Abstract
Objective
Study the pathological changes in gastric mucosa of Nyarwanda, Nkole (both with high prevalence of stomach cancer) and Ganda (with low prevalence of this cancer) ethnic groups in the presence of Helicobacter pylori (H. pylori) infection.
Research question
Do pathological changes accompanying H.pylori infection explain the varying prevalence of stomach cancer in these populations?
Design
Retrospective cross sectional study
Subjects
A total of 114 patients of the above ethnic groups with upper gastrointestinal symptoms who underwent endoscopic biopsy examination between January 1996 and June 2002 formed the basis of this study.
Results
The severity of gastritis correlated with the presence of H. Pylori in Ganda and Nyarwanda but not in Nkole. Intestinal metaplasia (IM) was observed in Nyarwanda and Nkole and in some of these cases there was H. pylori. Gastric atrophy (GA) was also commonly observed in Nkole and Nyarwanda and H. pylori was detected more in the severe form of GA. Lymphoid follicle formation was not associated with H. pylori infection in all study groups.
Conclusion
The major histological features relating stomach cancer to H. pylori in this study were presence of the infection in IM and GA that was observed mainly in Nyarwanda and Nkole.
The lack of association between presence of lymphoid follicle and H. pylori infection probably explains the rarity of MALT lymphoma in Africa as these tumours are said to arise from H. pylori associated lymphoid follicles.
Introduction
Despite the fact that in Africa prevalence of stomach cancer is generally low, there has been persistent reports of high prevalence of this cancer in areas of volcanic mountains particularly in the great lake region which comprises of the Kivu province of the Democratic Republic of Congo (former Zaire), Rwanda, Burundi, South Western Uganda and North Western Tanzania1, 2. Similar high prevalence of this tumour is also reported around Mount Kilimanjaro, Mount Kenya and Mount Elgon that are all volcanic in origin3, 4. No factors have yet been sought to explain this observation apart from earlier suggestion that lack of trace minerals in these volcanic soils may be responsible.
Recently the newly rediscovered H. pylori has been incriminated in the etiology of stomach cancer to such an extent that it is now considered type 1 carcinogen5. However, in Africa the incidence of gastric cancer seems not to be related to the prevalence of H. pylori, as this infection is quite prevalent. The aim of this study was therefore to determine the association between H. pylori infection and pathological changes of the gastric mucosa in Nyarwanda, Nkole (high gastric cancer prevalence) and Ganda (low gastric cancer prevalence) ethnic groups.
Materials and Methods
This was a retrospective cross sectional study in which Nyarwanda, Nkole and Ganda ethnic groups were investigated. Republic of Rwanda where Nyarwanda live and south-western Uganda where the Nkole live are parts of so-called stomach cancer region. The Ganda reside in central and southern Uganda in which the prevalence of stomach cancer is low. Paraffin blocks of endoscopic antral biopsies obtained from patients with upper gastrointestinal symptoms between January 1995 and June 2002 were retrieved from archives of Pathology departments of Makerere University, Mbarara University Faculties of Medicine and Private histopathology laboratory in Kampala, Uganda. These biopsies were studied for presence of H .pylori using the modified Giemsa stain as described by Gray et al6. The tissues were also studied for presence and severity of gastritis, atrophy, lymphoid follicles using the heamatoxylin and eosin histological technique and intestinal metaplasia using the Alcian Blue PAS technique of Mowry7.
Results
A total of 114 Nkole, Ganda and Nyarwanda patients were studied and table 1 shows the demographic characteristics of this study population. There was no difference in the sex distribution between the three ethnic groups and the Nyarwanda were significantly younger than the Nkole (P value = 0.016) but no difference in age between Nyarwanda and Ganda and between Nkole and Ganda (P values 0.34 and 0.26 respectively).
Table 1.
Showing the demographic characteristic of study population
|
Nkole (No.(%) |
Nyarwanda (No.(%) |
Ganda No.(%) |
|
| Sex | |||
| Male | 27(56) | 11(35) | 17 (48) |
| Female | 17(44) | 20(65) | 22 (52) |
| Age | |||
| < 21 | 1 | 7 | 4 |
| 25 – 49 | 21 | 18 | 23 |
| 50 – 74 | 16 | 6 | 9 |
| 75+ | 2 | 0 | 2 |
Table two shows the distribution of H. pylori infection in the study population according to sex. The infection was more frequent in Nyarwanda males a feature that was not observed in other ethnic groups.
Table 2.
