Abstract
Background and objectives of the study
Chronic kidney disease is a common cause of morbidity and mortality in Nigeria. This study aims at determining the pattern of chronic renal failure (CRF) in a Nigerian University Teaching Hospital.
Methods
The study was a 10-year retrospective study of consecutive cases of CRF seen at Olabisi Onabanjo University Teaching Hospital, Sagamu, Ogun State, Nigeria.
Results
The frequency of CRF in the population was 3.6% (182 of 5,107). There were 90 males and 63 females (1.42:1). The peak age was between 20–49 years, with a mean of 39.6±14.8 (range 14–72years). The commonest causes were chronic glomerulonephritis 63(41.2%), hypertensive nephrosclerosis 40(26.1%) and diabetes mellitus 20(13.1%). The commonest symptoms were oedema, vomiting, oliguria and dyspnea occurring at 118(77.1%), 96(62.7%), 89(58.2%) and 87(56.9%) respectively. The mean creatinine clearance value at presentation was 6.5±8.1mls/min, while the commonest complications were hypertension 68 (44.4%), biventricular failure 32 (20.9%) and urinary tract infection 29 (19%). The mean presenting systolic and diastolic blood pressures were 167.34±37.6mmHg and 106.03±28.9 mmHg respectively. The mean total haemodialysis session per patient was 3.5±1.6 (range 1–7sessions). Only 34(22.2%) of the patients were able to afford haemodialysis. The majority 21(61.8%) of these could only afford 3 haemodialysis sessions while only 2(5.9%) patients had up to 7 dialysis sessions in the center.
Conclusion
Chronic glomerulonephritis, hypertensive nephrosclerosis and diabetes mellitus are the commonest causes of chronic renal failure in Nigeria. Most of the patients presented late. Cardiovascular complications and infections were responsible for a greater morbidity among the patients.
Keywords: Chronic Renal Failure, Retrospective Study, Nigeria
Running title: Pattern of Chronic Renal Failure in Nigeria
Introduction
Chronic renal failure is defined as either a level of GFR <15 mL / min per 1.73 m2, which is accompanied in most cases by signs and symptoms of uraemia, or a need for initiation of renal replacement therapy1. It ultimately results into end stage renal failure (ESRF), with creatinine clearance <5ml/24hrs/1.73m2. Most forms of progressive renal disease lead to a common end point: the end stage kidney, which is usually fibrotic and reduced in mass. It is an important cause of morbidity and mortality in Nigeria.
Although end stage renal disease (ESRD) patients represent a small group of the total European countries population, (0.02% in UK and 0.06% in Italy), dialysis costs absorb 0.7–1.8% of the health-service budget1. In the United States, the expenditure on ESRD is estimated to double in the next year to more than US$28 billion by 20102. Similar figures are lacking in Africa and indeed, Nigeria. However, the United States Renal Data System Registry (USRDS)3 and the European Dialysis and Transplantation-European Renal Association (EDTA-ERA) reports4 are invaluable sources of data on trends in prevalence and incidence of ESRD worldwide1. The exact prevalence rate of Chronic Renal Disease (CRD) in Nigeria is not known. Hospital based data in Nigeria have reported prevalence rates expressed as ratios of hospital admissions of between 1.6 and 8%5,6,7,8. In the United States, the incidence of ESRD continues to grow increasingly at a rate of 8–10%, with about 220,000 patients presently undergoing dialysis, 80,000 living with functioning renal transplant and about 2 million people in various stages of CRF3. There are less detailed analyses for trends in Europe and Japan, but the data available indicate a similar pattern to that in the USA1.
ESRD disproportionately affects people in developing countries as well as ethnic and racial minorities in the United States. Data from the USRDS Annual Report demonstrates that African Americans make up to 29% of the ESRD cases and yet represent only 12% of the United States population9, with predominance in males. Similar gender differences have been documented among Nigerians7.
