Abstract
We have shown previously that human herpesvirus 8 (HHV8) seroconversion for antibodies to the latency-associated nuclear antigen encoded by ORF73 and/or the lytic capsid antigen (vp19) encoded by ORF65 is associated with orogenital contact and is strongly linked to the development of Kaposi's sarcoma among HIV-infected individuals in the Amsterdam Cohort Studies. Here, we investigate the relationship between seroconversion to these antigens and primary HHV8 infection. Between 1984 and 1997, 215 HHV8 seroconversions to ORF73 (106 cases or 49%) and/or to ORF65 (159 cases or 74%) were recorded in the cohort of homosexual men. The HHV8 seroconversion rate among HIV-infected homosexual men (6.2 per 100 person years) was consistently higher than among HIV-uninfected men (2.6 per 100 person years). In HIV-infected but not in uninfected individuals, seroconversion to ORF73/latency-associated nuclear antigen precedes that to ORF65/vp19. Antibody levels to both ORF65- and ORF73-encoded antigens were higher in HIV-infected than in HIV-uninfected men, and among HIV-seropositives, antibody levels to ORF65/vp19 rise even higher with declining CD4 cell counts and peak with Kaposi's sarcoma development, suggesting continuing and increasing viral replication. In 10.3% of HHV8 seroconversions, transient serum viremia could be demonstrated before or at seroconversion. Together with the previously reported link between unprotected orogenital sex and HHV8 seroconversion, our observations suggest that HHV8 seroconversions result from primary infections.
The human herpesvirus 8 (HHV8) or Kaposi's sarcoma-associated herpesvirus (KSHV) belongs to the gamma-2 or rhadinovirus sublineage of the Gammaherpesvirinae subfamily together with the Old World monkey viruses, rhesus monkey rhadinovirus, and retroperitoneal fibromatosis-associated herpesviruses (RFHV); the New World monkey viruses, herpesvirus saimiri (HVS), and herpesvirus ateles (HVA); equine herpesvirus type 2 (EHV2); and murine herpesvirus 68 (MHV68; refs. 1–6). HHV8 is strongly associated with Kaposi's sarcoma (KS) in HIV-infected individuals, body cavity-based lymphomas, and Castleman's disease (7–10). The only other human gammaherpesvirus, Epstein–Barr virus, is associated with lymphomas and nasopharyngeal carcinoma (11).
Tests for antibodies to both lytic and latent HHV8 antigens can identify not only most HIV-infected individuals diagnosed with KS but also those at increased risk to develop KS (12–18). Recently, we showed that seroconversion to a recombinant HHV8 lytic-phase capsid antigen, vp19, encoded by ORF65, and/or the latent-phase nuclear antigen (LANA) encoded by ORF73, is highly predictive of KS (19). Among HIV-infected persons, those who seroconvert for HHV8 after HIV infection are at higher risk to develop KS than those who seroconvert for HHV8 before HIV infection. Time-dependent adjustment for CD4+ cell count and HIV-1 RNA copy number have no impact on this additional risk, although the CD4+ cell count was an independent risk factor for KS (19).
The current study was designed to investigate the persistence of antibody responses to the lytic-phase capsid (ORF65) and latent-phase nuclear (ORF73) antigens and to assess whether seroconversion follows a burst in HHV8 production and is associated with clearance of serum viremia. In addition, we studied the impact of HIV and KS on the antibody response to ORF65/vp19 and ORF73/LANA to identify virus reactivation. Subsequently, we investigated the association between HHV8 seroconversion among HIV-seropositive and HIV-seronegative individuals and the practice of particular sexual behaviors over the course of the HHV8 epidemic.
Materials and Methods
Study Participants, Clinical Follow-Up, and Study Design.
Subjects for the present study enrolled in the Amsterdam Cohort Studies: 1,458 homosexual men and 1,167 injecting drug users as described by Renwick et al. (19). To determine whether participants were HHV8 seronegative or seropositive, their most recently obtained serum sample was tested by an enzyme immunoassay (EIA) involving recombinant HHV8 proteins (see below). If a sample tested negative, the individual was considered to have had no antibodies against HHV8 throughout his or her participation. If a sample tested positive, the sample taken at enrollment of the cohort study was tested to determine whether seroconversion had occurred during follow-up. If so, the year of seroconversion was determined by testing serum samples at yearly intervals and, within the year of seroconversion, at intervals of 3–6 months. The midpoint between the last negative sample and the first positive sample (seroconversion sample) was considered the date of HHV8 seroconversion. However, to investigate the potential for false negativity, the enrollment samples of 200 participants whose most recent sample had tested negative were evaluated with the EIA system. A positive result at entry was found for 9 of the 200, yielding a putative false negativity rate of 4.5% [95% confidence interval (CI): 2.1–8.4].
Detection of HHV8 Antibodies.
