Abstract
Background
Medical students in the course of their clinical work are at risk of acquiring hepatitis B virus (HBV) infection or transmitting it to their patients. HBV immunization for medical students in Uganda is recommended but not strictly enforced. It is important to assess the prevalence of HBV infection in medical students in order to improve on the interventions to control this infection among them.
Objectives
The objective of the study was to assess the seroprevalence rates of HBsAg and anti-HBc among clinical and pre-clinical medical students.
Methods
This was a cross-sectional study done over three months from November 2000 to January 2001 among Makerere University Medical students. A random sample of medical students was recruited from both the pre-clinical and clinical years. Blood samples from each participant were tested for HBsAg and anti-HBc.
Results
The overall prevalence was 11.0% for HBsAg and 65.9% for anti HBc. Nine pre-clinical students (12.2%) were positive for HBsAg compared to 11 (10.2%) clinical students. This difference was not statistically significant. However, clinical students were more likely to have been exposed to HBV with 86 (79.6%) testing positive for anti-HBc compared to 34 (45.9%) among pre-clinical students (p-value <001). Risk factors associated HBV infection included having a sexual relationship, accidental needlestick injuries, and unprotected exposure to patients' body fluids.
Conclusion
Medical students need to be offered more sensitization and support regarding prevention of HBV infection including vaccination and the use of universal precautions for infection control.
Introduction
HBV infection is endemic in East Africa 1. HBV immunisation is now part of the national routine immunisation programme for children in Uganda. HBV immunization in medical students and health workers in Uganda is recommended but not strictly enforced. As a result, individuals at high risk like healthcare workers and medical students have low immunisation rates. Therefore, HBV remains an occupational risk to which healthcare workers and medical students are exposed while at work.
Determining the prevalence of HBV infection in the medical students is important in planning for any intervention to control this infection among them. Furthermore, the information obtained may be used in a wider sense to create awareness among all categories of healthcare workers about the magnitude of the risk of contracting or transmitting HBV in the workplace.
The main objective of the study was to determine the prevalence of HBsAg and anti-HBc among the medical students. The other objectives were to compare the prevalence rates of HBsAg and anti-HBc between the pre-clinical and clinical medical students and to assess risk factors associated with presence of HBsAg and anti HBc among the students.
Methods
This was a descriptive -cross-sectional study carried out among medical students in Makerere Medical School over a period of three months, from November 2000 to January 2001. They were recruited from both the pre-clinical years (that is years I and II) and the clinical years (years III, IV and V). The study was approved by the Department of Medicine, the Faculty Research and Ethics Committee and the Dean of Makerere Medical School.
Students studying for the Bachelor of Medicine and Bachelor of Surgery (MBChB) or Bachelor of Dental Surgery (BDS) were approached and those who gave informed consent were recruited. Those who joined the medical school as mature entrants and were already employed in the paramedical or nursing fields were excluded as well as those studying for the pharmacy or nursing degree. Stratified random sampling was used. The estimate for the sample size was 170 students. However, a total of 182 medical students were eventually recruited, (74 pre-clinical and 108 clinical). Each student filled in a structured questionnaire covering demographic characteristics, medical history and risk factors to getting infected with HBV. Each student then underwent a venipuncture . Blood thus taken was analysed for HBsAg and anti-HBc using ELISA test kits sourced from Human Diagnostics®, Wiesbaden, Germany. The data initially recorded on the questionnaire forms were later entered on EPI-INFO 6 for analysis.
Informed written consent was obtained from each student. Before enrolment, the principal investigator (Bongomin Pido) organised two seminars and explained issues about hepatitis B, including its complications, management and prevention. Participation was completely voluntary and students who tested positive for HBsAg were individually counselled by the principal investigator. Those who tested negative for anti-HBc were counselled about measures to prevent exposure and the need to get vaccination against Hepatitis B as soon as possible.