Distribution of H. pylori infection in the study population
| Ethnic group |
Male | Female | Odds radio |
Confidence intervals |
| Nyarwanda | ||||
| H.pylori -ve | 1 | 12 | 0.007 | 0.00 – 0.73 |
| H.pylori +ve | 10 | 8 | ||
| Nkole | ||||
| H.pylori -ve | 13 | 6 | 0.042 | 0.09 – 1.82 |
| H.pylori +ve | 10 | 11 | ||
| Ganda | ||||
| H.pylori -ve | 8 | 11 | 1.0 | 1.00 – 4.48 |
| H.pylori +ve | 8 | 11 |
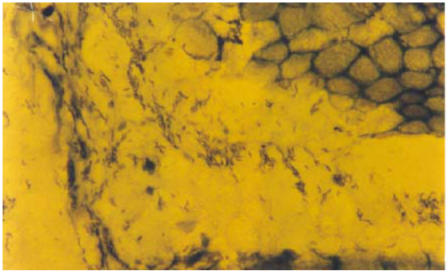
Table three shows the distribution of major pathological changes and their relationship to H. pylori infection. The severity of the infection, as shown in figure one, correlated well with gastritis in Ganda ethnic group in which the more severe the gastritis the more likely to find H.pylori (P value =0.036). In Nyarwanda the severity of gastritis was related to detection of the bacteria and this observation was nearly significant (P value = 0.052). In Nkole group, there was no significant difference between grade of inflammation and detection of the bacteria (P value = 0.74).
Table 3.
Distribution of major pathological changes and their relationship to H. pylori infection
| Type of lesions | Nkole | Nyarwanda | Ganda | |||
| No. of cases with lesions |
% with H.pylori |
No. of cases with lesions |
% with H.pylori |
No. of cases with lesions |
% with H.pylori |
|
| Gastritis | ||||||
| Mild/Nil | 22 | 50% | 16 | 87% | 26 | 36% |
| Severe | 19 | 57% | 14 | 100% | 12 | 75% |
| IM | 5 | 100% | 3 | 0% | 0 | |
| Gastric Atrophy | ||||||
| Mild/Nil | 28 | 46% | 25 | 56% | 36 | 47% |
| Severe | 12 | 67% | 5 | 56% | 2 | 100% |
| Lymphoid follicles | 3 | 33% | 3 | 33% | 5 | 60% |
Figure 1.
Severe H. pylori infection X 1000
Intestinal metaplasia was only observed in Nkole and Nyarwanda and in Nkole there was scanty H. pylori accompanying IM. Gastric atrophy was observed in most cases but as shown in table three, the severe form of GA was mainly observed in Nkole and Nyarwanda. H. pylori infection occurred more in severe atrophic mucosae than the mild or normal mucosae.
Lymphoid follicles were not frequently found in this study population and as shown in table three there was no association between H. pylori infection and presence of lymphoid follicles.
Discussion
Gastritis has been identified as one of the outcome of H. pylori infection and this study confirms the role of the bacteria in causing chronic gastritis particularly in Nyarwanda and Ganda where it was found that the more severe the gastritis the more likely to find the organisms. Dooley et al9 found the bacteria in all subjects with gastritis. Weir at el10 studied endoscopic materials from 47 Ugandan patients with dyspepsia and 29 Zimbabweans and H. pylori was detected in all the 47 Ugandan cases with gastritis and 24 of the 25 Zimbabwean cases with gastritis had H. pylori. It was surprising that in Nkole there was no relation between severity of gastritis and detection of the bacteria and no explanation could be provided for this observation. Chronic H. pylori associated gastritis has been put forward as a risk factor for development of GA and IM that are known premalignant lesions11. Intestinal metaplasia was only observed in Nkole and Nyarwanda and there were scanty organisms in a few of these IM mucosae. Severe GA was also mainly found in Nkole and Nyarwanda and H. pylori was observed more in the severe GA mucosae than those with mild GA or normal mucosae. It is believed that IM and GA lead to a rise in the intragastric pH, which is said to be unfavorable to the survival of H. pylori because of a high pH associated with these two lesions. The finding in this study in which H. pylori was found more in severe atrophy and in some IM is surprising from the above explanation but Rouvry et al12 while studying endoscopic biopsies from Nyarwanda patients with dyspepsia found 15 out of 18 (83%) patients with atrophy had H. pylori infection. Fox et al13 also found 89% of patients with chronic atrophic gastritis had H. pylori. This finding would suggest that the pH in these ethnic groups remain low despite development of GA and IM. It is possible that in these cases the infection is localized to the antrum and therefore does not affect the acid producing body and fundal regions of the stomach thereby keeping the pH low. In Ganda, it is possible that the infection may spread to body and fundal areas and accompanying gastritis may lead to a reduction in acid output and in turn reduce/eradicate the bacteria. Imai et al14 found antral atrophy commoner in populations with high gastric cancer incidence and it is probably the persistence of these bacteria in these severe atrophic mucosa that may explain the difference in prevalence rates of stomach cancer in various populations. Kuipers et al15 and MacFarlane et al16 also found development of gastric atrophy and intestinal metaplasia significantly associated with H. pylori and that these lesions were not related to severity of the gastritis. The finding in the present study appears to agree the finding of the above authors whereby in Nkole the infection was not related to the gastritis but to GA and IM.
