Abstract
We present a case of malignant fibrous histiocytoma accompanied by prolonged spiking fevers, which disappeared after tumor resection. Sarcoma with fever as a primary symptom is rare. Furthermore, in this case, fever was closely related to the clinical course of the tumor. In order to detect possible production of febriferous substance(s), we used blood and tumor tissue samples to investigate nine candidate cytokines possibly responsible for the fever. Expression of IL-8 mRNA was detected in preoperative peripheral blood mononuclear cells by RT-PCR. Expressions of IL-6, IL-8, IFN-γ and TNF-α mRNAs were also detected in tumor tissue, while IL-1α, IL-1β, IL-2, IL-4 and COX-2 mRNAs were not. We suspected IL-8 to be a causative factor, and examined its localization by immunohistochemical staining, paraffin sections of tumor tissue stained positive for IL-8. Since infiltrating mononuclear cells were positive for IL-8, this may explain the tumor-associated fever. This case involves intratumoral production of IL-8 as a causative factor, and IL-6, IL-8, IFN-γ and TNF-α cytokine production might have resulted from stimulation with a substance(s) derived from tumor tissue, since the fever disappeared postoperatively. To date the patient is alive and in good health for 7 years and 2 months since the surgery.
Keywords: malignant fibrous histiocytoma, fever, cytokine, interleukin-8
I. Introduction
Malignant tumors associated with prolonged fever have been reported for over 50 years [8, 10]. Some of the reported cases were associated with hematological malignancies [4], or carcinomas of the gastrointestinal or respiratory tracts [3, 11]. However, fever as a primary symptom in mesenchymal tumors, especially in soft tissue sarcomas, is a relatively rare event [1]. Some smooth muscle malignant tumors have been reported to be accompanied by fever of unknown origin [3]. Recently, increased production of proinflammatory cytokines and chemokines, such as interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and cyclooxygenase-2 (COX-2) messenger RNA (mRNA), have been reported and suggested to be febriferous in acute and chronic inflammatory diseases [2, 4, 5, 9, 12, 13, 16, 17]. These cytokines are produced by immune cells including various populations of lymphocytes macrophages and other cells. A pleomorphic type of malignant fibrous histiocytoma (MFH) presenting with fever was reported IL-6 [7]. Herein, we describe a case with inflammatory type MFH accompanied by prolonged fever. Using peripheral blood and tumor tissue from this patient, we investigated the expressions of IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IFN-γ, TNF-α and COX-2 mRNAs, which are reportedly febriferous in inflammation.
Case report
The patient was a 51-year-old male, who visited our hospital with a chief complaint of a medial mass in his left thigh that had increased in size over a period of one month. A CT-scan and MRI showed a large mass close to the femoral vessels (Fig. 1). Hematologic laboratory data on admission were as follow: Hb 10.0 g/dl, WBC 7.8×103 ml, Ht 29.7%, platelets 601×103/ml, CRP was 13.4 mg/dl, and tumor markers (CEA, AFP and CA19-9) were within normal limits. Gastroscopic and colonoscopic examinations, radiographs and CT-scan of chest, and abdominal echo and CT-scan showed no abnormalities. Though the patient had a fever spiking to more than 40°C for 6 weeks, we found no infectious lesions that might be responsible for the fever. The patient underwent open biopsy. Inflammatory cells, fibrous spindle cells and bizarre cells were identified histologically, and MFH was suspected (Fig. 2).
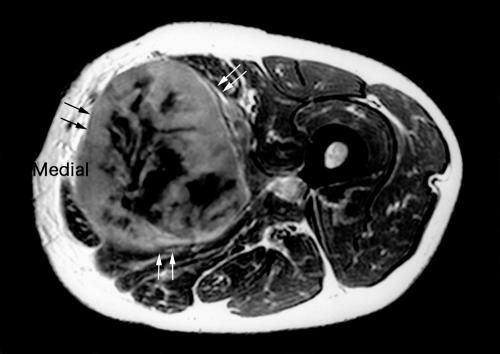
Fig. 1.
T2-weighted MRI shows a high intensity mass (black and white arrows).