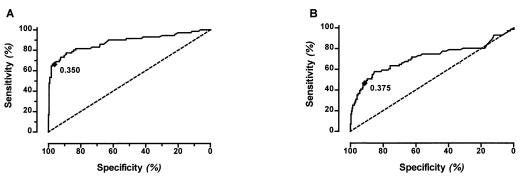
We used an EIA format as described earlier (13, 19) by utilizing either recombinant ORF65/vp19, associated with the lytic stage of HHV8 infection (13), or a carboxyl-terminal fragment of the LANA that is encoded by ORF73 (20). In the case of HHV8, we deal with imperfect reference standards because HHV8 cannot be cultured currently and HHV8 DNA is found only in 58–67% of peripheral blood mononuclear cells and 46% of serum from KS patients (21). For our analysis, results from the EIA system with an optical density of 0.350 or more for ORF65/vp19 or 0.375 or more for ORF73/LANA were considered HHV8-seropositive. These cut-off values were three times the SD of the mean optical density from a panel of 40 HIV-uninfected IV drug-using women representing low risk for KS. In addition, to evaluate the cut-off points in our population of interest, receiver operator characteristic curves for both antibody tests were calculated (Fig. 1), showing sensitivity and specificity according to different optical density cut-off points. The best theoretical cut-off value is the point on the curve that is closest to the upper left-hand corner. For this analysis, we used samples from 71 participants with KS of the homosexual men cohort as an index for true positivity, and from all 302 women from the drug user cohort as an index for true negativity, because no KS had occurred among these women in 12 years of follow-up. Based on these parameters, the sensitivity of the combined assay format (positive for either ORF65/vp19 or ORF73/LANA or both) was 81.7% (95% CI: 70.7–89.9) and the specificity was 90.76% (95% CI: 86.9–93.8).
Figure 1.
Receiver–operator characteristic curve for assays to detect antibodies against HHV8 lytic ORF65 (A) and latent ORF73 (B) antigens.
To facilitate comparison of antibody levels to ORF65 and ORF73, serum-over-cut-off (S/C) ratios were calculated. S/C ratios greater or equal to 1, representing positive antibody results, were compared among HIV-infected participants with KS before and after KS was diagnosed. S/C ratios were calculated in HIV-infected participants without KS according to the following five CD4 categories: 401–500, 301–400, 201–300, 101–200, and 1–100 cells/mm3.
Detection of HHV8 DNA.
To detect HHV8 DNA in serum, we developed a sensitive nested PCR. Primers were designed based on the HHV8 sequence in the plasmids used for the expression of the ORF65 and ORF73 antigens (13, 20). Both plasmids were purified and used in in vitro reconstruction experiments. The sensitivity of the assay was measured by limiting dilution of the purified linearized plasmids (cut with the restriction enzyme BamH1). Both PCRs were optimized to detect reproducibly an input of one to five plasmid copies. Nucleic acids were isolated by using a previously described method starting with 100 ml of serum (22). One-fifth of the isolated nucleic acids were amplified with ORF65- or ORF73-specific primers. The sense primer 5′-ORF65–1 (5′-GAGTTTCCGCGGCGTCGGCTTA) and the antisense primer 3′-ORF65–4 (GGGTTTCCTCGCGTCGGCCA) were used in the first PCR of ORF65, and sense primer 5′-ORF73–1 (5′-AGCTAGGCCACAACACATCT) and the antisense primer 3′-ORF73–4 (5′-ACAATAAGTTATGGGCGACT) were used in the first ORF73 reaction. Amplification was performed in our standard PCR mix containing buffer, dNTPs, Taq, and optimized MgCl2 in a total volume of 50 ml (23). One-twentieth of the amount used in the first reaction was subjected to the second round of amplification by using the sense primer 5′-ORF65–2 (5′-GACGTTCACCGTGCCTTCGA) and antisense primer 3′-ORF65–3 (5′-GGCCGTTTCCGTCGTGGATGA) for ORF65 detection and the sense primer 5′-ORF73–2 (5′-AGGATGGAAGACGAGATCCA) and the antisense primer 3′-ORF73–3 (5′-TCTGGTCCAGGGTGGGGCAA) for ORF73 detection. The amplified fragments (169 bp for ORF65 and 150 bp for ORF73) were identified by gel electrophoresis. Random PCR fragments were directly sequenced to confirm the origin of the amplified fragments and all belonged to the ORF65- or ORF73-encoded genome fragments, respectively.
PCR testing of serum samples of participants began with the first sample that tested positive for HHV8 and was extended to samples taken 3–6 months before and after the seroconversion point to determine whether the positive reaction was due to a reactivation or due to a primary infection. Samples were available for 175 of 215 homosexual men who were identified as seroconverter for HHV8. Although the nested PCR made detection possible to the one-molecule level, HHV8 DNA could be detected in only 18 of the 175 (10.3%) HHV8 seroconverters.
Statistical Analysis.
Levels of antibodies against antigens encoded by ORF65 and ORF73 were compared by using Student's t test among HIV-infected and HIV-uninfected participants who seroconverted for HHV8. This comparison was done for participants who seroconverted for ORF65/vp19 only, or ORF73/LANA only, and for those who seroconverted for both antigens. In the latter group the order of seroconversion was compared by using χ2 test.