In the United States (US), the average age of CRF patients was 58 in 1999 and 62 in 19983, reflecting the increasingly available renal replacement therapy. The average age of CRF patients among Nigerians lies between the third and the fourth decade6,7. In the US, diabetes mellitus and hypertension are the two leading causes of CRF10, while chronic glomerulonephritis and hypertension top the list in the tropics7,11,12,13(including Nigeria7). In Scotland however, chronic pyelonephritis and chronic glomerulonephritis are of equal aetiological significance14.
Although it has been recommended that CRF patients should be referred early to nephrologists15 to reduce complications (and hence early mortality), generally many patients are still referred late16,17,18, requiring dialysis within four months of presentation. Such complications include pulmonary oedema, severe hypertension, severe anemia, and septicemia16,17,18.
Few studies have been done on the pattern of CRF in South West Nigeria 6,7,19–27. No similar study has been carried out at the Olabisi Onabanjo University Teaching Hospital Sagamu, Nigeria. The hospital is a 185 bed facility, located in South West Nigeria and made up of 40 medical beds, 51 paediatric beds, 61 surgical beds and 33 obstetrics and gynaecological beds, excluding the accident and emergency unit16.
The objectives of this study were to:
Determine the frequency of CRF in the hospital and document the pattern and the clinical presentation of CRF in the Olabisi Onabanjo University Teaching hospital over a 10 year period and to
Methods
This study was carried out in the Medical Department of the Olabisi Onabanjo University Teaching Hospital, Sagamu, Nigeria, a tertiary health care center. The hospital serves as a major referral center for South West Nigeria, especially Ogun State and environs. The study is a retrospective study of chronic renal failure cases seen over a ten-year period (Jan 1992 to December 2002). Approval of the local ethical committee was obtained.
The inclusion criterion was a diagnosis of CRF, based on the history, physical examination, biochemistry and ultrasonography. Chronic renal failure was defined by persistent elevation of plasma creatinine above 2mg/dL (176.8ì mol/L), bilateral shrunken kidneys on ultrasound except in diabetic nephropathy, autosomal dominant polycystic kidney disease, obstructive nephropathy, sickle cell disease, renal tumors, where renal size may be normal / increased with loss of corticomedullary differentiation and increased parenchyma echogenicity and presence of clinical features of uraemia such as body weakness, anorexia, nausea and vomiting. Other features that suggest CRF included anaemia, hypertension and bone disease. The exclusion criteria include a diagnosis of ARF, with acute deterioration in renal function, normal sized kidneys and echogenicity on ultrasound.
Information was obtained from the medical outpatient register, the admission and discharges register on the medical wards, and the dialysis unit registers. Clinical data retrieved included the age and sex of the patients, presenting symptoms, complications and the aetiology of the chronic renal failure. Other information included number of patients dialyzed, frequency of dialysis and the duration of dialysis.
Patients with detectable proteinuria equal to or greater than 2+, age less than 35years, history of previous body swelling, presence of haematuria, red cell, and granular casts were classified as having chronic glomerulonephritis27. The diagnosis was taken as hypertensive nephrosclerosis if there was normal renal function at the time of initial diagnosis of hypertension, presence of other end organ of long standing hypertension and the absence of other risk factors for renal disease27. Patients with previous history of hypertension, presence of trace or 1+ proteinuria and no cast were also classified as having hypertensive nephrosclerosis. Patients with history of significant urinary obstruction were classified as having obstructive uropathy27. Patients with history of diabetes mellitus, proteinuria, diabetic retinopathy and normal or increased renal sizes on ultrasound were classified as having diabetic nephropathy27.
Data on laboratory parameters obtained include the presenting serum electrolyte, urea and creatinine, serum calcium and phosphate levels, packed cell volume and urine microscopy.
Quantitative data was expressed as mean ± standard deviation. Student's t-test was used to assess the difference between the various groups. Statistical significance was taken as p<0.05.
