Abstract
Background
Abnormal haemoglobin variants ( HbSS,AS,AC,SC,etc) have been known to be common among blacks. Patients with sickle cell disease are often faced with the risk of alloimmunization from allogeneic blood transfusion
Objectives
The study was designed to sample students population of African descents for the purpose of updating information on the prevalence of abnormal haemoglobin variants, ABO, and Rh blood groups and compare the results with previously published data
Methods
Standard electrophoretic and haemagglutination techniques were employed in testing the blood samples.
Results
Of the 620 students screened, 80.32% were HbAA and 19.68% HbAS. 22.9% were of blood group A, 17.10% group B, 4.84% group AB and 55.16% group O. 96.77% were Rh.D positive while 3.23% were Rh D negative. Sickle cell gene in homozygous state (HbSS) and other abnormal haemoglobin variants were not encountered in this students population,. Analysis of the students population revealed that 454(73.23%) were females while 166(26.77%) were males. Participants of the age group 26–30 years (35.7%) constituted the majority and in this age group, all blood groups were represented.
Conclusion
There is a gradual decline in the prevalence of abnormal haemoglobin variants in our black population. The frequencies of ABO and Rh blood groups however appeared to be stable and consistent with previous published data.
Keywords: Haemoglobin genotypes, blood groups, ABO, Rh, Nigeria
Introduction
Haemoglobin genotypes and blood groups are all inherited blood characters. The inherited disorders of haemoglobin are the most common gene disorders with 7% of the world's population being carriers1. It is on record that about 300,000 children are born with sickle cell disease (SCD) worldwide every year2. Sickling disorders are found very frequently in the Afro-Caribbean populations and sporadically throughout the Mediterranean region, India and the Middle East1. These sickling disorders include the heterozygous state for haemoglobin S or the sickle cell trait (AS), the homozygous state for HbS or sickle cell anaemia (SS) and the compound heterozygous state for HbS together with haemoglobin C, D, E or other structural variants. Haemoglobin S differs from haemoglobin A by the substitution of valine for glutamic acid at position 6 in the β - chain1,2.
The membrane of the human red blood cell (RBC) contains a variety of blood group antigens. The most important and best known of these is A and B antigens, which are actually complex oligosaccharides that differ in their terminal sugar. On RBCs, they are mostly glycosphingolipids. The antibodies against red cell antigens are called agglutinins and individuals are divided into four major blood groups A, B, AB and O according to the presence of these antigens and agglutinins3,4. In addition, human red cells that contain antigen D are known as Rhesus positive while those without antigen D in their RBC's are Rhesus negative4,5. The clinical relevance of these blood group systems relate to the capacity of alloantibodies (directed against antigens not possessed by the individual) to cause destruction of transfused red cells (ABO antibodies) or to cross the placenta and give rise to haemolytic disease of the newborn (HDN)5.
The frequencies of these three inherited characters have severally been reported to vary significantly in various populations and ethnic groups around the world [6–8]. In Nigeria, few recent published data have been encountered9,10 but none in Port Harcourt, South - South of Nigeria. This study was therefore designed to provide the frequencies of haemoglobin genotypes, ABO and Rh blood groups for reference purposes using students' population in Port Harcourt.
Materials and methods
Six hundred and twenty (620) apparently healthy subjects (males-166, females-454) representing about 24.8% of the entire students population, were selected randomly from a cross-section of college students. The institutional Ethical committee approved the study. All the participants gave their written informed consents and willingly presented themselves at the Medical Laboratory for sample collection. Their results were confidentially given to them. Their ages ranged from 17 – 48 years (x = 24.4 years).
Blood samples were collected by venipuncture into ethylenediamine tetracetic acid (EDTA) salt and used for haemoglobin genotype determination and red cell phenotyping. The method described by Brown [11] was used for haemoglobin electrophoresis. A small quantity of haemolysate of venous blood from each of the subjects was placed on the cellulose acetate membrane and carefully introduced into the electrophoretic tank containing Tris - EDTA - Borate buffer at pH 8.9. The electrophoresis was then allowed to run for 15 – 20 mins at an electro motive force (emf) of 160 V. The results were read immediately. Haemolysates from blood samples of known haemoglobin (i.e. AA, AS, AC) were run as controls.
