Abstract
Background
This study was to assess malaria prevalence in relation to rainfall pattern in different localities of Entebbe Municipality, Uganda. A cross sectional study was conducted amongst the local community residing in the area from January 2003 to January 2004 to investigate the incidence and intensity of malaria infection.
Methods
Thick and thin blood smears were made from each patient with fever (body temperature ≥ 37.5°C). The slides were examined microscopically for malaria parasites. A total of 616 residents aged 2 to 50 years were registered in the study by name, age, sex and residential location. Spearman correlation coefficient (r) was used to evaluate relationship between parasite density, age and body temperature on one hand and rainfall plus parasite density on the other hand.
Results
A direct relationship was observed between malaria transmission and monthly rainfall in Entebbe Municipality. About 69.8% of the patients had fever. Parasite density fluctuated according to monthly rainfall pattern. Two peaks of high parasite density was observed, each peak coinciding with a peak rainfall pattern of the bimodal annual rain seasons. There was a negative but significant correlation (r = -0.09271; p < 0.0214) between parasite density and age, suggesting that mature individuals clear parasites more effectively than children. Furthermore, we observed a bimodal peak of mean parasite density in children and adults; peak in children is higher than of adults, each peak coinciding with rainfall pattern. There is also a significant positive correlation between parasite density and body temperature (r = 0.1927; p = 0.0001). However, there is no significant variation in mean parasite densities in the different locations of Entebbe Municipality.
Conclusions
Our study confirms rainfall pattern and age influence parasite density and are important determinants of malaria infection and transmission in Entebbe Municipality. Increased parasite density in children is a useful indicator for monitoring intensity of infection. This information is valuable in policy formulation for control of malaria during periods of intense transmission.
Introduction
The occurrence of a major malaria disease is closely related to naturally existing environmental and climatic conditions. The incidence, severity and distribution of malaria is also affected substantially by human activities such water and agricultural developments and by urbanization. Estimates indicate that 90% of the global burden of malaria is attributable to environmental factors1. Recent projections on climatic changes like global warming, predict that there will be a significant increase in the territories where malaria can occur e.g. the El Nino weather phenomenon of 1997–1998 was associated with trends of both extensive flooding and severe droughts, as well as an increase in risk of malaria epidemics in semi-arid areas like Punjab and Southwestern Sri Lanka. The most pronounced effects of climate on vector-borne diseases such as malaria would undoubtedly occur in places where the disease is newly introduced and are at the edges of the vector range, and where the population have built little immunity against malaria2. In Africa this will be at higher altitudes that are formerly too cold to support malaria. Increasing numbers of malaria cases have already been reported in the highlands of Madagascar, Ethiopia and Kabale/Kisoro in Uganda as a result of climatic changes; in Rwanda, record high temperatures and rainfall experienced in 1987 brought malaria into the highlands where local residents had no immunity. These incidences have led public health officials to fear that relatively small increases in temperature from global worming could spread malaria into large urban centers such as Nairobi in Kenya and Harare in Zimbabwe, that currently lie just outside of the malaria range. Under a similar scenario, malaria could spread into large swaths of the temperate zone where populations now lack resistance3.
Several factors known to influence the incidence, transmission, infection intensity, and vector distributions in an area are known and have been described. The effect of recent changes in climatic and environmental conditions experienced in many malaria endemic countries, could affect disease incidences, prevalence, severity, transmission, and infection intensity amongst others. These aspects have not been sufficiently studied; in this study we investigated the malaria prevalence and intensity of infection in residents of eleven villages of Entebbe peninsula. We further, correlated common malaria indicators such as parasite density and fever to age/age groups of residents and to monthly rainfall, to get an insight of the peak period and time of occurrence of peak malaria transmission in the peninsula. The study aims at underlining some of the important indicators that could be useful for public health monitoring of malaria infection intensity.
Severe malaria caused by Plasmodium falciparum infection may occur as a result of delay in treating uncomplicated falciparum malaria. In children, severe malaria may develop very rapidly. Recognition and prompt treatment of uncomplicated P. falciparum malaria is therefore of vital importance in averting incidence of severe malaria. A patient with severe malaria may present with confusion or drowsiness with prostration. In addition, the patient may develop other symptoms such as cerebral malaria, generalized convulsions, severe anemia, hypoglycemia, acidosis, high fever, jaundice and hyper-parasitemia4,5,6,7. This severe manifestation may occur singly as a result of heavy parasitemia or more commonly in combination in some patients. Pregnant women constitute a special group with specific symptoms not expected in an immune adult due to lowered immunity coupled with parasitization of the placenta. In high transmission areas, the risk of severe falciparum malaria developing is greatest among young children and visitors of any age coming from non-endemic areas. In non-transmission and low-transmission areas the risk of developing severe malaria is greatest among travelers returning with undiagnosed malaria infection from areas where P. falciparum transmission has occurred7.