Results
One hundred and eighty two CRF patients were seen during the study period of whom one hundred and fifty-three had complete data. A total of 5,107 patients were seen on the medical wards and dialysis unit of the hospital during the study period giving a frequency of occurrence of CRF in the population studied to be 3.6%. Table 1 shows the clinical characteristics of the population at presentation. The patients include 90 males and 63 females with a male to female ratio of 1.42:1. The peak age was between 20–49 years, with a mean of 39.6±14.8 (range 14–72years).
Table 1.
clinical characteristics of the population at presentation
| Subjects with chronic renal failure | ||
| Number of patients (%) | 153 (100) | |
| Mean age (Years) | 39.6±14.8 | |
| Sex distribution (Male: Female) | 1.4:1 | |
| Hypertension (%) | 84(38.5%) | |
| Mean *SBP (mmHg) | 167.3±15.5 | |
| Mean +DBP (mmHg) | 106±28.9 | |
| Mean creatinine clearance (mls/min) | 6.5±8.1 | |
| Mean dialysis sessions | 3.5±1.6 | |
| Total number without clinical complications | 28(18.3%) | |
| Commonest symptoms | • Oedema | 118(77.1%) |
| • Vomiting | 96(62.7%) | |
| • Oliguria | 89(58.2%) | |
| • Dyspnea | 87(56.9%) | |
| Commonest complications | • Hypertension | 68(44.4%) |
| • Biventricular Failure | 32(20.9%) | |
| • Urinary Tract Infection | 29(19%) | |
| Commonest aetiological factors | • Systemic Hypertension | 27(17.6%) |
| • Chronic Glomerulonephritis | 4(2.6%) | |
SBP-Systolic Blood Pressure;
DBP-Diastolic Blood Pressure
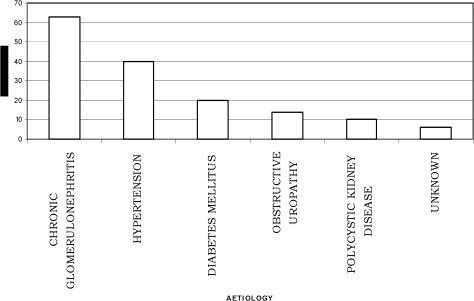
Fig 1 shows the distribution of the etiological factors of CRF in the population. The commonest causes of chronic renal disease among the patients were chronic glomerulonephritis 63(41.2%), hypertensive nephrosclerosis 40(26.1%) and diabetes mellitus 20(13.1%). Fig 2 shows the distribution of haemodialysis sessions the patients had. The mean total number of haemodialysis sessions per patient was 3.5±1.6 (range 1–7sessions). Only 34(22.2%) of the patients were able to afford haemodialysis. Majority 21(61.8%) of these could only afford 3 haemodialysis sessions haemodialysis sessions while only 2(5.9%) patients had up to 7 dialysis sessions in the center. There are no facilities presently for peritoneal dialysis in the hospital.
Figure 1.
Aetiological factors of chronic renal failure
The symptomatology at presentation is shown in Fig 3. The commonest symptoms were oedema, vomiting, oliguria and dyspnea occurring at 118(77.1%), 96(62.7%), 89(58.2%), 87(56.9%) respectively. The mean presenting systolic and diastolic blood pressures were 167.34±37.6mmHg and 106.03±28.9 mmHg respectively (range systolic blood pressure of 90–260mmHg and diastolic blood pressure of 50–190 mmHg (Table 2). Most of the patients presented in Stage 2 hypertension.
Table 2.