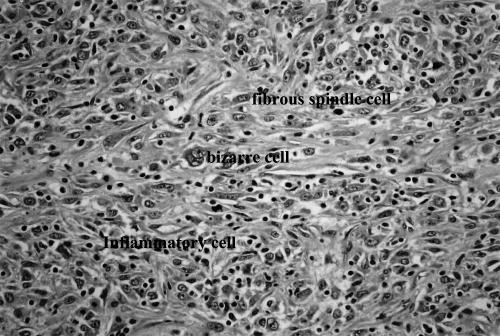
Fig. 2.
Atypical cells undergoing mitosis and many infiltrating inflammatory cells in the tumor mass (arrows) (H&E: high power view).
Wide excision of the tumor with accompanying vessels was performed, followed by reconstruction using the contralateral saphenous vein as the graft vessel. Inflammatory MFH was diagnosed, based on histology and the results of immunohistochemistry (Table 1) and electron microscopy (Fig. 3). The spiking fevers disappeared 2–3 days postoperatively. This patient has remained in good health for 7 years and 2 months to date since surgery.
Table 1.
Immunohistochemical results
| Antibody | Methods* | Results** |
|---|---|---|
| Vimentin | ABC | +++ |
| Actin | ABC | +++ |
| HAM56 | ABC | + |
| S-100 protein | ABC | + |
| α-1-antitrypsin | ABC | + |
| α-1-antichymotrypsin | ABC | + |
| Desmin | ABC | − |
| Myosin | ABC | − |
| Myoglobin | ABC | − |
ABC=avidin-biotin peroxidase complex.
+++, many tumor cells stained; ++, some tumor cells stained; +, few tumor cells stained; −, no tumor cell staining.
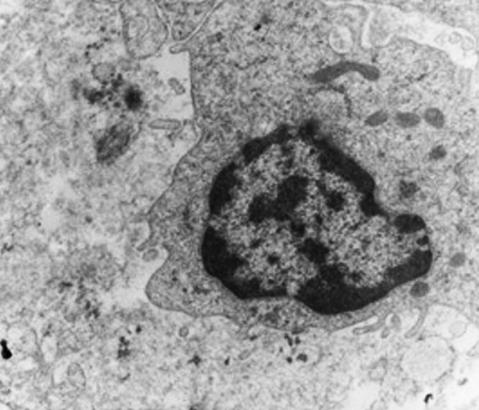
Fig. 3.
Ultrastructural examination reveals no myofilaments (×5000).
II. Materials and Methods
Preoperative peripheral blood and the excised tumor tissue were submitted for reverse transcription and polymerase chain reaction (RT-PCR) as decribed elsewhere. Briefly, surgical samples were taken during the operation, and snap frozen in liquid nitrogen. From peripheral blood samples, mononuclear cells were prepared using Lymphoprep (Nycomed, Torshov, Norway) gradient centrifugation. Negative control lymphocytes were collected from healthy volunteers. Informed consent was obtained from the patient before sampling. RNA was extracted using an RNAzolTM (Biotecx, Houston, TX, USA) rapid extraction kit. A single strand complementary DNA (cDNA) copy was made from total RNA using random hexamer primers and AMV reverse transcriptase. Oligonucleotide primers complementary to human IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IFN-γ, TNF-α and COX-2 mRNAs for PCR amplification were prepared using a DNA synthesizer (Applied Biosystems 381A, Foster City, CA, USA). PCR amplification was performed using a DNA thermal cycler (PerkinElmer Life and Analytical Science, Wellesley, MA, USA). After 25, 30 and 35 cycles of amplification, samples were taken to examine amplification products. This established the linear phase of amplification for each PCR product relative to internal control β-actin. Each cycle consists of 1 min at 94°C to denature double stranded DNA, 1 min at 60°C for the primers to anneal to their complementary sequence and 1 min at 72°C for extension of DNA strands. The resulting PCR products were size fractionated on 2% agarose gels and visualized by ethidium bromide staining under ultraviolet light. Relative quantitation of the PCR products was accomplished by comparing the signals densitometrically.
Next, we immunohistochemically examined the localization of the proinflammatory cytokine, IL-8 (Abcam Ltd, Cambridge, UK), using frozen and formalin-fixed paraffin-embedded surgical specimens. Signals were detected employing the avidin-biotin peroxidase complex (ABC) technique using an ABC kit (Vector Laboratories, Burlingame, CA, USA).