Seroconversion rates for HIV or HHV8 per calendar year were calculated and expressed as cases per 100 person years (pyrs) of follow-up, or incidence density (ID), and 95% CI. Polynomial smoothing was applied to ID curves to illustrate the change or stability of the ID over time.
To determine the year that HHV8 was introduced in the community, the estimation of the survival distribution for HHV8 seroconversion was done among persons starting HHV8-seronegative until the known date of HHV8 seroconversion or censored for the other statuses or lost to follow-up, by using the exponential form of this survival distribution. The validity of the exponential form was checked by fitting a Weibull distribution and testing whether or not the scale parameter could be assumed to equal 1 (meaning an exponential distribution). After estimating the incidence per year, as a parameter of the exponential survival distribution, and the HHV8 seroprevalence at the start of the cohort, we computed the year at which the virus could have been introduced, at the latest. For this purpose we used the well established relation between the Poisson distribution and the number of events up to a certain time and the gamma distribution (i.e., exponential in this case) of that time to event (24). Thus, for example, if the incidence is 50 per year and there are 100 cases, 2 years are required to reach this prevalence and even longer if some of the 100 patients died.
Results
HHV8 Antibody Profiles in the Seroconverter Population.
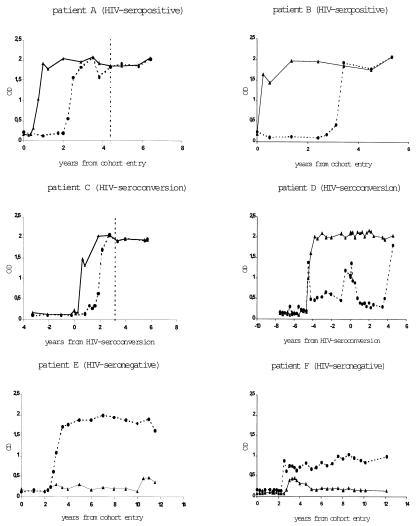
A total of 215 HHV8 seroconverters were identified among homosexual men. Of these, 106 (49%) seroconverted for latent antigen ORF73, 159 (74%) seroconverted for lytic antigen ORF65, and 50 (23%) seroconverted for both antigens. Fig. 2 shows the seroconversion in six persons with different antibody patterns. The antibody levels for both ORF65 and ORF73 antigens are persistently elevated, although with different heights. In patient D, the seroconversion for HIV was associated with a temporary increase in antibodies against ORF65/vp19. Among the 50 individuals seroconverting for both ORF65/vp19 and ORF73/LANA, HIV-seronegative individuals seroconverted in 37.5% of cases (9 of 24) for ORF65/vp19 before ORF73/LANA, in 33.3% (8 of 24) for ORF73/LANA before ORF65/vp19, and simultaneously in 7 cases. In contrast, HIV-seropositive individuals seroconverted in only 15.4% of cases (4 of 26) for ORF65/vp19 before ORF73/LANA, in 65.4% (17 of 26) for ORF73/LANA before ORF65/vp19, and in 5 cases simultaneously (P = 0.066).
Figure 2.
Antibody responses against HHV8 lytic ORF65 and latent ORF73 antigens in six homosexual individuals, either HIV-infected (patients A and B), HIV seroconverters (patients C and D), or HIV-seronegative (patients D and E); dashed line, OD of ORF65; solid line, OD of ORF73; vertical, dashed line, KS diagnosis.
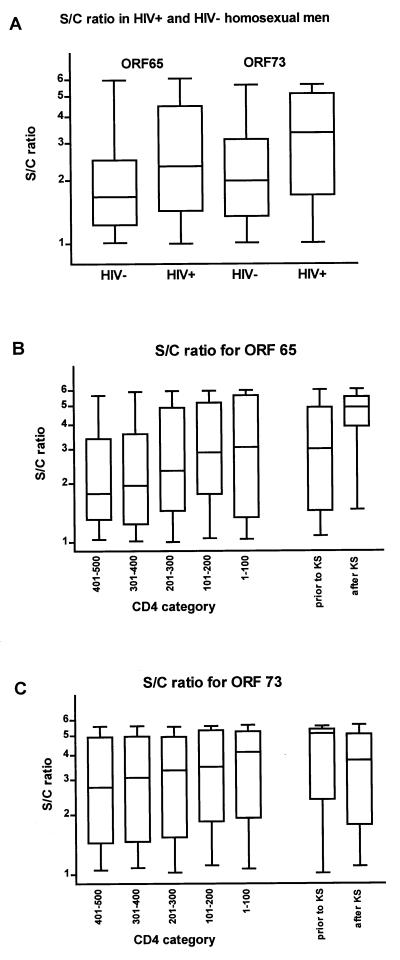
HIV-infected and HIV-uninfected persons seroconverting for either ORF65 or ORF73 antigens had comparable antibody levels at the first sample that tested positive. However, higher antibody levels against both ORF65/vp19 and ORF73/LANA were observed during follow-up in HIV-infected persons (Fig. 3A). For ORF65/vp19, the median S/C ratio in HIV-infected individuals is 2.3 vs. 1.6 for non-HIV-infected individuals (P = 0.0001). For ORF73/LANA, these figures are 3.3 and 1.9, respectively (P = 0.0001). To investigate the impact of a KS event, we compared HHV8 antibody levels in samples taken before and after KS development in HIV-infected individuals. The median S/C ratio for antibodies against ORF65/vp19 was 3.0 (interquartile range 1.5–4.7) before and 4.9 (3.9–5.5) after KS was diagnosed (Fig. 3B; P = 0.004). Concurrently, the median CD4 cell count was 290 (140–430) cells/mm3 before and 75 (10–200) cells/mm3 after KS was diagnosed. The median S/C ratio for antibodies against ORF73/LANA was 4.8 (2.0–5.3) and 3.7 (1.8–5.0), respectively (Fig. 3C; P = 0.28), and median CD4 cell count at the time samples was obtained among the positive tested persons was 380 (265–635) cells/mm3 before and 30 (10–160) cells/mm3 after KS was diagnosed.