Blood pressure pattern at presentation
| Classification | Blood pressure range (mmHg) |
Number of patients (%) |
| Normal | SBP<120 | 10 |
| DBP<80 | 21 | |
| Pre-hypertension | SBP 120–139 | 12 |
| DBP 80–89 | 2 | |
| Stage 1 hypertension | SBP 140–159 | 19 |
| DBP 90–99 | 14 | |
| Stage 2 hypertension | SBP ≥160 | 59 |
| DBP ≥100 | 63 | |
| Mean SBP | 167.34±37.6 | |
| Mean DBP | 106.03±28.9 |
SBP-Systolic Blood Pressure
DBP-Diastolic Blood Pressure
Fig 4 shows the distribution of complications present at presentation. Twenty-eight (18.3%) patients presented without complications. The commonest complications were hypertension 68 (44.4%) biventricular failure 32 (20.9%) and urinary tract infection 29 (19%). The commonest complications usually combined were acute pulmonary oedema and hypertension.
Table 3 shows the mean biochemistry of the patients at presentation. The mean value of serum urea, creatinine, potassium and bicarbonate are 218.1±97.7mg%, 7.0±4.8 mg%, 4.7±1.3 mmol/L and 17.8±9.9 mmol/L respectively. The mean creatinine clearance value was 6.5±8.1mls/min.
Table 3.
The mean serum biochemistry at presentation.
| Parameter (Unit) | Mean±SD |
| Sodium mmol/L | 129.41±3.7 |
| Potassium mmol/L | 4.7±1.3 |
| Bicarbonate mmol/L | 17.8±9.9 |
| Urea mmol/L | 218.1±97.7 |
| Creatinine mg% | 7.0±4.8 |
| Calcium mg% | 7.5±1.8 |
| Phosphate mmol/L | 6.6±3.5 |
| Chloride mmol/L | 99.7±11.6 |
| Creatinine clearance | 6.5±8.1 |
| Uric Acid mg% | 10.4±4.1 |
| Serum albumin mg% | 3.6±1.2 |
| HDL mg% | 32.2±16.7 |
| LDL mg% | 82.7±36.1 |
| VLDL mg% | 132.5±59.7 |
| Cholesterol mg% | 172.6±50.4 |
The pattern of monthly clinic attendance of chronic renal failure patients and the effects of meteorological parameters has been reported elsewhere28. The month of January recorded the highest admissions rate of 23 while September recorded the lowest of 5 cases p<0.05. The mean monthly admission from November to March was 13.8 while it was 12 from April to October.
Discussion
The mean age of the patients was 39.6+14.8 years (range 14–72years), with a peak between 20–49 years. Our observations are in keeping with earlier studies 6,7,19,23, which showed that the peak incidence of ESRD in Nigeria is between the 3rd and 5th decade of life. This corresponds to the productive years of these patients leading to economic and human resource wastage. In developed countries however, the prevalence of CRF increases with age29,30. The incidence of ESRD is 6- to 10- times higher in patients between 70 and 90 years of age compared with those aged between 30 and 50 years. In Japan, two-thirds of total dialysis patients are >60 years and 50 % are > 65 years31,32.
The commonest causes of chronic renal disease among the patients were chronic glomerulonephritis 63(41.2%), hypertensive nephrosclerosis 40(26.1%) and diabetes mellitus 20(13.1%). Generally, chronic glomerulonephritis and hypertension are principal causes of CRF in tropical Africa and East Africa33. The commonest causes of chronic renal diseases in Nigeria from earlier studies were also chronic glomerulonephritis, hypertension, diabetes mellitus and obstructive uropathy7. We may have inadvertently included patients that developed hypertension as a result of the CRF in the analysis of the aetiological factors since this was a retrospective study. Hypertension in CRF is very common and contributes to morbidity and mortality34. About 85% of patients with ESRD have hypertension34, which is in part responsible for the high incidence of cardiovascular events and deaths in these patients. It accelerates progression of Chronic Renal Disease (CRD) in humans, whether it results from, or causes, the renal disease34. Activation of the reninangiotensin system (RAS) in conjunction with sodium retention and volume expansion has been recognized as the most important factors34. In North Africa, the incidence of kidney diseases is much higher than that in West Africa35 and the principal causes of ESRD are interstitial nephritis (14 to 32%), glomerulonephritis (11 to 24%), diabetes (5 to 20%) and hypertensive nephrosclerosis (5 to 21%)35. Hypertension has become a significant problem in many developing countries experiencing epidemiological transition from communicable to noncommunicable chronic diseases36,37,38. The emergence of hypertension and other CVDs as a public health problem in these countries is strongly related to the aging of the populations, urbanization, and socioeconomic changes favoring sedentary habits, obesity, alcohol consumption, and salt intake, among others39,40. A cost-effective use of health services to control these emerging chronic diseases is particularly needed in developing countries because resources are limited and generally must be shared with the concurrent burden of persistent communicable diseases.