Another unexpected finding in this study was lack of association between H.pylori and presence of lymphoid follicle that is in contrast with the general belief that lymphoid follicles are virtually restricted to H. pylori infection. In the most comprehensive study by Genta et al17, lymphoid follicles were found in all patients with H.pylori but absent in normal controls. In Herrera-Geopfert et al18 series lymphoid follicles were found a reliable indicator of H. pylori infection. The possible explanation is that the present study dealt with mainly adult Africans who may have adapted to the infection over time. Meining et al19 clearly demonstrated severe presence of lymphoid follicles in children as compared to adults and thought that this could be a child-specific immune response. It is therefore possible that if the infection is acquired during adulthood, as it commonly occurs in western world, then patients acquire this child-like immune response. Whereas if H.pylori infection is acquired in childhood like in Africa, the body become adapted as a result of repeated infections thereafter the therefore does not elicit similar immune response. Lymphoid follicles are acclaimed pathophysiological substrate for MALT-lymphoma and these reactive follicles are integral to this lymphoma20. The present study may therefore provide better explanation for the rarity of MALT lymphoma in Africans.
References
- 1.Hutt MSR, Burkitt DP, Shepherd DJ. Malignant tumours of the gastrointestinal tract in Uganda. Nat Cancer Inst Monograh. 1966;25:41–47. [PubMed] [Google Scholar]
- 2.Hiza PR. Malignant disease in Tanzania. E Afr Med J. 1976;53:82–95. [PubMed] [Google Scholar]
- 3.Bourdenx L, Renard F, Gigase PL. Mextal L'incidence des cancers a l'hospital de Katana, Kivu Est. Zaire de 1983 a 1986. Am Soc Belge Med Trop. 1988;68:141–156. (Fre). [PubMed] [Google Scholar]
- 4.Cook Paul, Burkitt DP. An epidemiological study of seven malignant tumours in East Africa. Cyclostyled booklet. Medical Research Council; 1970. Jan, [Google Scholar]
- 5.Intenational Agency for Research on Cancer, author. Schistosomes, Liver flukes and Helicobacter pylori. IARC Monographs Eval Carcinog Risks Hum. 1994;61 [PMC free article] [PubMed] [Google Scholar]
- 6.Gray SF, Wyatt JI, Ralhbone BJ. Simplified techniques for identifying Compylobacter pylorids. J Cli Pathol. 1986;39:1230. doi: 10.1136/jcp.39.11.1279-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madan E, Hemp J, Westblom TU, et al. Evaluation of staining methods for dentifynng Camplylobacter pylori. Am J Clin Pathol. 1988;90:450–453. doi: 10.1093/ajcp/90.4.450. [DOI] [PubMed] [Google Scholar]
- 8.Mowry RW. Observation on the use of sulphuric ether for sulphation of hydroxyl groups in tissue sections. J Histochem Cytochem. 1956;4:407. [PubMed] [Google Scholar]
- 9.Dooley CP, Cohen N, Filzgibbons PL, et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Eng J Med. 1989;321:1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 10.Weir WRC, Goodgame R, Kiire CF, Lucas B. Campylobacter-like otganisms and gastritis in Africa. Trans Roy Soc Trop Med Hyg. 1988;82:172. doi: 10.1016/0035-9203(88)90300-8. [DOI] [PubMed] [Google Scholar]
- 11.Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet. 1975;11:58–59. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 12.Rouvroy D, Bogaerts J, Nsengiumwa O, et al. Campylobacter pylori, gastritis and peptic ulcer disease in central Africa. BMJ. 1978;296:1174–1174. doi: 10.1136/bmj.295.6607.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox JG, Correa P, Taylor NS, et al. High prevalence and persistence of cytotoxin positive Helicobacter pylori strains in a population with high prevalence of atrophic gastritis. Am J Gastroenterol. 1992;87:1554–1560. [PubMed] [Google Scholar]
- 14.Imai T, Kubo T, Watanabo H. Chronic gastritis in Japanese with reference to high incidence of gastric carcinoma. J Nat cancer Inst. 1971;47:179–195. [PubMed] [Google Scholar]
- 15.Kuipers EJ, Uyterlinde AM, Pena AS, et al. Longterm sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 16.McFarlane GA, Wyatt J, Forman D, Lachlan GW. Trends overtime in Helicobacter pylori gastritis in Kenya. Eur J Gastroenterol Hepatol. 2000;12:617–621. doi: 10.1097/00042737-200012060-00007. [DOI] [PubMed] [Google Scholar]
- 17.Genta RM, Hammer HW, Graham DY. Gastric Lymphoid follides in H.pylori infections, frequency, distribution and response to triple therapy. Human Pathol. 1993;29:577–583. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- 18.Herrera-Goepfert R, Garcia-Marcano R, Zeichner-Gancz I. Helicobacter pylori and lymphoid follicles in primary gastric MALT - lymphoma in Mexicol. Rev Invest Chin. 1996;48:261–265. [PubMed] [Google Scholar]
- 19.Meining A, Behrens R, Lehn N, et al. Different expression of Helicobacter pylori gastritis in children, evidence for a specific peadiatric diseases. [DOI] [PubMed] [Google Scholar]
- 20.Isaacson PG. B-Cell lymphoma of mucosa associated lymphoid tissue (MALT) Bull Cancer Paris. 1991;78:203–205. [PubMed] [Google Scholar]