Red cell phenotyping was carried out with standard tube techniques as described by Judd12 and Brecher 13. For ABO blood grouping, a drop of anti-A, anti-B, and anti-AB (Biotec, Ipswich, UK) each was placed in clean test tubes labelled 1,2,3. To each tube was added a drop of 5% red blood cell suspension in saline. The contents were gently mixed together and centrifuged for 30 seconds at 1000g. The cell buttons were resuspended and observed for agglutination. Agglutination of tested red cells constituted positive results. A smooth cell suspension after resuspension followed by a microscopic confirmation constituted negative test results.
For Rhesus D typing, a drop of anti-D serum (Biotec, Ipswich, UK) was placed in a clean labelled test tube and a drop of control placed in a second tube. 1 drop of 5% RBC suspension in saline was then added and incubated at 37°C. At the end of the incubation period, the contents of the tube were mixed gently and centrifuged for 30 seconds at 1000g. Agglutination was read macroscopically and microscopically in doubtful cases. All negative results were confirmed using the indirect antiglobulin test (IAT) procedure (also for confirmation of weak D).
Data were analysed using the computer statistical analytical software. (SAS). Frequency procedure of SAS was employed for the frequency distribution of blood groups and haemoglobin genotypes
Results
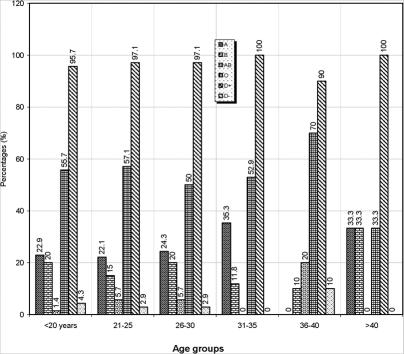
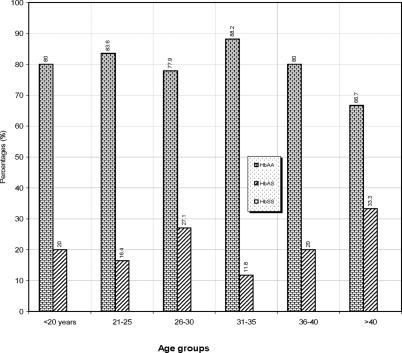
The overall frequencies of the various haemoglobin genotypes, ABO and Rh blood groups in this study are shown in table 1. Of the 620 subjects screened, 80.32% were HbAA, 19.68% were HbAS. 22.9% were of blood group A, 17.10% group B, 4.84% group AB and 55.16% group O. 96.77% were Rh D positive while 3.23% were Rh D negative. Of the 620 people tested, 166 (26.77%) were males while 454 (73.23%) were females. The percentage distribution of the age groups used for this study is shown in Figure 1. Majority of the participants were of the age group 26 – 30 years (35.7%). Figure 2 shows the distribution of ABO and Rh blood groups according to their various age groups used for the study. All blood groups were represented within the ages of £ 20 – 30 years. Figure 3 shows the distribution of haemoglobin genotypes among the students. Majority of he participants with HbAA were found in the 31 – 35 years age group while most participants with HbAS were found within the 26 – 30 years age group.
Table 1.
Frequencies of ABO, Rh and haemoglobin genotypes among 620 health students.
| Number tested | Percentage frequency | |
| ABO Blood group | % | |
| A | 142 | 22.90 |
| B | 106 | 17.10 |
| AB | 30 | 4.84 |
| O | 342 | 55.16 |
| Rh Blood group | ||
| D+ | 600 | 96.77 |
| D− | 20 | 3.23 |
| Hb Genotypes | ||
| AA | 498 | 80.32 |
| AS | 122 | 19.68 |
Figure 1.