Clinical manifestation of the symptoms of malaria is a result of parasitization and destruction of the red blood cells. The developmental stages of the parasite in the liver, or its persistence as hypnozoites, mainly in P. vivax and P. ovale, do not produce any symptoms8.
Initial symptoms of the disease are quite variable, particularly in children, and may include irregular fever, general malaise, headache, muscular pains, sweats, chills, nausea, vomiting, and sometimes diarrhea. If untreated, the fever may progress to periodic bouts alternating with less or no fever. The fever paroxysms depends on the species of the malaria parasite and goes through three stages of: cold shivering rigor, hot burning fever with dry skin temperatures reaching up to 40–42°C, intense sweating and lowering of body temperature. In P. vivax and P. ovale the cycle of fever is referred to as tertian while in P. malariae it is quartan and P. falciparum malignant. Plasmodium vivax and P. ovale selectively invade young erythrocytes and P. malariae selects the old erythrocytes, while P. falciparum in discriminatively invades any red blood cell. The untreated acute attack of P. falciparum is shorter than that of P. vivax, in fatal cases death often happens 2–3 weeks, although in some cases it may occur as early as 2–3 days after onset of symptoms. Repeated infection gives rise to immune responses of the host, which eventually controls the disease and the infection.
Most anti-malarial drugs are effective against erythrocytic stage of the parasites, but not against the hyponozoites in the liver and gametocytes in the blood9, so that while P. falciparum and P. malariae could be fully cured, P. vivax and P. ovale may produce true relapses by new invasion of the blood from latent hypnozoites, even after complete clearance of parasites from the blood. The elimination of hyponozoites and gametocytes requires long treatment periods (14 days or more) using primaquine or related drugs. Any untreated or incompletely treated infections will therefore produce several recrudescences. In the absence of reinfection, untreated P. falciparum may persist for 1–2 years, P. vivax for 3–4 years and P. malariae recrudescence up to 52 years 10.
Material and Methods
We collected a total of 616 blood samples from consenting residents of both sexes and aged between 2 to 50 years residing in different locations of Entebbe Municipality from January 2003 to January 2004. The residents in this study were registered by name, age, sex and residential location in the Municipality. Patients who presented with fever (i.e. body temperatures = 37.5°C, WHO Scale) from the eleven different locations of Entebbe Municipality were recorded and blood samples were taken from them through the fingertips using sterile disposable lancets. In the case of infants, blood was taken from the heels or toes. The patients (mostly malaria patients) were either referred to our Laboratory from other Private Health Care facilities within Entebbe or came directly to our center for routine medical examinations. Our center is a Registered Clinical Diagnostic Laboratory located at Kitoro New Taxi Park in Entebbe Municipality. The center served as a field station for screening and for all laboratory analysis. For each blood sample taken, three smears both thin and thick were made on microscope slides. The slides were air dried and accordingly stained. Thin smears were fixed in absolute methanol (99%), and stained with 30% Geimsa stain, for 10 minutes. Thick smears were stained in diluted 10% Geimsa stain without fixing. The slides were examined by a trained and qualified microscopist using binocular microscope (LEITZ Carl ZEST + S GS, GERMANY) and examined using x100 objective and x10 eyepiece. The thin smears were used to identify the species of the malaria parasite while the thick smears were used to estimate the parasite density per microliter of blood. Two hundred leukocytes plus the number of malaria parasites seen in the same field were recorded. The number of malaria parasites per microliter (parasite/µL) of blood was expressed as the reciprocal of the mean counts in the three slides divided by the leukocyte counts, multiplied by a factor of 8000 i.e.
Ethics
This study was conducted in conformity to the Helsinki Declaration of 1975, which gave the right of the patients to protection against publicity. Adult patients who had fever were told the objective of the study and their informed consent to participate in the study was sought. For infants and children ≤15 years, consent was sought from the parent or guardian.
