Abstract
Introduction
The tuberculin skin test is one of the most valuable tests for demonstrating tuberculous infection in both symptomatic and asymptomatic children. However, its application is often undermined by difficulties in interpretation of results arising from its low sensitivity and specificity.
Objectives
This review aimed to use the concept of induration distribution analysis to estimate the induration size demarcating positive from negative results in a group of children with suspected tuberculosis, and to compare this cut-off with available guidelines for interpretation of the Mantoux test in the diagnosis of tuberculosis in children.
Methods
The results of Mantoux tests of children presenting with suspected tuberculosis over a 12-month period were retrospectively reviewed and plotted on a frequency distribution curve. The distribution was used to define a demarcation between positive and negative reactions. The resultant cut-off was compared with currently published guidelines for interpretation of the Mantoux test.
Results
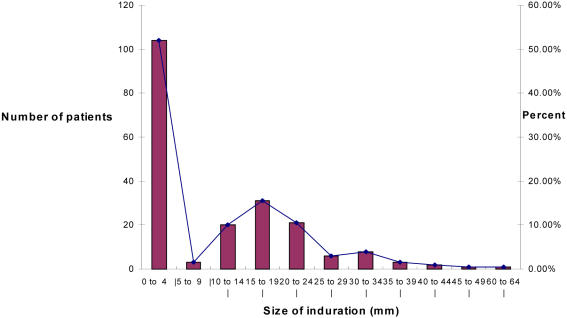
Two hundred (200) Mantoux results were analysed out of 202 records reviewed. Induration sizes ranged from 0 to 60 mm, with a mean of 9.4 mm. The induration distribution showed a bimodal pattern, with 103 patients showing no reaction (0 mm), and 96 (48%) patients with an induration size of 3 5 mm, with the second mode at 15–19 mm. The demarcating antimode was at 5 mm.
Conclusions
The induration distribution showed that a cut-off induration size of 5 mm was appropriate for this group of patients. This was in agreement with currently published guidelines for the interpretation of the Mantoux test in the diagnosis of tuberculosis in children.
Keywords: Mantoux, induration distribution, tuberculosis, children
Introduction
Tuberculosis remains a leading cause of morbidity and mortality in the world especially in developing countries. A combination of factors including high costs, limited resources and the poor performance of various diagnostic tests make the diagnosis of tuberculosis in children difficult in the developing countries. The problem is compounded by the frequent co-infection with HIV in children suspected for tuberculosis, which affects both the clinical presentation as well as the interpretation of the common diagnostic tests.1, 2 Despite this, the number of children treated for tuberculosis has been shown to be on the increase.3, 4
Short of demonstrating viable organisms in body tissues and fluids the tuberculin skin test is the only method of detecting M. tuberculosis infection in an individual, and is used in the diagnosis of tuberculosis in individual patients, as well as in epidemiological settings, to measure the prevalence of tuberculous infection in populations.5 However, various factors both in the host and inherent in the test lower both its specificity and sensitivity.6, 7 Consequently, its application in any group of patients will usually yield a wide range of results, from the presence of a reaction in un-infected children to the complete absence of a reaction in some children with confirmed TB disease.8, 9 The distribution of results generally falls into one of two patterns depending on the rate of false-positive (cross-reactions from other mycobacterial infections) in the population.6
For the test to be useful in the clinical setting, an appropriate cut-off needs to be defined to demarcate positive and negative reactions.
We aimed to examine the distribution of reactions to the standard Mantoux test in a group of children with suspected tuberculous disease, to use this to estimate a useful cut-off, and to examine how this compares with current guidelines for the interpretation of the result of Mantoux testing.
Methods
Over a period of 12 months (July 2001 – June 2002), children with suspected tuberculous disease were registered, investigated and treated in the routine children's TB clinic. Suspected TB was defined according to standard guidelines, and included both children with symptoms and signs suspicious of TB as well as those investigated as household contacts of adult TB patients.10
Mantoux testing was performed using 5 TU of tuberculin PPD RT23. Three attending doctors who were responsible for the care of the children did the testing. Results were read between 48 and 72 hours and recorded as the transverse diameter (in millimetres) of palpable induration. For purposes of diagnosis and treatment indurations of 5 mm or more were interpreted as positive, regardless of nutritional status, history of BCG vaccination and HIV status where it was known.