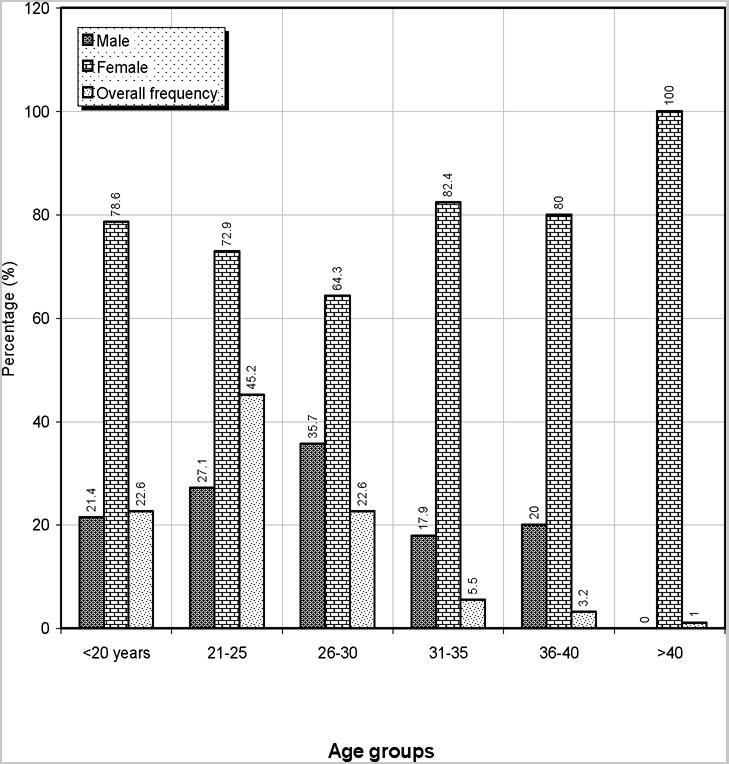
Frequencies of male and female students according to their age groups
Figure 2.
Distribution of ABO and Rh blood groups among different age groups.
Figure 3.
Distribution of haemoglobin genotypes among students in relation to their age groups.
Discussion
The prevalence of HbSS among the black population in the United State, was reported to be 9% and 30 – 40% generally for Africans [14, 15]. In another report, the geographical distribution of sickle cell anaemia (SS) was given as follows: 3 – 9% for USA black Americans, 1 – 8% for USA whites, 3 – 7% for Europe (UK, Pakistanis - Blacks), 2 – 8% for other European countries (Mediterranean), 1 – 3% for Caribbeans, 1 – 3% for Middle East, 1 – 10% for Africa. The frequency of sickle cell trait (AS) was equally reported as follows: 8 – 16% for USA blacks, 8 – 10% USA (whites), 6 – 15% for Europe (UK, Pakistanis - Blacks), 1 – 15% for Europe (Mediterranean), 3 – 8% for Caribbeans, 7 – 8% for Middle East, 15 – 30.5% for Africa and 40.5% for West Africa and Nigeria16.
In this study, the frequency of HbAA was 80.32% while HbAS was found to be 19.68%. HbSS and other haemoglobin variants did not occur among the 620 participants in this study thus presenting a wide variation from previous published reports. However, this study is in agreement with a study carried out in Kenya, East Africa, where the prevalence of HbSS was equally zero. A value of 74% and 97% for HbAA in lowland and highland areas and 26% and 3% for HbAS in lowland and highland areas respectively were obtained in that study17. The zero frequencies observed in these studies possibly imply that the sickling gene pool is gradually reducing in our African population. It may not mean a complete absence because there are other published reports in Nigeria that carry 3.0% HbSS, 2.0% SC and 0.3% CC in the South-West region of Nigeria9. Another paper also reported 4% HbSS and 1% AC in parts of South-South region of Nigeria but not Port Harcourt10. The low prevalence of HbSS in the study population could be attributed to increased awareness of the disease, improved socio-economic conditions and other environmental and genetic factor which have an overall effect on the sickling gene pool. It is equally possible that the hardy - Weinberg equilibrium must have been disturbed which has led to more people acquiring normal haemoglobin gene and sickle cell trait while the homozygous sickle cell gene is gradually tending to zero. The observed frequency of HbAA is above the normal range of 55 – 75% earlier reported for Blacks18. The frequency of HbAS in this study corresponds to the value, 20 – 30% quoted for Nigeria and 20 – 40% in Africa in general8,16,18.