Statistical analysis
Statistical analyses were carried out using Graph Pad Prism version 3.0 Software package (Graph pad Software, Inc., San Diego CA, USA) and Microsoft Excel 2000. Correlations between parasite density, monthly rainfall, age/age-groups and temperature were calculated using Spearman rank correlation coefficient.
Results
The geometric means of malaria parasite density exhibited two major peaks in May and November, which corresponds with the bimodal pattern of equatorial rainfall seasons in the months of February–June and September–December (Figure 1). This rain pattern creates several breeding sites for mosquitoes. As the vector population increases, the transmission of malaria increases. The result further shows that children below 15 years have higher peaks of mean parasite density than adults (Figure 2). The geometric mean parasite density however decreases with individual age and age groups of the study subject (Figure 3a and 3b). Infants, and adolescents harbour higher parasites in their blood than adults.
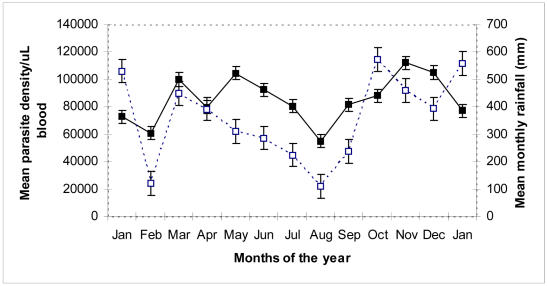
Figure 1. Mean parasite density versus monthly rainfall from January 2003 to 2004.
Parasite density increases with increasing rainfall between February and March to a peak value in May. It declines to the lowest value 55,000 parasites/mL in August, but again rises to the maximum in November following the start of the second rain in September. Mean parasite density (■), Mean monthly rainfall (□).
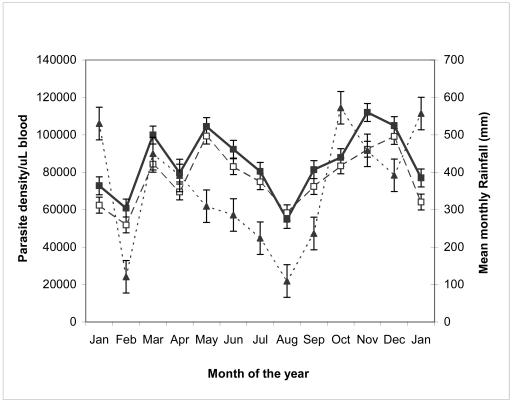
Figure 2. Mean parasite density of adults and children versus monthly rainfall from January 2003 to 2004.
Mean parasites density in children = ■; Mean parasites density in adults = □ and bi-modal pattern of rainfall = ▲.
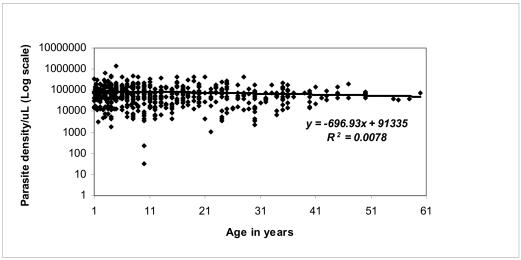
Figure 3a. Distribution of parasite density/mL of blood according to individual age of the study subjects.
A negative linear correlation R2 = 0.0078 was observed between parasite density and age.
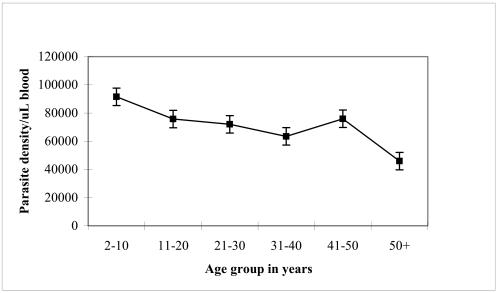
Figure 3b. Mean parasite density versus age group of study subjects.
The mean parasite density decreases with age group.