The nutritional status at the time of investigation was recorded as the weight-for-age standard deviation (Z-) score.
Analysis
Data were entered into a computer and analysed using the Epi Info version 6.04 statistical analysis package. Induration sizes, grouped in a range of 5 mm, were plotted on a frequency distribution graph. A curve was superimposed onto the graph and the modes and antimode identified. The antimode was taken to represent the demarcation between negative and positive results.
The nutritional status of the group, presented as Z-scores, was plotted on a distribution curve allowing comparison with a National Centre for Health Statistics (NCHS) standard reference population.
The analysis of this data was retrospective and did not affect the treatment of this particular group of patients.
Results
Over the 12-month period reviewed 202 patients were registered for treatment for tuberculosis treatment. Two hundred (200) of these had results of Mantoux tests recorded. 104 patients were male and 96 female.
Age distribution
The age distribution of the patients is shown in figure 1. Patients ranged from 2 months to 12 years, with a mean age of 46.3 months (S.D. = 40.8).
Figure 1.
Age distribution of patients
Mantoux results
Two hundred patients had results of Mantoux tests recorded. Results ranged from 0 to 60 mm, with a mean of 9.4 mm. The majority (103 patients) had no detectable induration reaction (recorded as 0 mm). Positive reactions (≥ 5 mm) were recorded in 96 (48%) patients.
Figure 2 shows a graphic presentation of the frequency distribution of Mantoux results. The distribution shows a bi-modal pattern with the primary mode at 0 mm and a second mode at 15–19 mm. The antimode between the two modes is at 5–9 mm. From this distribution the 5 mm point demarcated the negative from the positive reactions.
Figure 2.
Frequency distribution of M antoux results
Weight statistics
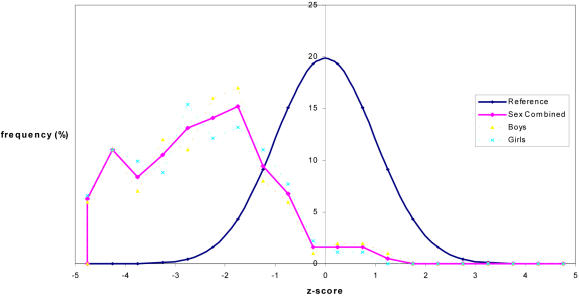
The distribution of weight-for-age Z-scores in relation to a reference population (NCHS) is shown in figure 3.
Figure 3.
Weight-for-age z-score distribution for study patients
The z-scores ranged from -5.70 to +4.91, with a median of - 2.45.
The vast majority of patients (63%) had a weight-for-age Z-score less than - 2.00.
The nutritional status, expressed as the weight-for-age Z score, was not directly (linearly) related to the size of reaction to tuberculin (r = 0.27, r2 = 0.07, confidence interval - 0.07 to 0.21). However patients with a Z-score < -2.00 were significantly more likely to have a negative reaction (78/126 vs. 26/74, rel. risk 1.76, 95% CI 1.26 – 2.47).
Discussion
A correctly applied Mantoux test can be invaluable in the assessment of a child with suspected tuberculosis. The interpretation of the result, however, is often difficult, with different workers using different induration sizes to indicate a positive reaction.9, 12, 13
Various guidelines have been developed to improve the predictive value of the test by recommending varying the cut-off induration size with the perceived epidemiologic risk for true tuberculous infection in each individual (table 1).14,15,16, 17 However, it is not always practical for the practitioner to estimate a different level of such risk for each individual patient.
Table 1.