The frequency of ABO blood groups varies from race to race. Among Western Europeans, 42% have group A, 9% group B, 3% group AB and the remaining 46% group O. However, some Eastern Europeans have a higher proportion, upto 40% of group B blood while pure native American Indian belong exclusively to blood group O 3. American blacks generally have frequencies of A, B, AB and O blood groups of 27%, 20%, 4% and 49% respectively4. In Nigeria, few published works on the frequencies of the ABO blood groups among ethnic groups/tribes were encountered. The first was that of Worlledge et al19 who reported the frequencies among Yoruba and Hausa as follows, 21% for group A, 17% for group B, 2% for group AB and 58% for group O. All previous reports are in agreement with the frequencies obtained in this study and goes to confirm that group O appears to show predominance over the other blood groups. An exception to this is the Gwari tribe of Abuja and the Rubuka tribe of Plateau state of Nigeria, where group B has been reported to show predominance over the other blood groups in those states21. The high frequency of group O in this population provides an advantage in terms of availability of blood for blood transfusion especially in emergencies. However, some level of caution has to be exercised since some group O blood is known to contain potent immune haemolytic antibodies (haemolysins). Routine haemolysin test on every group O blood should be encouraged to reduce the risk of transfusion reaction.
The frequency of Rhesus D antigen in this study was 96.77%. This is also in agreement with 96.7% recorded for the Ibos by Ukaejiofor20 and similar to 95% found in Port Harcourt recently22. It is striking in this study that the 3.23% Rh D negatives were all women. This percentage of D negative women stands the risk of developing anti-D which can cause both moderate and severe form of haemolytic disease of newborn. In this study, sex, age and disease conditions were not considered critical; rather the results depended on the genetic constitution of the subjects. However, the distribution of the blood groups and haemoglobin genotypes among different age groups were analysed. It was discovered that majority of the students fell into the 26 – 30 years group and in this group, the blood groups were better distributed than other age groups. Most of the people with HbAA were within 31 – 35 years. There was no test of significance between groups so it is difficult to state statistically whether age has any influence on these inherited characters.
Acknowledgment
The author wishes to acknowledge the efforts of the staff of the Medical Laboratory Science department of the College of Health Science and Technology, Port Harcourt for assisting in sample collections and laboratory analysis.
References
- 1.Weatheral DJ. Genetic disorders of haemogloobin. In: Hoffbrand AV, Lewis SM, Tuddenham EGD, editors. Postgraduate haematology. 4th ed. London, UK: Arnold Publishers; 2001. pp. 91–119. [Google Scholar]
- 2.Okpala I, Thomas V, Westerdale N, Jegede T, Raj K, Daley S, Costello - Binger H, Mullen J, Rochester - Peart C, Helps C, Tulloch E, Akpala M, Dick M, Bewley S, Davies M, Abbs I. The comprehensive care of sickle cell disease. Eur J Haematol. 2002;68(3):157–162. doi: 10.1034/j.1600-0609.2002.01523.x. [DOI] [PubMed] [Google Scholar]
