestern University for the plasma hormone analyses. We would like to thank Dr. George Bentley and Dr. Susan Fraley for their critical comments on this research and manuscript. We would also like to thank Ken Kuper and Forrest Powers for their assistance with behavioral experiments in the Fraley lab. We extend great appreciation to the NIH (K01 DK066238-01A1 to GSF) for supporting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation. Lessons learned from lesioning studies. Physiol Behav. 2002;76(3):431–42. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006 doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol. 2003;15(8):794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- Chan YY, Clifton DK, Steiner RA. Role of NPY neurones in GH-dependent feedback signalling to the brain. Horm Res. 1996;45(Suppl 1):12–4. doi: 10.1159/000184820. [DOI] [PubMed] [Google Scholar]
- Chan YY, Steiner RA, Clifton DK. Regulation of hypothalamic neuropeptide-Y neurons by growth hormone in the rat. Endocrinology. 1996;137(4):1319–25. doi: 10.1210/endo.137.4.8625906. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Allard J, Truitt WA, McKenna KE. Central regulation of ejaculation. Physiol Behav. 2004;83(2):203–15. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ. Galanin-like peptide as a link between metabolism and reproduction. J Neuroendocrinol. 2004;16(8):717–23. doi: 10.1111/j.1365-2826.2004.01221.x. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ, Clifton DK, Steiner RA. Leptin's actions on the reproductive axis: perspectives and mechanisms. Biol Reprod. 1999;60(2):216–22. doi: 10.1095/biolreprod60.2.216. [DOI] [PubMed] [Google Scholar]
- Fabbri A, Jannini EA, Gnessi L, Ulisse S, Moretti C, Isidori A. Neuroendocrine control of male reproductive function. The opioid system as a model of control at multiple sites. J Steroid Biochem. 1989;32(1B):145–50. doi: 10.1016/0022-4731(89)90155-6. [DOI] [PubMed] [Google Scholar]
- Finn PD, Pau KY, Spies HG, Cunningham MJ, Clifton DK, Steiner RA. Galanin's functional significance in the regulation of the neuroendocrine reproductive axis of the monkey. Neuroendocrinology. 2000;71(1):16–26. doi: 10.1159/000054516. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Kuenzel WJ. Immunocytochemical and histochemical analyses of gonadotrophin releasing hormone, tyrosine hydroxylase, and cytochrome oxidase reactivity within the hypothalamus of chicks showing early sexual maturation. Histochemistry. 1993;99(3):221–9. doi: 10.1007/BF00269140. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Scarlett JM, Shimada I, Teklemichael DN, Acohido BV, Clifton DK, Steiner RA. Effects of diabetes and insulin on the expression of galanin-like peptide in the hypothalamus of the rat. Diabetes. 2004;53(5):1237–42. doi: 10.2337/diabetes.53.5.1237. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Shimada I, Baumgartner JW, Clifton DK, Steiner RA. Differential patterns of Fos induction in the hypothalamus of the rat following central injections of galanin-like peptide and galanin. Endocrinology. 2003;144(4):1143–6. doi: 10.1210/en.2002-0114. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Thomas-Smith SE, Acohido BV, Steiner RA, Clifton DK. Stimulation of sexual behavior in the male rat by galanin-like peptide. Horm Behav. 2004;46(5):551–7. doi: 10.1016/j.yhbeh.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA. Galanin-like peptide as a link in the integration of metabolism and reproduction. Trends Endocrinol Metab. 2004;15(5):215–21. doi: 10.1016/j.tem.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, Kitada C, Masuo Y, Asano T, Matsumoto H, Sekiguchi M, Kurokawa T, Nishimura O, Onda H, Fujino M. A prolactin-releasing peptide in the brain. Nature. 1998;393(6682):272–6. doi: 10.1038/30515. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2(10):703–8. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- I'Anson H, Foster DL, Foxcroft GR, Booth PJ. Nutrition and reproduction. Oxf Rev Reprod Biol. 1991;13:239–311. [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20(1):68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Nutritional infertility: the role of the interconnected hypothalamic neuropeptide Y-galanin-opioid network. Front Neuroendocrinol. 1996;17(4):371–401. doi: 10.1006/frne.1996.0010. [DOI] [PubMed] [Google Scholar]
- Krasnow SM, Fraley GS, Schuh SM, Baumgartner JW, Clifton DK, Steiner RA. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology. 2003;144(3):813–22. doi: 10.1210/en.2002-220982. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103(7):2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behav Neurosci. 1997;111(6):1361–7. doi: 10.1037//0735-7044.111.6.1361. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Noguchi J, Takatsu Y, Horikoshi Y, Kumano S, Ohtaki T, Kitada C, Itoh T, Onda H, Nishimura O, Fujino M. Stimulation effect of galanin-like peptide (GALP) on luteinizing hormone-releasing hormone-mediated luteinizing hormone (LH) secretion in male rats. Endocrinology. 2001;142(8):3693–6. doi: 10.1210/endo.142.8.8432. [DOI] [PubMed] [Google Scholar]