Other known causes of hypertension such as Conn's syndrome, phaeochromocytoma renal artery stenosis, which may lead to CRF, are also notably rare in Nigerians. We may have also inadvertently excluded patients with secondary hypertension in this study due to the lack of appropriate facilities in our setting to screen for urinary metanephrines, plasma aldosterone and plasma cortisol; and to perform diethylenetriamine pentaacetic acid technicium scan and renal angiogram.
Notably rare amongst our patients is chronic pyelonephritis as a cause of CRF. A number of predisposing factors to CPN such as analgesic abuse, anatomical abnormality of urinary tract and vesicoureteral reflux are rare among the Africans7, and may thus account for the low prevalence of CPN.
Most of the patients in this study were referred late to the hospital and had profoundly altered plasma biochemistry. The mean creatinine clearance value at presentation was 6.5±8.1mls/min. Thus, many cases of CRF with relatively stable renal function may not have been included in this study; hence our conclusions may apply more to CRF cases with acute deterioration. Given the fact that early referral to the nephrologist is likely to result in optimal pre-dialysis care, the recommendation is now that referral of a patient to a renal team should occur at a serum creatinine of 1.5mg%(132.6Umol/L) in women and 2.0mg%(176.8Umol/L) in men15. Recently, other workers17,18 have recommended that all patients with an established, progressive increase in serum creatinine level should be followed up by a nephrologist early, since adequate preparation for dialysis or transplantation (or both) requires at least 12 months of relatively frequent contact with a renal care team. Economic and clinical considerations strongly argue in favour of early referral of chronic uraemic patients.
The mean value of serum urea and creatinine at presentation were 218.1±97.7mg% and 7.0±4.8 mg% respectively. These figures are significantly lower than figures documented in other centres in South West Nigeria and may be difficult to explain since it has been shown that most CRF patients present late to the hospital.
Only twenty-eight (18.3%) patients presented without complications. The commonest complications at presentation were hypertension 68 (44.4%), biventricular failure 32 (20.9%) and urinary tract infection 29 (19%). This is similar to findings from an earlier study in the hospital16. However, there are no similar studies available from other Nigerian health institutions addressing this issue.
Seasonality was demonstrated in the distribution of the CRF patients and has been reported elsewhere28. The month of January (dry season) recorded the highest admissions rate of 23 while September (wet season) recorded the lowest of 5 cases p<0.05. This may be explained by the hot and dry climate that occurs during the day during the dry seasons. Much fluid is lost during this period, which may result in significant dehydration further deterioration in the precarious renal blood flow, hence acute deterioration of chronic renal disease during the dry season. It is also known that blood pressures are higher during the colder months41 (which occur at nights during the dry seasons) and may cause further deterioration in renal function.