Cut-off size of reaction for a positive Mantoux test
| ≥5 mm | ≥10 mm | ≥15 mm |
| Children in close contact with patients with infectious tuberculosis |
Children with increased risk of disseminated disease |
Children with no risk factors |
| Children with clinical evidence of possible tuberculosis |
Children with increased risk of exposure to tuberculosis |
|
| Children with radiological features suggestive of previous or active tuberculosis |
||
| Children with immune-suppressive conditions (including HIV) |
* Source 14
The utility of the tuberculin skin test depends on the prevalence of tuberculosis infection in the particular group on the one hand and the relative prevalence of cross-reactions from other mycobacterial infections on the other. A group of patients with suspected clinical tuberculosis (as opposed to a population survey, for instance) would inevitably have a high prevalence of true M. tuberculosis infection and thus a low risk of false positive reactions. Therefore, although the test itself is neither 100% sensitive nor 100% specific, the predictive value of a positive reaction is very high in such a group.8
The technique of induration distribution analysis is more often applied to community surveys seeking to estimate the prevalence of tuberculosis infection in populations. It is recognised that when applied to patient groups, the distribution is similar to that obtained in populations with a low rate of false-positives (i.e. the test is highly specific).8 In both cases the results usually show a bi-modal distribution with a clear anti-mode demarcating positive from negative reactions. If such a distinct anti-mode is present it may be used as a rational cut-off for that particular group.5, 8
Results from this group of patients showed the expected bi-modal distribution, with a large proportion having no or mild reactions (0–4 mm) and the rest having stronger, more clearly positive reactions ranging from 5 to 60 mm. The anti-mode was at 5 mm indicating that this would be a reasonable cut-off for this patient group.
All the patients, having initially presented with clinical features suspicious of possible TB or having had recent contact with a case of active TB, would have been considered to have a high risk for tuberculosis infection. Furthermore, most patients had additional risk factors such as malnutrition and HIV infection. Most children in this group were significantly malnourished, and although HIV status was not specifically recorded for this study, previous studies have shown that the majority of them would be expected to be HIV infected.3, 18 Therefore, according to the common guidelines they would all be in category 1 (see table 1), and a reaction of ≥5 mm would have been considered positive. It is, therefore, interesting to find that the results of the induration distribution analysis appear to agree with the current guidelines.
Malnutrition has previously been shown to affect the results of tuberculin testing. As in other studies, underweight children in this study were significantly more likely to have a negative Mantoux result. 2, 11, 18
As in many other studies, the majority of children did not have any reaction to tuberculin despite having received BCG immunisation soon after birth. The reasons for this are not always clear but clearly whatever tuberculin sensitivity BCG might have caused could not have been significant or persistent. This agrees with the current recommendation that for patients with a high risk for tuberculosis the history of BCG vaccination should not be a consideration in the interpretation of the tuberculin test.14
References
- 1.Chintu C, Bhat G, Luo C, et al. Seroprevalence of human immunodeficiency virus type 1 infection in Zambian children with tuberculosis. Pediatr Infect Dis J. 1993;12:499–504. doi: 10.1097/00006454-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Kiwanuka J, Graham SM, Coulter JBS, et al. Diagnosis of pulmonary tuberculosis in children in an HIV-endemic area, Malawi. Ann Trop Paediatr. 2001;21:5–14. [PubMed] [Google Scholar]
- 3.Raviglione MC, Snider DE, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 4.Narain JP, Raviglinone MC, Kochi A. HIV-associated tuberculosis in developing countries; epidemiology and strategies for prevention. Tuber Lung Dis. 1992;73:311–321. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 5.Arnadottir T, Rieder HL, Trebucq A, Waaler HT. Guidelines for conducting tuberculin skin test surveys in high prevalence countries. Tubercle and Lung Disease. 1996;(77 Suppl):1–20. doi: 10.1016/s0962-8479(96)90127-6. [DOI] [PubMed] [Google Scholar]
- 6.Sepulvida RL, Ferrer X, Latrach C. The influence of Calmette-Guerin bacillus immunization on the booster effect of tuberculin testing in healthy young adults. Am Rev Respir Dis. 1990;142:24–28. doi: 10.1164/ajrccm/142.1.24. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society, author. The tuberculin skin test. Am Rev Respir Dis. 1981;124:356–363. [Google Scholar]
- 8.Huebner RE, Schein MF, Bass JB. The Tuberculin Skin Test. Clin Infect Dis. 1993;17:968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 9.Steiner P, Rao M, Victoria MS, Jabbar H, Steiner M. Persistently negative tuberculin reactions. Am J Dis Child. 1980;134:747–750. doi: 10.1001/archpedi.1980.02130200017007. [DOI] [PubMed] [Google Scholar]