Figure 3.
S/C ratios for antibody responses to HHV8 lytic ORF65 and latent ORF73 antigens in HIV-infected and uninfected homosexual men (A), in HIV-infected men without KS according to five CD4 categories (401–500, 301–400, 201–300, 101–200, and 1–100 cells/mm3), and in HIV-infected men with KS before and after KS was diagnosed [ORF65 (B) and ORF73 (C)]. Of 71 patients with KS, 69 had samples taken before and after KS was diagnosed. Of these, 41 and 44 tested positive for antibodies against ORF65 before and after KS was diagnosed, respectively (B); 36 and 33 tested positive for antibodies against ORF73 before and after KS was diagnosed, respectively (C).
For five CD4 cell count categories in HIV-infected participants without KS, the median S/C ratios of the positive test results were: 1.8, 2.0, 2.3, 2.9, and 3.0 for ORF65/vp19 and 2.8, 3.1, 3.3, 3.5, and 4.1 for ORF73/LANA in patients with CD4 ranging from 401 to 500, 301 to 400, 201 to 300, 101 to 200, and 1 to 100, respectively (Fig. 3 B and C). The S/C ratios for ORF65/vp19 showed a statistically significant increase when CD4 cell declined, but the increase for ORF73/LANA S/C ratios was not significant (P = 0.04 and P = 0.38, respectively).
HHV8 DNA in Serum During Seroconversion.
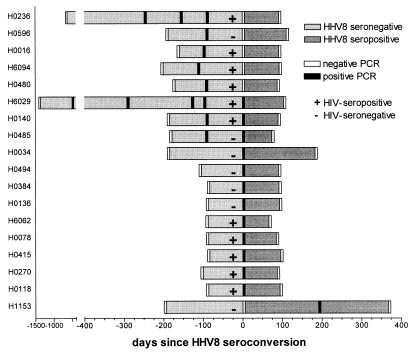
We detected HHV8 DNA in 18 of 175 HHV8 seroconverters (10.3%; 95% CI: 5.8–14.8%), with comparable proportions of HIV-infected men in the group with and without demonstrable HHV8 DNA (61% and 54%, respectively). The DNA could be measured either before (n = 8), at (n = 9), or after (n = 1) HHV8 seroconversion (Fig. 4). HHV8 DNA positivity did not persist, although four cases were positive on two or more consecutive occasions. The coding region for ORF65, ORF73, and both ORF65 and ORF73 was detected by PCR in 11 of 18 cases, 4 cases, and 3 cases, respectively. The period from the last negative until the first positive PCR ranged from 63 to 189 days and from the first positive PCR until the first subsequent negative PCR ranged from 65 to 182 days, bracketing a maximum period of HHV8 serum DNA positivity of 364 days. The window phase from HHV8 DNA positivity until HHV8 antibody positivity ranged from 0 to 112 days. However, because of the sampling frequency of 3 months, duration of DNA presence of less than 3 months could not be estimated.
Figure 4.
HHV8 antibody status and PCR positivity for HHV8 DNA in serum among HIV-seropositive (+) and HIV-seronegative (−) homosexual men.
HHV8 and HIV Seroconversion Rates.