Unlike in developed countries, most of our patients were able to afford only three sessions of haemodialysis because the total cost of dialysis is borne by the patients. In another Nigerian study, 70.8% of the patients were able to remain on dialysis for less than 1 month, 12.7% for between 3 and 6 months, 5.1% for between 7 and 12 months and only 1.9% remained on dialysis for over 12 months23. In developed countries, the costs of dialysis are borne by the government with only small costs imposed on the patients as copay. Since 1972, the Medicare ESRD program has borne 80% of the costs of dialysis and transplantation for approximately 93% of patients in the United States allowing near universal access to treatment for kidney failure1. In Japan, the government pays US$40,000 to 50,000 per year to the dialysis provider per patient either on HD or PD32. In China and Brazil also, RRT is accessible to all those in need of it since the government covers most expenses related to RRT42. The situation in most developing countries is different in that the patient provides the bulk of the fund for renal replacement therapy13. This makes the treatment unavailable to most patients and also makes it impossible for patients to have long-term dialysis treatment. The majority of those with ESRD in developing countries die because of lack of funds, as very few can afford regular maintenance dialysis20,21,23,26,33.
The magnitude of the existing burden of illness caused by renal failure, the projections for increasing incidence of ESRD, and the limitations of the existing treatments for renal insufficiency in Nigeria all point to the need for clinical and population - based interventions aimed at prevention of ESRD26. The cost of treating this condition is enormous. Appropriate management should preferably involve a multifactorial approach targeting the control of hypertension, dyslipidaemia, proteinuria, obesity, avoidance of low birth weight, smoking and use of heavy metals containing products such as lead, which have been identified as intervention strategies for preventing progression of renal diseases34. Pre-employment urinalysis screening and health education will go a long way in educating, increasing awareness and preventing the deleterious complications of uncontrolled hypertension, diabetes mellitus and glomerulonephritis. There is a need to embark on a massive health education campaign and screening of the study populace for early detection of kidney diseases. Finally, with the introduction of the National Health Insurance Scheme (NHIS) in 198443, the NHIS is expected to make health services more available to the generality of the Nigerian populace.
References
- 1.National Kidney Foundation-K/DOQI, author. Clinical Practice Guidelines for chronic kidneydisease, evaluation, classification and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 2.Collins A, Xue JL, Ma JZ, Louis T. Estimating the number of patients and medicare cost for end stage renal disease in the US to the year 2010. J Am Soc Nephrol. 2000;11:133A. doi: 10.1681/ASN.V12122753. [DOI] [PubMed] [Google Scholar]
- 3.The USRDS and its products. Unite States Renal Data System. Am J Kidney Dis. 1998;32(suppl):S20–S194. doi: 10.1053/ajkd.1998.v32.pm9713405. Anon. [DOI] [PubMed] [Google Scholar]
- 4.Valderrabano F, Berthoux FC, Jones EH, Mehls O. Report on management of renal failure in Europe, XXV, 1994 end stage renal disease and dialysis report. The EDTA-ERA Registry. Nephrol Dial Transplant. 1996;11(suppl):2–21. doi: 10.1093/ndt/11.supp1.2. [DOI] [PubMed] [Google Scholar]
- 5.Analysis of medical admissions to Adeoyo state hospital Ibadan. Nig Med J. 1973;3(1):5–12. [PubMed] [Google Scholar]