Results
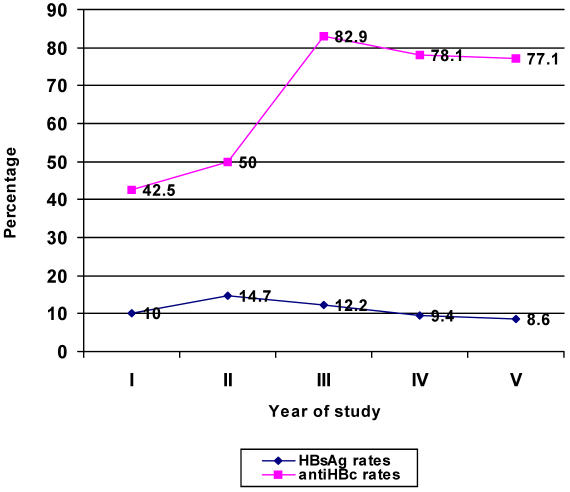
Socio-demographic characteristics of the study subjects are shown in table 1. No student was below 18 years of age and the oldest was 39 years old. There were 76 females and 106 males, giving a female to male ratio of 1:1.4. The majority of the students were from the central and western regions of Uganda and only 10 were foreign students. Of the 182 students tested, 20 were positive for HBsAg, giving an overall prevalence rate of 11.0%. This represents the proportion of students with current infection. There were 120 students positive for anti-HBc, putting the overall prevalence of past infection to HBV at 65.9%. When seroprevalence rates were compared between clinical and pre-clinical students, the difference in rates of current infection was not statistically significant (p-value = 0.859). However, significantly more clinical students had been exposed to HBV than pre-clinical students (p-value < 0.001). This is depicted in table 2. Figure 1 shows the trend of HBV seroprevalence according to the year of study. Current infection (positive HBsAg) rates were similar in all the years but evidence of past exposure to HBV (positive anti-HBc) sharply rose in the clinical years of study.
Table 1.
Comparison of socio-demographic characteristics of study subjects
| Variable | Pre-Clinical N=74 | Clinical N=108 | ||
| Number | Percentage | Number | Percentage | |
| Age distribution | ||||
| 19 – 20 | 30 | 40.5 | 3 | 2.8 |
| 21 – 22 | 28 | 37.8 | 35 | 32.4 |
| 23 – 24 | 4 | 5.4 | 41 | 38.0 |
| >24 | 12 | 16.2 | 29 | 26.9 |
| Sex | ||||
| Male | 40 | 54.1 | 66 | 61.5 |
| Female | 34 | 45.9 | 42 | 38.9 |
| Region | ||||
| Northern | 14 | 18.9 | 15 | 13.9 |
| Central | 21 | 28.4 | 37 | 34.3 |
| Eastern | 15 | 20.3 | 21 | 19.4 |
| Western | 21 | 28.4 | 28 | 25.9 |
| Foreign | 3 | 4.1 | 7 | 6.9 |
Table 2.
Prevalence rates of HBV markers among pre-clinical and clinical students
| Marker | Pre-clinical N=74 Number (%) |
Clinical N=108 Number(%) |
Odd Ratios (95% CI) |
p-value |
| HBsAg | 9 (12.2) | 11(10.2) | 1.22 (0.42–3.44) | 0.859 |
| Anti-HBc | 34 (45.9) | 86(79.6) | 0.22 (0.11–0.44) | <0.001 |
Figure 1.
Trend of HBV sero-prevalence rates according to year of study
As shown in tables 3, clinical students were significantly more likely to have a sexual relationship than their pre-clinical counterparts. Furthermore, significantly more clinical students had a history of either accidental needlestick injuries or unprotected exposure to patients' body fluids compared to pre-clinical students. Over 90% of the students had no history of prior hepatitis B immunisation.
Table 3.
Baseline risk factors for HBV infection among the students
| Year of study | Pre-clinical N=74 Number (%) |
Clinical N=108 Number(%) |
Odd Ratios (95% CI) |
p-value |
| Having a sexual relationship |
24 (32.4) | 52 (48.1) | 0.52 (0.27–1.00) | 0.035 |
| History of STDs | 7 (9.6) | 3 (2.8) | 3.66 (0.79–22.51) | 0.105 |
| History of blood transfusion |
4 (5.4) | 7 (6.6) | 0.82 (0.17–3.39) | 0.988 |
| History of surgery | 37 (50.7) | 67 (65.7) | 0.61 (0.32–1.16) | 0.066 |
| History of injections | 64 (86.5) | 96 (88.9) | 0.80 (0.30–2.20) | 0.797 |
| Not immunized for HBV | 72 (98.6) | 101(93.5) | 2.50 (0.46–25.19) | 0.203 |
| History of unprotected exposure to body fluids /needlestick injury |
11 (14.9) | 82 (75.9) | 0.06 (0.02–0.13) | <0.001 |
Among the students with current infection (positive HBsAg), those from the Central region, had the lowest prevalence rate of 8.6%. By comparison, students from the Northern parts of Uganda had the highest HBsAg prevalence rate of 17.2%. Students from Western Uganda had HBsAg rates of 12.2% while those from the East had 11.1%.