- 3.Pramanik T, Pramanik S. Distribution of ABO and Rh blood groups in Nepalese Medical Students: a report. East Mediterr Health J. 2000;6(1):156–158. [PubMed] [Google Scholar]
- 4.Conteras M, Lubenko A. Immunohaematology: Introduction. In: Hoffbrand AV, Lewis SM, Tuddenham EGD, editors. Postgraduate haematology. 4th ed. London UK: Arnold Publishers; 2001. pp. 165–181. [Google Scholar]
- 5.Knowles S, Poole G. Human blood group systems. In: Murphy MF, Pamphilon DH, editors. Practical transfusion medicine. 1st ed. London, UK: Blackwell Science; 2002. pp. 24–31. [Google Scholar]
- 6.Schneider RG, Hightower B, Hosty TS, Ryder H, Tomlin G, Atkins R, Brimhall B, Jones RT. Abnormal haemoglobin in a quarter million people. Blood. 1976;48(5):629–637. [PubMed] [Google Scholar]
- 7.Graham RS. Sickle cell disease. New York. USA: Oxford University Press; 1988. [Google Scholar]
- 8.Reid HL, Famodu AA. Spectrophotometric quantification of haemoglobin fraction in heterozygous sickle cell trait (HbAS) Med Lab Sci. 1988;45(2):143–145. [PubMed] [Google Scholar]
- 9.Bakare AA, Azeez MA, Agbolade JO. Gene frequencies of ABO and Rhesus blood groups and haemoglobin variants in Ogbomosho, South - West, Nigeria. Global J Med Sci. 2004;3(3):17–22. [Google Scholar]
- 10.Nwafor A, Banigo BM. A comparison of measured and predicted haemoglobin genotype in a Nigerian population in Bonny, Rivers State, Nigeria. J Appl Sci Environ Manage. 2001;5:79–81. [Google Scholar]
- 11.Brown BA. Hematology: Principles and procedures. 6th edn. Philadelphia, USA: Lea and Febiger; 1993. [Google Scholar]
- 12.Judd JW. Methods in Immunohematology. 2nd edn. Durham, USA: Montogomery Scientific Publications; 1994. [Google Scholar]
- 13.Brecher M. Technical Manual. 14th ed. Bethesda, USA: American Association of Blood Banks; 2002. [Google Scholar]
- 14.Richard AW. Textbook of Black related diseases. New York, USA: McGraw Hill; 1975. [Google Scholar]
- 15.Lewis RA. Sickle cell: Clinical features in West Africans. Accra, Ghana: Ghana University Press; 1970. [Google Scholar]
- 16.Sinou MT. Antenatal screening of sickle cell disease. 8th postgraduate course for training in reproductive medicine and reproductive biology. Cameroon. 2003 [Google Scholar]
- 17.Moormann AM, Embury PE, Opondo J, Sumba OP, Ouma JH, Kazura JW, John CC. Frequencies of sickle cell trait and glucose - 6-dehydrogenase deficiency differ in highland and nearby lowland malaria - endemic areas of Kenya. Trans R Soc Trop Med Hyg. 2003;97(5):513–514. doi: 10.1016/s0035-9203(03)80010-x. [DOI] [PubMed] [Google Scholar]
- 18.Fleming AF, Lehman H. Sickle cell disease: A handbook for General clinician. Edinburgh: Churchill Livingstone; 1982. [Google Scholar]
- 19.Worlledge S, Ogiemudia SE, Thomas CO, Ikoku BN, Luzzatto L. Blood group antigens and antibodies in Nigeria. Ann Trop Med Parasitol. 1974;68(3):249–264. doi: 10.1080/00034983.1974.11686948. [DOI] [PubMed] [Google Scholar]
- 20.Ukaejiofor EO, Okonkwo WC, Tagbar EN, Emeribe AO. Blood Transfusion in the Tropics. Nigeria: (Ukaejiofor EO)Salem Press; 1996. ABO and Rhesus in a Nigerian population; pp. 1–22. [Google Scholar]
- 21.Onwukeme KE. Blood group distribution in blood donors in a Nigerian population. J Physiol Sci. 1990;6(1):67–70. [Google Scholar]
- 22.Jeremiah ZA, Buseri FI. Rh antigens and phenotype frequencies and probable genotypes for the four main ethnic groups in Port Harcourt Nigeria. Immunohematology. 2003;19(3):86–88. [PubMed] [Google Scholar]