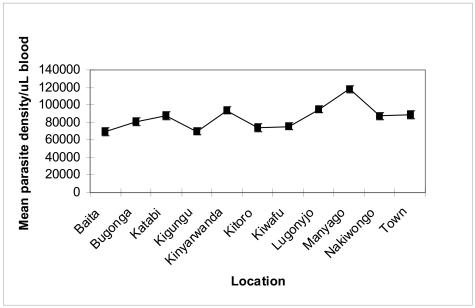
Of the 616 study subjects, 69.8% had body temperatures above 38°C classified as having high fever, while only 30.2% had variable body temperatures ≤ 37.5 – 38° C and were classified as mild fever. The body temperatures correlated positively with geometric mean of parasite density (Figure 4). Individuals with higher body temperatures had higher mean parasite densities than those with lower or normal body temperatures. There were however, no significant variations observed between the means of parasite density for the different location of Entebbe Municipality (p = 0.4405). Our data further indicates a generally high mean parasite density for all the eleven locations of Entebbe municipality, the highest peaks occurring in Manyango and Kinyarwanda sub-locations (Figure 5).
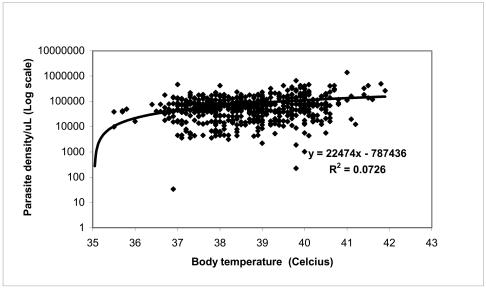
Figure 4. Parasite density per milliliter of blood versus body temperature of the subjects.
Parasite density is positively correlated to body temperature R2= 0.0726.
Figure 5.
Mean parasite density per milliliter of blood versus location in Entebbe Municipality
Discussion
The present study shows that there is a statistically significant relationship between mean parasite density (PD) and the annual pattern of rainfall January 2003 to January 2004 (Figure 1). Parasite density rises soon after the start of the first rain season in February reaching first peak in May, because the rains provide good breeding sites for mosquito vectors. As vector population increases, transmission of infection subsequently rises hence the increase in parasite density. As rainfall decreases and breeding grounds of mosquito vector dries up at the end of the first rain season around (June/July), parasite density accordingly falls reaching a minimum in August, probably due to a reduction in mosquito vector population. This is followed by another rise and peak in mean parasite density in November, shortly after the beginning of the second rainy season in September. The finding in this study corroborates our laboratory case records, which indicate that two weeks following the fall of the first rains usually registers increased cases of malaria infection.
The higher peaks of mean parasite density observed in children (Figure 2) is a result of a less developed immune system, which is incapable of clearing parasites more effectively as in adults. Therefore, parasite densities in children seems to give a true picture of the intensity of an infection than of adults, this may be a useful indicator for purposes of monitoring disease intensity.
Descriptive epidemiological studies of malaria infection in areas of stable transmission have revealed distinct age-specific pattern of parasite prevalence and density as detected by positive blood slide smears. Data from our study is consistent with the age-related patterns of prevalence of infection for a typical endemic area (Figure 3a & 3b). The prevalence of infection and parasite density decreased with increasing age or age group. The observed decline in parasite density is most likely due to the development of non-sterile clinical immunity over time11. This background immunity regulates infection and is usually pronounced in children above 15 years and in adults. These are people who have been exposed to mosquito bites over the years, hence to malaria many more times. Such limited immunity enables the individuals to tolerate severe malaria infection without getting ill even though they may get malaria fever11,12.
Furthermore, this study found that (69.8%) of the total patients screened had body temperatures ≥ 38°C, indicative of fever. The high body temperature was observed mainly in babies and adolescents. This observation was supported by the positive and significant correlation between parasite density, body temperature and age (r = 0.2695; p < 0.0001). Fever is a common sign and symptom of malaria and has been described as a natural host response to malaria. Even if high fever (38 – 40°C) is especially common in children and may lead to convulsion and altered unconsciousness, we did not encounter this in our study. The 68.9% of the patients were mainly children and babies aged < 15 years, who came to our laboratory presenting with high body temperatures (> 40° C), shivering and sweating (Data not included). They carried clinical notes, which indicated delayed diagnosis, treatment and treatment failures. The explanation to such clinical presentations and responses to untreated malaria or treatment failures is a classical fever paroxysm, which may ensue every two or three days depending on the parasite species. This malaria pathogenesis leading to fever is due to rupturing of erythrocytic shizonts releasing their pyrogens in the blood13.