Table 4 shows the risk factors that were associated with current infection. Having a sexual relationship, unprotected exposure to patients' body fluids and needlestick injuries were significantly associated with HBsAg carriage.
Table 4.
Risk factors for HBV infection among students with current infection (positive HBsAg)
| Variable | Pre-clinical N=74 Number (%) |
Clinical N=108 Number(%) |
Percentage | Odd Ratios (95% CI) |
p-value |
| Having a sexual relationship | |||||
| Yes | 76 | 16 | 21.1 | ||
| No | 106 | 4 | 3.8 | 6.8 (2.04–28.95) | 0.0002 |
| History of an STD | |||||
| Yes | 10 | 3 | 30 | ||
| No | 172 | 16 | 9.4 | 4.18 (0.63–20.44) | 0.074 |
| History of blood transfusion | |||||
| Yes | 11 | 1 | 9.1 | ||
| No | 171 | 19 | 11.2 | 0.8 (0.02–6.22) | 1.000 |
| History of Surgery | |||||
| Yes | 104 | 11 | 10.6 | ||
| No | 71 | 9 | 12.7 | 0.81 (0.29–2.37) | 0.668 |
| History of injections | |||||
| Yes | 160 | 17 | 10.6 | ||
| No | 22 | 3.0 | 13.6 | 0.75 (0.19–4.39) | 0.952 |
| History of HBV immunization |
|||||
| Yes | 8 | 2 | 25.0 | ||
| No | 174 | 18 | 10.4 | 2.89 (0.26–17.65) | 0.216 |
| History of unprotected body fluids exposure/needlestick injury |
|||||
| Yes | 93 | 16 | 15.1 | ||
| No | 89 | 4 | 6.74 | 4.42(1.34–18.80) | 0.006 |
Among students with past infection (positive anti-HBc), the highest exposure rate of 82.8% was seen among the students from the north of the country while those from the central region had the lowest rate of 44.8%. Risk factors significantly associated with past exposure to HBV were similar to those for current HBV infection. These include having a sexual relationship, unprotected exposure to patients' body fluids and needlestick injuries (Table 5).
Table 5.
Risk factors for HBV infection among students with past infection (anti-HBc positive)
| Variable | Pre-clinical N=74 Number (%) |
Clinical N=108 Number(%) |
Percentage | Odd Ratios (95% CI) |
p-value |
| Having a sexual relationship | |||||
| Yes | 76 | 72 | 94.7 | ||
| No | 106 | 48 | 45.3 | 21.8 (7.23–86.48) | <0.001 |
| History of an STD | |||||
| Yes | 10 | 6 | 60.0 | ||
| No | 172 | 113 | 66.5 | 0.78 (0.18–3.93) | 0.979 |
| History of blood transfusion | |||||
| Yes | 11 | 5 | 45.5 | ||
| No | 171 | 114 | 67.5 | 0.42 (0.10–1.72) | 0.268 |
| History of Surgery | |||||
| Yes | 104 | 69 | 66.3 | ||
| No | 71 | 45 | 63.4 | 1.14 (0.57–2.24) | 0.808 |
| History of injections | |||||
| Yes | 160 | 104 | 65.0 | ||
| No | 22 | 16 | 72.2 | 0.70 (0.21–2.01) | 0.633 |
| History of HBV immunization |
|||||
| Yes | 8 | 4 | 50.0 | ||
| No | 174 | 114 | 65.9 | 0.53 (0.09–2.94) | 0.602 |
| History of unprotected body exposure/needlestick injury |
|||||
| Yes | 93 | 72 | 77.4 | ||
| No | 89 | 48 | 53.9 | 2.93 (1.48–5.87) | <0.001 |
Discussion
The overall prevalence rate of HBsAg was 11.0% among the medical students. Lule found the HBsAg carrier rate of 18% among medical students in Kenyatta National Hospital 1. In Nigeria2, Olubuyide found the HBsAg carrier rate of 39.0% among doctors and dentists compared to the national average of 20.0%.