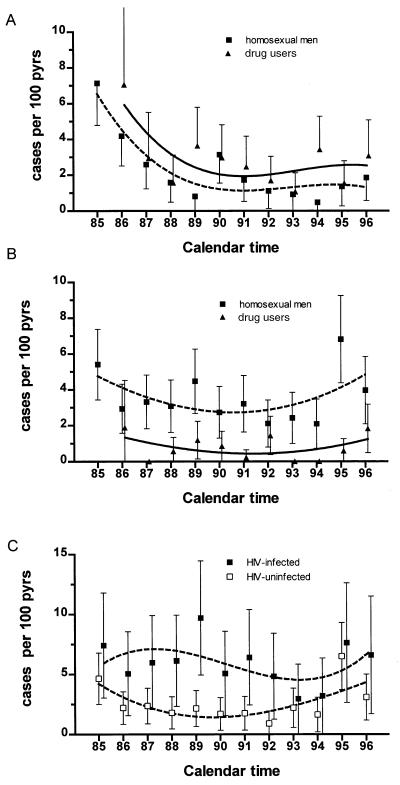
Within the Amsterdam Cohort Studies, the HIV seroconversion rate among homosexual men has dropped between 1984 and 1996 from 7.1 to 1.8 per 100 pyrs and among IV drug users between 1985 and 1996 from 7.1 to 3.1 per 100 pyrs (Fig. 5A). In contrast, the HHV8 seroconversion rate has remained relatively stable both for homosexual men, at 3.6 per 100 pyrs, and injecting drug users, at 0.7 per 100 pyrs (Fig. 5B). In the cohort of homosexual men, the HHV8 seroconversion rate among those with HIV infection is consistently higher (6.0 per 100 pyrs) than in those without HIV infection (2.6 per 100 pyrs) (Fig. 5C), but this difference is not seen among the men and women in the drug-using cohort. Survival analysis indicated that HIV seropositivity negatively influenced HHV8-free survival of homosexual men, but that CD4+ cell counts had no impact on the rate of HHV8 seroconversion, either in the HIV-seropositive or in the HIV-seronegative group. HIV-infected homosexual men with more than 15,850 HIV-1 RNA copies had no increased risk over men with low HIV-1 RNA copy numbers for seroconversion for HHV8 (relative risk = 0.96, 95% CI: 0.58–1.63; P = 0.91).
Figure 5.
Seroconversion rate (incidence) per 100 pyrs of HIV (A) and HHV8 (B) among homosexual men (■, dashed line) and drug users (▴, bold line). (C) HHV8 seroconversion rate per 100 person years among HIV-infected (■) and HIV-uninfected (□) homosexual men.
To compute the latest point in time at which the HHV8 could have been introduced in both the homosexual community and injecting drug users, an exponential survival curve was fitted for HIV-seronegative individuals, excluding the potential effect of HIV. We found the exponential curve, implying a constant hazard, justified, because the Weibull curve did not yield a better fit. We estimated the latest year of introduction by dividing the prevalence in a given year by the incidence rate. This is justified by the correspondence of the exponential distribution and the Poisson distribution (24). HHV8 was introduced in the community of homosexual men no later than 1956 (95% CI for 1951–1961), and for injecting drug users this was 1983 (95% CI for 1982–1984). However, it must be stressed that this is an approximation, as the prevalence is used, instead of an exact, true cumulative incidence. Thus, the denominator might be underestimated especially in cases in which a longer period exists between the introduction of the virus and start of the observation (calculated prevalence); for that reason, the estimate for the homosexual men should be regarded with caution.
HHV8 Seroconversion Rate and Sexual Behavior in the Course of the AIDS-KS Epidemic.
As we have reported previously (25), the one behavioral parameter associated with HHV8 seroconversion in the homosexual cohort was the number of partners with whom orogenital sex was practiced (P = 0.04), whereas no such association was found for anogenital or oroanal sex. The odds ratio for orogenital sex with more than five partners is 4.13 (95% CI: 2.34–7.31). When adjusted for HIV seropositivity, this figure increased in HIV-seronegative individuals to 5.95 (95% CI: 2.88–12.29), as was reported by Dukers et al. (25). In this study, the risk to seroconvert for HHV8 among HIV-seronegative individuals increased with the number of sexual partners with whom orogenital sex was practiced, whereas in HIV-infected men such a dose-response effect was not observed.
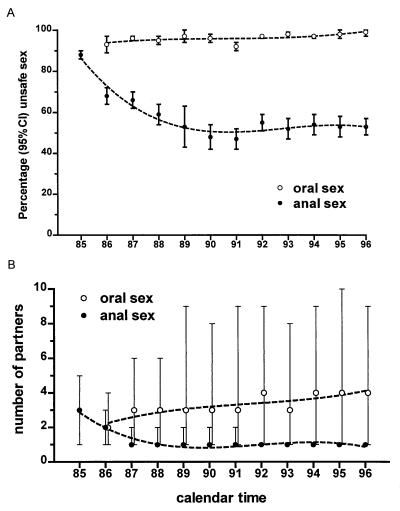
The dynamics of unprotected oral and unprotected anal sexual behavior was studied in the course of the epidemic (Fig. 6). Whereas the proportion of cohort participants reporting unprotected anogenital intercourse declined from 88% in 1985 to 57% in 1996, no decline was noted in the proportion of participants reporting unprotected oral sex (93% in 1986 vs. 98% in 1996) (Fig. 6A). Likewise, the number of partners with whom unprotected oral sex was practiced remained stable or increased slightly, whereas a negative trend was seen regarding partners in unprotected anal intercourse (Fig. 6B). In 1985, 50% practiced unprotected anal sex with three or more partners and 25% did so with five or more, whereas in 1996, 75% practiced unprotected anal sex with only a single partner or not at all. Unprotected orogenital sex was practiced by 50% with two or more people and was practiced by 25% with four or more in 1986 compared with 50% with four or more and 25% with nine or more people 10 years later.
Figure 6.
(A and B) Unprotected anogenital vs. orogenital sexual behavior over time in the Amsterdam Cohort of homosexual men. (A) Percentage of participants with at-risk contacts (95% CI). (B) Number of partners with whom at-risk behavior was practiced (median and interquartile ranges).