- 6.Oyediran AB, Akinkugbe OO. Chronic Renal Failure in Nigeria. Trop Geog Med. 1970;22:41–45. [PubMed] [Google Scholar]
- 7.Akinsola W, Odesanmi WO, Ogunniyi JO, Ladipo GOA. Diseases causing chronic renal failure in Nigerians - a prospective study of 100 cases. Afr J Med Sci. 1989;18:131–137. [PubMed] [Google Scholar]
- 8.Ogun SA, Adelowo OO, Familoni OB, Jaiyesimi AEA, Fakoya EAO. Pattern and outcome of medical admissions at the Ogun State University Teaching Hospital Sagamu - a three years review. WAJM. 2000;19(4):304–308. [PubMed] [Google Scholar]
- 9.USRDS 1991 Annual Data Report. Bethseda, Md: National Institute of Diabetes and Digestive and Kidney diseases, National Institutes of Health; 1991. Aug, US Renal Data System. [Google Scholar]
- 10.Kobrin S, Aradhye S. Preventing progression and complications of renal disease. Quadrant Health Com, Inc. Hospital Medicine. 1997;33(11):11–12. 17–18, 20, 29–31, 35–36, 39–40. [Google Scholar]
- 11.Gold CH, Isaacson C, Levin J. The pathological basis of ESRD in blacks. S African Med J. 1982;61:263–265. [PubMed] [Google Scholar]
- 12.Hutt MSR. Renal disease in tropical environment. Trans R Soc Tropical Med Hyg. 1980;74:17–21. doi: 10.1016/0035-9203(80)90004-8. [DOI] [PubMed] [Google Scholar]
- 13.Rostand GS, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for ESRD. New Eng J med. 1982;306(12):76–79. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 14.Pandreigh DM, Howitt LF, Macdougall AI, et al. Survey of Chronic Renal Failure in Scotland. Lancet. 1972;I:304–307. doi: 10.1016/s0140-6736(72)90304-2. [DOI] [PubMed] [Google Scholar]
- 15.Obrador GT, Pereira BJ. Early referral to the nephrologist and timely initiation of renal replacement therapy: a paradigm shift in the management of patients with chronic renal failure. American Journal of Kidney Diseases. 1998;31(30):398–417. doi: 10.1053/ajkd.1998.v31.pm9506677. [DOI] [PubMed] [Google Scholar]
- 16.Alebiosu CO. Detrimental effects of late referral for dialysis. African Journal of Health Sciences. 2001 January–June;8(1–2)(19):78–81. [PubMed] [Google Scholar]
- 17.Jungers P, Zingraff J. Detrimental effects of late referral in patients with chronic renal failure: a case-control study. Kidney International. 1993;43:S170–S173. [PubMed] [Google Scholar]
- 18.Roderick P, Jones C, Drey N, Blakeley S, Webster P, Goddard J, Garland S, Bourton l, Mason J, Tomson C. Late referral for end-stage renal disease: a region-wide survey in the south west of England. Nephrology, Dialysis and Transplantation. 2002;17:1252–1259. doi: 10.1093/ndt/17.7.1252. [DOI] [PubMed] [Google Scholar]
- 19.Salako BL, Kadiri S, Etok V, Adesola K. The clinical and laboratory evaluation of patients with chronic renal failure in UCH Ibadan before their first dialysis. Dokita. 1995;22(1):56–59. [Google Scholar]
- 20.Odutola TA, Ositelu SB, D'Almeida EA, Mabadeje AF. Five years experience of haemodialysis at the Lagos University Teaching Hospital—November 1981 to November 1986. Afr J Med Med Sci. 1989 Sep;18(3):193–201. [PubMed] [Google Scholar]
- 21.Mabayoje MO, Bamgboye EL, Odutola TA, Mabadeje AF. Chronic renal failure at the Lagos University Teaching Hospital: a 10-year review. Transplant Proc. 1992 Oct;24(5):1851. discussion 1852. [PubMed] [Google Scholar]
- 22.Bamgboye EL, Mabayoje MO, Odutola TA, Mabadeje AF. Acute renal failure at the Lagos University Teaching Hospital: a 10-year review. Ren Fail. 1993;15(1):77–80. [PubMed] [Google Scholar]
- 23.Arije A, Kadiri S, Akinkugbe OO. The viability of hemodialysis as a treatment option for renal failure in a developing economy. Afr J Med Med Sci. 2000 Sep–Dec;29(3–4):311–314. [PubMed] [Google Scholar]