For ultrastructural examination, sample tissues were fixed with 2% osmium tetroxide and embedded in Epon 812. Ultrathin sections were cut and stained with uranyl acetate and lead citrate and observed with a Hitachi H-800 electron microscope (Hitachi Ltd, Tokyo, Japan).
III. Results
The specimen excised from the muscle of the left thigh consisted of a solid yellow-white tumor measuring 18×10×10 cm, with central necrosis. On histopathological examination, the tumor section showed proliferation of atypical cells composed of spindle cells and multi- or mono-nuclear giant cells with prominent nucleoli. Numerous inflammatory cells infiltrating the tumor were evident, and mitosis and necrotic areas were recognized in the tumor. Eosinophilic cytoplasm was found in some tumor cells, raising the possibility of rhabdomyosarcoma in the differential diagnosis. However, tumor cells were negative for desmin, myocin and myoglobin, while being strongly positive for vimentin and actin and slightly positive for HAM56, suggesting a histiocytic origin (Table 1). No myofilaments were found in tumor cells on ultrastructural examination. The tumor was ultimately diagnosed as MFH of the inflammatory type, and the surgical margin was negative.
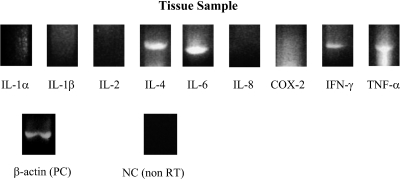
On RT-PCR analysis of preoperative peripheral blood mononuclear cells, only IL-8 mRNA resulted in a visible product, while mRNAs of other proinflammatory cytokines were negative (Fig. 4). On the other hand, IL-6, IL-8, IFN-γ and TNF-α mRNAs were expressed in tumor tissue. The mRNAs of other cytokines, IL-1α, IL-1β, IL-2, IL-4 and COX-2, were not identified in a tumor tissue sample (Fig. 5). Sequences and lengths of oligonucleotide primers are shown in Table 2.
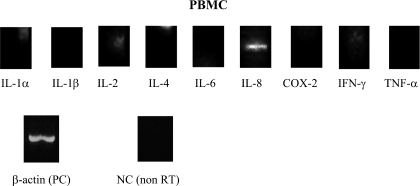
Fig. 4.
RT-PCR findings, obtained with preoperative peripheral blood mononuclear cells, were positive only for IL-8 (243 bp) mRNA.
Fig. 5.
Expressions of IL-6 (610 bp), IL-8 (243 bp), IFN-γ (330 bp) and TNF-α (429 bp) mRNAs were detected in a tumor tissue sample. No IL-1α, IL-1β, IL-2, IL-4 or COX-2 mRNA was detected in either sample.
Table 2.
Sequences and lengths of oligonucleotide primers
| Sequence (5'→3') | Fragments (bp) | ||
|---|---|---|---|
| IL-1α | sense | GTAAGCTATGGCCCACTCCAT | 408 |
| antisense | TGACTTATAAGCACCCATGTC | ||
| IL-1β | sense | GACCTGGACCTCTGCCCTCTG | 408 |
| antisense | AGGTATTTTGTCATTACTTTC | ||
| IL-2 | sense | AACTCCTGTCTTGCATTGCA | 441 |
| antisense | GTGTTGAGATGATGCTTTGAC | ||
| IL-4 | sense | CAACTTTTGTCCACGGACAC | 345 |
| antisense | TCCAACGTACTCTGGTTGG | ||
| IL-6 | sense | AACTCCTTCTOCACAAGCG | 610 |
| antisense | TGGACTGCAGGAACTCCTT | ||
| IL-8 | sense | CTTAGATGTCGATGCATAAAGACATACT | 243 |
| antisense | ACACCTCTTTCAAAACTTCTCCCGACTC | ||
| IFN-γ | sense | TGTTACTGCCAGGACCCA | 330 |
| antisense | GCGTTGGACATTCAAGTC | ||
| TNF-α | sense | CAGGCAGTCAGATCATCATCT | 429 |
| antisense | ATAGTCGGGCCGATTGAT | ||
| COX-2 | sense | GGTCTGGTGCCTGGTCTGATGATG | 569 |
| antisense | GTCCTTTCAAGGAGAATGGTGC | ||
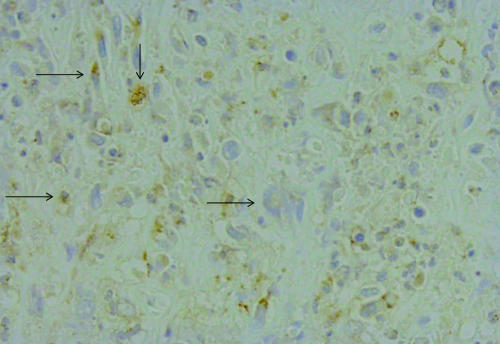
Immunohistochemically stained frozen and paraffin sections from tumor tissue were positive for IL-8, in the cytoplasm of tumor tissue (Fig. 6).