Discussion
In this study we sought to investigate events around the point of HHV8 seroconversion and to provide evidence that seroconversion reflects, in general, primary HHV8 infection. Because our EIA, based on two recombinant HHV8 proteins, has a sensitivity of 81.7%, it is likely that we missed some seroconverters in our retrospective study. In some cases, primary infection may have happened sometime before seroconversion, and two examples for this are shown in Fig. 4. However, in most seroconverters with detectable viremia (Fig. 4), virus was detectable close to the point of seroconversion. The overall persistence of HHV8 antibodies points to persistent antigen presentation even when HHV8 DNA cannot be detected in blood except in HHV8-seropositive individuals progressing to or diagnosed with KS. This suggests that the virus remains present somewhere in the body in a latent or lytic stage of replication, boosting the antibody levels continuously, as found previously in the case of the mouse gammaherpesvirus, MHV68 (26). We have demonstrated that HIV infection boosts HHV8 antibody levels, as reflected in S/C ratios, to both the lytic cycle capsid protein encoded by ORF65 and the latent nuclear antigen encoded by ORF73. Among HIV-infected individuals, CD4 cell decline and KS development are associated with a further rise in ORF65 antibodies, suggesting promotion of active virus replication. HHV8 antibody levels to ORF65/vp19 are higher in people developing KS compared with those with low CD4 levels developing an opportunistic infection (Fig. 3B). The reason that we do not see, on average, an increase in ORF73/LANA antibody titers after the development of KS development may be that the S/C ratios of ORF73/LANA antibodies are already boosted to their peak levels by HIV infection. This notion is corroborated by the observation that among HIV-infected individuals, HHV8 seroconversion tends to occur to ORF73/LANA before ORF65/vp19 (Fig. 2). Possible explanations include a lack of T cell-mediated control of ORF73/LANA-expressing, latently infected cells or a role of increased inflammatory cytokine levels in HIV-infected patients in increasing its expression. In patients with nasopharyngeal carcinoma, reactivation of Epstein–Barr virus is reflected by elevated titers of antibodies to both viral capsid and nuclear antigens that rise with severity of the disease (27); the same might be true for HHV8 during disease progression.
During primary HHV8 infection, the period of virus production before antibody development and the subsequent clearance of virus from the blood apparently takes only months instead of years. This relatively short window phase and rapid control of viremia is seen also in infections with MHV68 and other rhadinoviruses (28). Serum HHV8 viremia in HHV8-infected individuals is short-lived even during the acute phase of infection.
The low rate of HHV8 serum viremia seen in this study therefore may be explained at least partly by the fact that samples had been collected only in 3 monthly intervals. The level of viremia may be expected to be somewhat higher in peripheral blood mononuclear cells, although in our experience the copy numbers are still very low, i.e., about 10–100 copies/ml of plasma vs. 100–1,000 per 106 peripheral blood mononuclear cells in KS patients (29). This accords with recent data reported by LaDuca et al. (30).
Taken together, our data support the assumption that most, if not all, HHV8 seroconverters have primary infections. Based on this assumption, HHV8 is estimated to have been introduced in the Dutch homosexual community in about 1956. Because of the relatively low sensitivity of the assay (81.7%), an underestimation of the incidence as well as the prevalence might have occurred. An underestimated incidence results in an underestimation of the risk of HHV8 infection. This potential error together with the underestimation of the prevalence subsequently will be compensated in the estimation of the introduction time because this is a ratio of both underestimated figures. In a separate study (25), we demonstrated that HHV8 seroprevalence was higher in men of Southern European origin enrolled in our cohort. Dutch companies actively recruited Southern Europeans to the Netherlands in the 1950s. The first to immigrate, from 1949 on, were Italians (31). Only in the 1960s did substantial numbers of individuals immigrate from Spain, Portugal, Greece, and the former Yugoslavia (32). The earliest immigrants, who settled largely in Amsterdam, came from northern Italy and from Sardinia, an island that is noted for HHV8 endemicity (33–35).
The data reported here and in previous communications (25) strongly indicate that the stable HHV8 seroconversion rate among homosexual men participating in the Amsterdam Cohort Studies is related to the persistence of unprotected orogenital sex in this group. An earlier report on a cohort of Danish homosexual men (17) had reported a marked decrease in HHV8 incidence between 1981 and 1984, i.e., before the start of our cohort, which could explain this apparent difference. Our observations fit with the finding that the oropharynx serves as the source of HHV8, rather than the port of entry, taking into account the substantially higher HHV8 load in saliva compared with semen (30). The findings are reminiscent of Epstein–Barr virus, for which intimate oral contact with the exchange of saliva was first established as the major route of transmission by Hoagland (37) and confirmed by Evans (38). Additional evidence for the oral shedding of infectious virus comes from the animal rhadinovirus group. MHV68 reaches the highest titers in the respiratory tract and causes disease after experimental infection by the intranasal route but not by the i.v. route (39). The highest frequency of isolation of EHV2, another member of the rhadinovirus group, is from the bronchial lymph nodes as well as nasal swabs of naturally infected horses (40). Infectious virus was isolated from the lungs of mice experimentally infected with EHV2, but no infectious virus was found in their blood or lymphoid tissues (41). HVS is highly frequent among both captive and feral squirrel monkeys, in which virus was consistently isolated from oropharyngeal secretions (42).