- 24.Akinola O, Adelekun TA, Akinsola W. Jugular venous access for short-term haemodialysis. Ife experience. West Afr J Med. 1999 Jul–Sep;18(3):175–178. [PubMed] [Google Scholar]
- 25.Salako BL, Kadiri S, Arije A, Obisesan K. Short-term haemodialysis in pregnant patients with acute renal failure: a report of two cases. Afr J Med Med Sci. 2002 Sep;31(3):271–273. [PubMed] [Google Scholar]
- 26.Bamgboye EL. Hemodialysis: management problems in developing countries, with Nigeria as a surrogate. Kidney Int Suppl. 2003 Feb;(83):S93–S95. doi: 10.1046/j.1523-1755.63.s83.19.x. [DOI] [PubMed] [Google Scholar]
- 27.Salako BL, Ayodele OE, Kadiri S, Arije A. Prevalence of hepatitis B and C viruses in pre-dialysis patients with chronic renal failure. Afr J Med Med Sci. 2002;31:311–314. [PubMed] [Google Scholar]
- 28.Alebiosu CO, Ayodele OE, Adigun AB, Jaiyesimi AEA. Nigerian Journal Of Internal Medicine, Unilag. Meteorological parameters and the pattern of chronic renal failure clinic attendance in a tropical African population. In Press. [Google Scholar]
- 29.Feest TG, Mistry CD, Grimes DS, Mallick NP. Incidence of advanced chronic renal failure and the need for end-stage renal replacement treatment. BMJ. 1990;301:897–900. doi: 10.1136/bmj.301.6757.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mc Geown MG. Prevalence of advanced renal failure in Northern Ireland. BMJ. 1990;301:900–903. doi: 10.1136/bmj.301.6757.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iseki K, Kawazeo N, Osawa A, Fukiyama K. Survival Analysis of Dialysis Patients in Okinawa, Japan. Kidney Int. 1993;43:404–409. doi: 10.1038/ki.1993.59. [DOI] [PubMed] [Google Scholar]
- 32.Kurokawa K, Nangaku M, Saito A, Inagi R, Miyata T. Current issues and future perspectives of chronic renal failure. J Am Soc Nephrol. 2002;13:S3–S6. [PubMed] [Google Scholar]
- 33.Saraladevi Naicker. End-stage renal disease in sub-Saharan and South Africa. KI. 2003;63:119. doi: 10.1046/j.1523-1755.63.s83.25.x. [DOI] [PubMed] [Google Scholar]
- 34.Alebiosu CO. An update on progression promoters in chronic renal disease. J Natl Med Assoc USA. 2003;95:30–42. [PMC free article] [PubMed] [Google Scholar]
- 35.Barsoum RS. End-stage renal disease in North Africa. KI. 2003;63:111. doi: 10.1046/j.1523-1755.63.s83.23.x. [DOI] [PubMed] [Google Scholar]
- 36.Dodu SRA. Emergence of cardiovascular diseases in developing countries. Cardiology. 1988;75:56–64. doi: 10.1159/000174349. [DOI] [PubMed] [Google Scholar]
- 37.Nissinen A, Bothig S, Granroth H, Lopez AD. Hypertension in developing countries. World Health Stat Q. 1988;41:141–154. [PubMed] [Google Scholar]
- 38.World Health Organization, author. Cardiovascular diseases in developing countries. World Health Stat Q. 1993;46:90–150. [PubMed] [Google Scholar]
- 39.Omran AR. The epidemiological transition: a theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;4:509–538. [PubMed] [Google Scholar]
- 40.Akinkugbe OO. World epidemiology of hypertension in blacks. J Clin Hypertens. 1987;3:1S–8S. [PubMed] [Google Scholar]
- 41.Brennan PJ, Greenberg G, Miall WE, Thompson SG. Seasonal variations in arterial blood pressure. BMJ. 1982;235:919–923. doi: 10.1136/bmj.285.6346.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zatz R, Romão JE, Jr, Noronha IL. Nephrology in Latin America, with special emphasis on Brazil. Kidney Int. 2003;63:108. doi: 10.1046/j.1523-1755.63.s83.28.x. [DOI] [PubMed] [Google Scholar]
- 43.Campbell PC. A decade of National Health Insurance Scheme in Nigeria part 1. Nig Q J Hospital Medicine. 1998;8(1):60–64. [Google Scholar]