Advanced studies on endogenous pyrogens by Dinarello, revealed them as subsets of cytokines that act on the thermoregulatory center in the hypothalamus to promote fever,14. The body's thermostat becomes set at a higher point and the thermoregulatory center acts to keep the temperature at precisely this point. At the end of the fever paroxysm, the thermoregulatory center returns the body thermostat to normal set point, and the individual feels hot and perspires profusely until temperature has fallen accordingly. The subsets of cytokines that are thought to act on the hypothalamic thermostat in this manner include tumor necrosis factor (TNF), interleukin-1 beta (IL-1β), interleukin-1 (IL-1α), interleukin -6 (IL-6), interferon-α and lymphotoxin-α and macrophage inflammatory protein-114. The pyrogenic properties and roles of these cytokines in the pathogenesis of malaria fever have been well demonstrated in animal models (Dinarello et al., 1986). Therefore, the significant correlation observed between parasite density and body temperature in this study demonstrate that the fevers are malaria induced rather than of other causes.
Although all the eleven locations are within Entebbe peninsula, the exceptionally high peaks observed in Manyago and Kinyarwanda may be attributed to the closer proximity to the lakeshore and surrounding swamps. Of particular importance, in this study is the increased parasite density recorded in children, which could be a useful indicator for purposes of public health monitoring of disease intensity. Adults usually have a more mature immune system, which can maintain parasite densities to a minimum level and hence may give a misleading indication of the infection intensity in an area.
Acknowledgments
We thank all those who voluntarily participated in this study. We in particular, thank the parents and guardians who willingly consented on behalf of their infants and children. Thanks also go to all the staff of St. Jude Diagnostic Laboratory for the tireless and active participation in carrying out all the diagnosis. Lastly but no least, we are grateful to the Assistant Commissioner Meteorological Department Mr. George Obua for the data on Rainfall records 2003–2004.
Footnotes
This paper is dedicated to our good friend Late George Ssegwanyi who helped us in parasite species identification. George died while this paper was being prepared. May his soul rest in peace
References
- 1.WHO, author. A vision for all. Geneva, Switzerland: 1998. The World Health report, Life in the 21st century. [Google Scholar]
- 2.Brewster D R, Kwiatkowski D, White N J. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039–1990. doi: 10.1016/0140-6736(90)92498-7. [DOI] [PubMed] [Google Scholar]
- 3.Bilal Z. Global Climate Change and Human Health. IFMSA Vagus. 2001;2:23–25. [Google Scholar]
- 4.Dinarello C A. Interleukins, tumor necrosis factors (cachectin), and interferons as endogenous pyrogens and mediators of fever. Lymphokines. 1987;14:1–31. [Google Scholar]
- 5.Dinarello C A, Cannon J G, Wolff S M, Bernheim H A, Beutler B, Cerami A. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin-1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenwood B, Marsh K, Snow R. Why do some children develop severe malaria? Parasitlogy Today. 1991;7:430–440. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- 7.Golgi C. Sul ciclo evolutio dei parassiti malarici nella febbre terzana: diagnosi differenziale tra I parassiti endoglobulari malarici della terzana e quelli della quartana (On the cycle of development of malarial parasites in tertian fever: differential diagnosis between the intracellular parasites of tertian, quartan fever. Extract reprinted in ‘Tropical medicine and parasitology: classic investigations. Vol 1. In: Kean B H, Mott K E, Russell A J, editors. Archivo per le Scienze Mediche. Vol. 13. Ithica: Cornell University, Press; 1889. pp. 173–196. (Fre). (1987) [Google Scholar]
- 8.Kluger M J. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh K. Malaria- a neglected disease? Parasitology. 1992;104:53–69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- 10.Marsh K, Forester D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol G, Snow R. Indicators of life-threatening malaria in African children. New Eng J Med. 1995;332(21):1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 11.Markell E K, John DT, Krotoski W A. Text Book Medical Parasitology. Eight edition. Philadelphia: W. B. SAUNDER COMPANY; 1998. p. 119. [Google Scholar]
- 12.Molineaux L. The epidemiology of human malaria as an explanation of its distribution, including some implication for its control. In: Wernsdorfer W H, McGregor I, editors. Malaria Principles and Practice of Malariology. Vol. 2. London: Churchill Livingstone; 1988. pp. 913–999. [Google Scholar]
- 13.Molineaux L, Grammiccia G. The Garki Project: Research in the epidemiology and control of malaria in the Sudan savanna of West Africa. Geneva: WHO; 1980. WHO Geneva. [Google Scholar]
- 14.Najera J A, Hempel J. The Burden of malaria. WHO. 1996 CTD/Mal 96.10 WHO Geneva. [Google Scholar]