There was no significant difference in the HBsAg carrier rates between pre-clinical and clinical students and yet the latter were more exposed to hepatitis B. Similar findings were observed by Khurana. in Maulana Azad Medical College-New Delhi, India 3. It is possible that most of the clinical students were healthy and fought off the hepatitis B infection despite being more exposed. It is known that spontaneous recovery after acute infection with HBV occurs in 95–99% of previously healthy adults 4. It is also possible that some students might have got occult HBV infection. This could only be revealed by performing highly sensitive molecular techniques which would show persistence of HBV genomes in HBsAg negative individuals 5. The magnitude of occult HBV infection was not assessed in the study.
There are several possible reasons why clinical students were more exposed to HBV. Firstly, they reported significantly more unprotected exposure to patients' body fluids and needlestick injuries. Indeed among the clinical students, those in the third year of study had the highest rates of past exposure to HBV (anti-HBc positive). This group represents students joining the wards for the first time and are inexperienced and therefore prone to getting accidents such as needlestick injuries while doing invasive procedures 6. Secondly, clinical students were more sexually active than the pre-clinical group and this could have contributed to their higher anti-HBc rate observed.
Pre-clinical students joined medical school when a sizeable proportion (45.9%) had already been exposed to hepatitis B infection. Effective prevention of hepatitis B infection should therefore be targeted to children much earlier before they join medical school. In the meantime medical students should be encouraged and supported to take all precautions necessary to prevent and control hepatitis B infection, especially before beginning clinical rotations 7,8. These include vaccination and the use of universal precautions for infection control. Mechanisms for enforcement and follow up of infection control among medical students need to be strengthened.
Acknowledgments
We are very grateful to the Dean, Makerere University Medical School for permission to carry out this study. We thank Dr. Moses Kamya for advice and support in this study. We would like to thank the students who kindly accepted to participate in the study. Part of the funding for this study was provided by Smith-Kline Beecham to cover the costs of the ELISA test kits.
References
- 1.Lule G N, Okoth F, Ogutu E O, Mwai S J. HBV markers (HBsAg, anti-HBc, anti-HBs) among 160 medical students at Kenyatta National Hospital. East Afr Med J. 1989;66(5):315–318. [PubMed] [Google Scholar]
- 2.Olubuyide I O, Ola S O, Aliyu B, Dosumu O O, Arotiba J T, Olaleye O A, et al. Prevalence and epidemiological characteristics of hepatitis B and C infections among doctors and dentists in Nigeria. East Afr Med J. 1997;74:357–361. [PubMed] [Google Scholar]
- 3.Khurana V, Kar P, Mansharamani N, Jain V, Kanodia A. Differences in hepatitis B markers between clinical and pre-clinical healthcare personnel. Trop Gastroenterol. 1997;18(2):69–71. [PubMed] [Google Scholar]
- 4.Heathcote J, Elawaut A, Fedail S, et al., editors. Management of Acute viral hepatitis: http//www.omge.org. 2003. Dec, OMGE Practice guidelines. [Google Scholar]
- 5.Raimondo G, Pollicino J, Squadrito G. What is the clinical impact of occult hepatitis B virus infection? Lancet. 2005;365:638–639. doi: 10.1016/S0140-6736(05)17961-6. [DOI] [PubMed] [Google Scholar]
- 6.Riddell L A, Sherrard J. Blood borne virus infection: the occupational risks. Int J STD-AIDS. 2000;11(10):632–639. doi: 10.1258/0956462001914986. [DOI] [PubMed] [Google Scholar]
- 7.Hurley J L, Turner H S, Butler K M. Planning and execution of a successful hepatitis B immunisation programme. J Am Coll Health. 2001;49(4):189–191. doi: 10.1080/07448480109596302. [DOI] [PubMed] [Google Scholar]
- 8.Bonanni P, Bonaccorsi G. Vaccination against hepatitis B in healthcare workers. Vaccine. 2001;19(17–19):2389–2394. doi: 10.1016/s0264-410x(00)00460-6. [DOI] [PubMed] [Google Scholar]