- Osugi T, Ukena K, Bentley GE, O'Brien S, Moore IT, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol. 2004;182(1):33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Newman SW. Fos-immunoreactivity within the extended amygdala is correlated with the onset of sexual satiety. Horm Behav. 1998;34(1):17–29. doi: 10.1006/hbeh.1998.1459. [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Catzeflis C, Aebi AC, Rivier JE, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle inhibits both the pituitary-testicular axis and growth hormone and insulin-like growth factor I secretion in intact adult male rats. Endocrinology. 1996;137(1):3–12. doi: 10.1210/endo.137.1.8536627. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16(9):1077–88. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Stanley S, Dhillo W, Murphy K, Ghatei M, Bloom S. Effects of galanin-like peptide on food intake and the hypothalamo-pituitary-thyroid axis. Neuroendocrinology. 2003;77(2):125–31. doi: 10.1159/000068648. [DOI] [PubMed] [Google Scholar]
- Stoyanovitch AG, Johnson MA, Clifton DK, Steiner RA, Fraley GS. Galanin-like peptide rescues reproductive function in diabetic rats. Diabetes. 2005a;54:2471–2476. doi: 10.2337/diabetes.54.8.2471. [DOI] [PubMed] [Google Scholar]
- Stoyanovitch AG, Johnson MA, Clifton DK, Steiner RA, Fraley GS. Galanin-like peptide rescues reproductive function in the diabetic rat. Diabetes. 2005b;54(8):2471–6. doi: 10.2337/diabetes.54.8.2471. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Sato M, Takahashi H, Ukena K, Tsutsui K, Furuse M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005;1050(12):94–100. doi: 10.1016/j.brainres.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Takenoya F, Hirayama M, Kageyama H, Funahashi H, Kita T, Matsumoto H, Ohtaki T, Katoh S, Takeuchi M, Shioda S. Neuronal interactions between galanin-like-peptide- and orexin- or melanin-concentrating hormone-containing neurons. Regul Pept. 2005;126(12):79–83. doi: 10.1016/j.regpep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138(11):5063–6. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–7. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ukena K. Review: Hypothalamic LPXRF-amide peptides in vertebrates: Identification, localization and hypophysiotropic activity. Peptides. 2005 doi: 10.1016/j.peptides.2005.06.036. in press. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci U S A. 2005;102(8):3052–7. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T, Ueno M, Ukena K, Tsutsui K. Developmental changes in gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica) hypothalamo-hypophysial system. J Endocrinol. 2003;178(2):311–8. doi: 10.1677/joe.0.1780311. [DOI] [PubMed] [Google Scholar]
- Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002;512(13):255–8. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- Ukena K, Tsutsui K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: Structure, localization, and function. Mass Spectrom Rev. 2004 doi: 10.1002/mas.20031. [DOI] [PubMed] [Google Scholar]
- Ukena K, Tsutsui K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: structure, localization, and function. Mass Spectrom Rev. 2005;24(4):469–86. doi: 10.1002/mas.20031. [DOI] [PubMed] [Google Scholar]
- Ukena K, Ubuka T, Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res. 2003;312(1):73–9. doi: 10.1007/s00441-003-0700-x. [DOI] [PubMed] [Google Scholar]
- Valcourt RJ, Sachs BD. Penile reflexes and copulatory behavior in male rats following lesions in the bed nucleus of the stria terminalis. Brain Res Bull. 1979;4(1):131–3. doi: 10.1016/0361-9230(79)90068-6. [DOI] [PubMed] [Google Scholar]
- Veening JG, Coolen LM. Neural activation following sexual behavior in the male and female rat brain. Behav Brain Res. 1998;92(2):181–93. doi: 10.1016/s0166-4328(97)00190-3. [DOI] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70(5):306–16. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Yano T, Iijima N, Hinuma S, Tanaka M, Ibata Y. Developmental expression of RFamide-related peptides in the rat central nervous system. Brain Res Dev Brain Res. 2004;152(2):109–120. doi: 10.1016/j.devbrainres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, Ibata Y. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res. 2003;982(2):156–67. doi: 10.1016/s0006-8993(03)02877-4. [DOI] [PubMed] [Google Scholar]
- Yin H, Ukena K, Ubuka T, Tsutsui K. A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): identification, expression and binding activity. J Endocrinol. 2005;184(1):257–66. doi: 10.1677/joe.1.05926. [DOI] [PubMed] [Google Scholar]