Fig. 6.
Immunohistolochemically, paraffin sections of tumor tissue stained positive for IL-8 (immunohistochemical staining: high power view).
IV. Discussion
Irrespective of tumor histogenesis, the mechanism and causative factors underlying tumor-associated fevers usually remain undetermined. In the present case, the patient manifested a prominent prolonged fever with a spiking characteristic. The tumor raised suspicion of inflammatory MFH based on routine clinical, pathological, immunohistochemical and ultrastructural findings. Taking these observations together, we concluded that this patient suffered from tumor-related fever arising from a malignant mesenchymal tumor. As expected, the fever promptly disappeared after tumor resection. The factor(s) responsible for tumor-associated fever were of interest. The most probable explanation was in situ production of febriferous substance(s) by the tumor. We examined the expressions of several proinflammatory cytokine candidates by RT-PCR and detected expressions of IL-6, IL-8, IFN-γ and TNF-α mRNAs.
It was recently reported that the mRNAs of multiple proinflammatory cytokines, such as IL-1α, IL-1β, IL-6, IFN-γ and TNF-α, are associated with fever and other generalized symptoms. Several articles have described local production of cytokines by malignant tumors or tumor-infiltrating immune cells. For example, neuroblastoma [6], osteosarcoma [15] and MFH [7] have all been reported to produce IL-6, and this production was up-regulated by TNF-α and IL-1β. Among the many proinflammatory cytokines, IL-1 is thought to be among the factors most potently producing fever, via the induction of prostaglandins [14]. Its action is mediated through COX-2 induction [2]. In our present case, we detected expressions of IL-6, IL-8, IFN-γ and TNF-α mRNAs in tumor tissue, while no IL-1α, IL-1β, IL-2, IL-4 or COX-2 mRNA expression was found.
As the fever disappeared after tumor resection, it was reasonable to conclude in this case that tumor tissues were closely associated with the fever. IL-8 was suspected to be the febriferous substance based on the RT-PCR results from the patient’s preoperative blood and tumor tissue samples. However, in situ immunohistochemical staining of tumor tissue sections revealed infiltrating tumor cells to be positive for IL-8. Intratumoral production of IL-8 and other proinflammatory cytokines (IL-6, IFN-γ and TNF-α) from inflammatory cells was speculated to be responsible for the fever in this case, leading us to further hypothesize that the production of these cytokines might be stimulated by a substance(s) derived from tumor tissue. Alternatively, our results might be attributable to stimulation from a causative factor produced by tumor tissue, since the fever disappeared after tumor resection.
V. Acknowledgments
The authors thank Dr. Christine A. Towle (Orthopaedic Laboratory of Massachusetts General Hospital) for help in preparing the manuscript.