However, transmission of HHV8 among homosexual men by oral sex obviously does not represent the usual route of HHV8 transmission in endemic countries, where most transmission appears to occur in childhood (43–46). In this setting, early studies suggest HHV8 transmission in families, perhaps from mother to child or among siblings (43–46). Such a route of transmission also could involve saliva, and the importance of oral sex among gay men reported here and previously (25) thus could represent an unusual way of saliva-mediated transmission. We conclude that most if not all cases of HHV8 seroconversion among HIV seronegatives reflect primary HHV8 infection from orogenital sex. Seroconversion appears to follow replication and seeding of HHV8 in a new host and result in rapid control of viremia. HIV-induced immunodeficiency may further enhance HHV8 replication, which boosts the antibodies to lytic antigen, an effect that is even more pronounced after development of KS, and also accelerates the appearance of antibodies to the latent nuclear antigen.
Acknowledgments
This study was performed as part of the Amsterdam Cohort Studies on AIDS, a collaboration between the Municipal Health Service, the Academic Medical Centre, and the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands. It was supported in part by the National Health Service Biomedical R&D fund (RDO22/09). Editorial review was provided by Lucy D. Phillips.
Abbreviations
- pyrs
person years
- HHV8
human herpes virus type 8
- KS
Kaposi's sarcoma
- MHV68
murine herpesvirus 68
- EIA
enzyme immunoassay
- S/C ratio
serum-over-cut-off ratio
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Desrosiers R C, Sasseville V G, Czajak S C, Zhang X, Mansfield K G, Kaur A, Johnson R P, Lackner A A, Jung J U. J Virol. 1997;71:9764–9769. doi: 10.1128/jvi.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose T M, Strand K B, Schultz E R, Schaefer G, Rankin G W J, Thouless M E, Tsai C C, Bosch M L. J Virol. 1997;71:4138–4144. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melendez L V, Daniel M D, Hunt R D, Garcia F G. Lab Anim Care. 1968;18:374–381. [PubMed] [Google Scholar]
- 5.Plummer G, Waterson A P. Virology. 1963;12:412–416. doi: 10.1016/0042-6822(63)90083-7. [DOI] [PubMed] [Google Scholar]
- 6.Blaskovic D, Stancekova M, Svobodova J, Mistrikova J. Acta Virol. 1980;24:468. [PubMed] [Google Scholar]
- 7.Desrosiers R C, Bakker A, Kamine J, Falk L A, Hunt R D, King N W. Science. 1985;228:184–187. doi: 10.1126/science.2983431. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 9.Said J W, Chien K, Takeuchi S, Tasaka T, Asou H, Cho S K, de Vos S, Cesarman E, Knowles D M, Koeffler H P. Blood. 1996;87:4937–4943. [PubMed] [Google Scholar]
- 10.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M-F, Clauvel J-P, Raphael M, Degos L, et al. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 11.Rickinson A B, Kieff E. In: Fields Virology. Fields B N, Howley P M, Knipe D M, editors. Philadelphia: Lippincott; 1996. pp. 2397–2446. [Google Scholar]
- 12.Martin J N, Ganem D E, Osmond D H, Page-Shafer K A, Macrae D, Kedes D H. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 13.Simpson G R, Schulz T F, Whitby D, Cook P M, Boshoff C, Rainbow L, Howard M R, Gao S-J, Bohenzky R A, Simmonds P, et al. Lancet. 1996;348:1133–1138. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- 14.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 15.Gao S J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 16.Gao S J, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo C R, Saah A, Phair J, et al. Nat Med. 1996;2:926–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 17.Melbye M, Cook P M, Hjalgrim H, Begtrup K, Simpson G R, Biggar R J, Ebbesen P, Schulz T F. Int J Cancer. 1998;77:543–548. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien T R, Kedes D, Ganem D, Macrae D R, Rosenberg P S, Molden J, Goedert J J. J Infect Dis. 1999;180:1010–1017. doi: 10.1086/315039. [DOI] [PubMed] [Google Scholar]
- 19.Renwick N, Halaby T, Weverling G J, Dukers N H T M, Simpson G R, Coutinho R A, Lange J M A, Schulz T F, Goudsmit J. AIDS. 1998;12:2481–2488. doi: 10.1097/00002030-199818000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S J, Stoiber H, Herrington C S, Moore P S, Schulz T F. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz T F. Adv Cancer Res. 1999;76:121–160. doi: 10.1016/s0065-230x(08)60775-7. [DOI] [PubMed] [Google Scholar]
- 22.Boom R, Sol C J, Salimans M M, Jansen C L, Wertheim-van Dillen P M, van der Noordaa J. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelissen M, Mulder-Kampinga G, Veenstra J, Zorgdrager F, Kuiken C, Hartman S, Dekker J, van der Hoek L, Sol C, Coutinho R, Goudsmit J. J Virol. 1995;69:1810–1818. doi: 10.1128/jvi.69.3.1810-1818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mood A M, Graybill F A, Boes D C. In: Introduction to the Theory of Statistics. Blackwell D, Solomon H, editors. New York: McGraw–Hill; 1974. pp. 114–122. [Google Scholar]
- 25.Dukers N H T M, Renwick N, Prins M, Geskus R B, Weverling G J, Coutinho R A, Goudsmit J. Am J Epidemiol. 2000;151:213–224. doi: 10.1093/oxfordjournals.aje.a010195. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson P G, Doherty P C. J Virol. 1998;72:943–949. doi: 10.1128/jvi.72.2.943-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Schryver A, Klein G, Henle G, Henle W, Cameron H M, Santesson L, Clifford P. Int J Cancer. 1972;9:353–364. doi: 10.1002/ijc.2910090214. [DOI] [PubMed] [Google Scholar]
- 28.Sarawar S R, Brooks J W, Cardin R D, Mehrpooya M, Doherty P C. Virology. 1998;249:359–366. doi: 10.1006/viro.1998.9309. [DOI] [PubMed] [Google Scholar]
- 29.Wit F W N M, Sol C J A, Renwick N, Roos M T L, Pals S T, van Leeuwen R, Goudsmit J, Reiss P. AIDS. 1997;12:218–219. [PubMed] [Google Scholar]
- 30.LaDuca J R, Love J L, Abbott L Z, Dube S, Friedman-Kien A E, Poiesz B J. J Infect Dis. 1998;178:1610–1615. doi: 10.1086/314514. [DOI] [PubMed] [Google Scholar]
- 31.Schneider M. Ph.D. dissertation. Amsterdam: Univ. of Amsterdam; 1984. [Google Scholar]
- 32.Lindo F, Pennings T. Zuideuropeanen in Nederland. Volksgezondheid en Cultuur: Ministerie van Welzijn; 1988. [Google Scholar]
- 33.Calabro M L, Sheldon J, Favero A, Simpson G R, Fiore J R, Gomes E, Angarano G, Chieco-Bianchi L, Schulz T F. J Hum Virol. 1998;3:207–213. [PubMed] [Google Scholar]
- 34.Angeloni A, Heston L, Uccini S, Sirianni M C, Cottoni F, Masala M V, Cerimele D, Lin S-F, Sun R, Rigsby M, et al. J Infect Dis. 1998;177:1715–1718. doi: 10.1086/517429. [DOI] [PubMed] [Google Scholar]
- 35.Whitby D, Luppi M, Barozzi P, Boshoff C, Weiss R A, Torelli G. J Natl Cancer Inst. 1998;90:395–397. doi: 10.1093/jnci/90.5.395. [DOI] [PubMed] [Google Scholar]
- 36.Beral V, Peterman T A, Berkelman R L, Jaffe H W. Lancet. 1990;335:123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 37.Hoagland R J. Am J Med Sci. 1955;229:262–272. doi: 10.1097/00000441-195503000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Evans A S. Am J Hyg. 1960;71:342–362. doi: 10.1093/oxfordjournals.aje.a120118. [DOI] [PubMed] [Google Scholar]
- 39.Sunil-Chandra N P, Efstathiou S, Arno J, Nash A A. J Gen Virol. 1992;73:2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 40.Palfi V, Belak S, Molnar T. Zbl Vet Med. 1978;25:165–167. doi: 10.1111/j.1439-0450.1978.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 41.Rizvi S M, Slater J D, Slade A J, Field H J. J Gen Virol. 1997;78:1119–1124. doi: 10.1099/0022-1317-78-5-1119. [DOI] [PubMed] [Google Scholar]
- 42.Falk L A, Nigida S, Deinhardt F, Cooper R W, Hernandez-Camacho J I. J Natl Cancer Inst. 1973;51:1987–1989. doi: 10.1093/jnci/51.6.1987. [DOI] [PubMed] [Google Scholar]
- 43.Mayama S, Cuevas L E, Sheldon J, Omar O H, Smith D H, Okong P, Silvel B, Hart C A, Schulz T F. Int J Cancer. 1998;77:817–820. doi: 10.1002/(sici)1097-0215(19980911)77:6<817::aid-ijc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 44.Sitas F, Newton R, Boshoff C. N Engl J Med. 1999;340:1923. doi: 10.1056/NEJM199906173402414. [DOI] [PubMed] [Google Scholar]
- 45.Gessain A, Mauclere P, van Beveren M, Plancoulaine S, Ayouba A, Essame-Oyono J L, Martin P M, de The G. Int J Cancer. 1999;81:189–192. doi: 10.1002/(sici)1097-0215(19990412)81:2<189::aid-ijc4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 46.Lyall E G, Patton G S, Sheldon J, Stainsby C, Mullen J, O'Shea S, Smith N A, De Ruiter A, McClure M O, Schulz T F. Pediatr Infect Dis J. 1999;18:795–799. doi: 10.1097/00006454-199909000-00010. [DOI] [PubMed] [Google Scholar]