VI. References
- 1.Browder A. A., Huff J. W., Petersdorf R. G. The significance of fever in neoplastic disease. Ann. Intern. Med. 1961;55:932–942. doi: 10.7326/0003-4819-55-6-932. [DOI] [PubMed] [Google Scholar]
- 2.Chishima F., Hayakawa S., Sugita K., Kinukawa N., Aleemuzzaman S., Nemoto N., Yamamoto T., Honda M. Increased expression of cyclooxygenase-2 in local lesions of endometriosis patients. Am. J. Reprod. Immunol. 2002;48:50–56. doi: 10.1034/j.1600-0897.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 3.Dendale P., Devis G., Goossens A. Leiomyosarcoma of the small intestine presenting as fever of unknown origin. Gut. 1992;33:411–413. doi: 10.1136/gut.33.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denz H., Fuchs D., Huber H., Nachbaur D., Reibnegger G., Thaler J. Correction between neopterine, interferon-gamma and haemoglobin in patients with hematological disorders. Eur. J. Haematol. 1990;44:186–189. doi: 10.1111/j.1600-0609.1990.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 5.Fryling C., Dombalagian M., Burgess W., Hollander N., Schreiber A. B., Haimovich J. Purification and characterization of tumor inhibitory factor-2: Its identity to interleukin-1. Cancer Res. 1989;49:3333–3337. [PubMed] [Google Scholar]
- 6.Goillot E., Combaret V., Ladenstein R., Baubet D., Blay J. Y., Philip T., Favrot M. C. Tumor necrosis factor as an autocrine growth factor for neuroblastoma. Cancer Res. 1992;52:3194–3200. [PubMed] [Google Scholar]
- 7.Hamada T., Komiya S., Hiraoka K., Zenmyo M., Morimatsu M., Inoue A. IL-6 in a pleomorphic type of malignant fibrous histiocytoma presenting high fever. Hum. Pathol. 1998;29:758–761. doi: 10.1016/s0046-8177(98)90288-x. [DOI] [PubMed] [Google Scholar]
- 8.Harsha W. N. Fever in malignant disease. Am. Surg. 1952;18:229–235. [PubMed] [Google Scholar]
- 9.Isshiki H., Akira S., Tanabe O., Nakajima T., Shimamoto T., Hirano T., Kishimoto T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1 responsive element in the IL-6 gene. Mol. Cell. Biol. 1990;10:2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly C. M., Cain J. C., Ellis F. H., Soule E. H. Leiomyosarcoma of the duodenum: Report of case with fever and abdominal pain for 7 years. Mayo Clin. Proc. 1957;32:712–716. [PubMed] [Google Scholar]
- 11.Klastersky J., Weerts D., Hensgens C., Debusscher L. Fever of unexplained origin in patients with cancer. Eur. J. Cancer. 1973;9:649–656. doi: 10.1016/0014-2964(73)90007-8. [DOI] [PubMed] [Google Scholar]
- 12.Masuda H., Iwai S., Tanaka T., Hayakawa S. Expression of IL-8, TNF-α and IFN-γ mRNA in ulcerative colitis, particularly in patients with inactive phase. J. Clin. Lab. Immunol. 1995;46:111–123. [PubMed] [Google Scholar]
- 13.Means R. T., Dessypris E. N., Krantz S. B. Inhibition of human erythroid colony-forming units by tumor necrosis factor requires accessory cells. J. Clin. Invest. 1990;86:538–541. doi: 10.1172/JCI114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morimoto A., Morimoto K., Watanabe T., Sakata Y., Murakami N. Does an increase in prostaglandin E2 in the blood circulation contribute to a febrile response in rabbits? Brain Res. Bull. 1992;29:189–192. doi: 10.1016/0361-9230(92)90025-s. [DOI] [PubMed] [Google Scholar]
- 15.Motoyama T., Hotta T., Watanabe H., Kumanishi T., Ichikawa T., Sekiguchi M. Differential production of interleukin-6 in human osteosarcoma cells and the possible effects on neoplastic bone metabolism. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993;63:277–281. doi: 10.1007/BF02899273. [DOI] [PubMed] [Google Scholar]
- 16.Nakamori T., Morimoto A., Yamaguchi K., Watanabe T., Murakami N. Interleukin-1β production in the rabbit brain during endotoxin-induced fever. J. Physiol. 1994;476:177–186. [PMC free article] [PubMed] [Google Scholar]
- 17.Osaka S., Fujimoto Y., Yamazaki H., Suzuki K., Sawada S., Hayashi N. Interleukin-1α producing synovial sarcoma with prolonged fever: a case report. Jpn. J. Clin. Oncol. 1998;28:436–440. doi: 10.1093/jjco/28.7.436. [DOI] [PubMed] [Google Scholar]