Abstract
Screening of different extracts and fractions from the plant Bidens pilosa Linn. var. (Asteraceae) has been conducted using the in - vitro comet assay for anticancer and the antipyretic action, which was done with in - vivo models. The extract from whole plant was extracted with n - hexane, chloroform and methanol extract (E1 – E3). The extracts were fractioned by column chromatography method and fractioned with ethyl acetate, acetone and water (F1 – F3). All the extracts and fractions were tested for anticancer and antipyretic activity. Among extracts E1 shows remarkable anticancer activity and E3 bears maximum antipyretic activity. In the antipyretic activity, paracetamol was used as the standard test drug. The most promising material (LC50 < 1500 µg / ml) was F1 ethyl acetate fractions of methanolic extract and methanolic crude extract of whole plants. However, little correlation was observed in the degree of antipyretic activity between the test drug and standard drug. In conclusion, the extract obtained from the whole plant of Bidens pilosa showed a significant cytotoxic effect to methanolic extract against Hela cells by in vitro method and showed a comparable antipyretic activity effect to paracetamol in rabbit pyrogen test.
Keywords: Bidens pilosa, Comet assay, Antipyretic, Anticancer, MTT assay
Introduction
The use of bioassay offers various scientific strategies like screening of extracts, fractions and compounds obtained from plants, which are often used in phytochemical research. In the evaluation of in vitro methods for natural products the biological activity has changed in the past few years, one of the recent developments is comet assay, which gives a ratio between the viable cells in the cell culture to total cells in the culture1. These techniques are considered rapid and economical for the evaluation of anticancer2 compounds. Antipyretic activity3 of large number of natural products easily allows to guide the isolation and purification of biologically active principles4. The genus Bidens [Asteraceae] is used in some countries to treat different diseases5. Previous studies have confirmed that some of the species produces compounds that exert some pharmacological activities like Phenylheptatriyne, linolic acid and linolenic acid, which have antimicrobial activities, friedelin and friedelan - 3 beta - ol, as well as several of the flavonoids found are anti - inflammatory agents and detection of these compounds in extracts from Bidens pilosa may rationalize the use of this plant in traditional medicine in the treatment of wounds, against inflammations and against bacterial infections of the gastrointestinal tract6.
Bidens pilosa grows rarely in the south of India being known as “Ottrancedi” and is frequently used in traditional medicine as a remedy to treat Glandular sclerosis, wounds, Colds and flu, Acute or chronic hepatitis and Urinary tract infections7. Finally, new biological activities of natural products have been just studied8. Therefore, one of the objectives of our study was to evaluate the anticancer activity of some species of Asteraceae. Isolated compounds from the plant material have been evaluated for both the activities. The aim of the present study is to evaluate the anticancer activity by in vitro method on Hela9 (human, black, cervix, carcinoma, epitheloid) and KB (a human nasopharyngeal epidermal carcinoma cell line) 10 carcinoma cell lines in vitro and antipyretic activity by in vivo11. The anticancer potency found for some of these compounds may be explained by the capacity that they have for DNA strand breaks and obvious application of the comet assay is the study of apoptosis12.
Materials and methods
Materials
Bidens pilosa was collected in Kerala, South India, in October 2003 and identified by a systemic botanist, Dr. B. Sampath Kumar, Department of Botany, Annamalai University. A voucher the specimen was also deposited in Department of Pharmacy, Annamalai University under number DP - XX - F. No.: 0102.
Extraction and Isolation
The shade dried parts of the whole plant were coarsely powdered and extracted with n - hexane, chloroform and methanol respectively for 48 hours in soxhlet apparatus. After evaporation of the solvent under reduced pressure, the respective extracts were obtained. Considering that the methanolic extract suits for better activity, it was successively partitioned with ethyl acetate and acetone affording 0.150 gm and 0.200 gm (residue dry) of each fraction respectively.
The ethyl acetate fraction exhibited anticancer activity (Table 1) and was selected for phytochemical studies.
Table 1.
Anticancer activity of Bidens pilosa extracts and fractions on Hela and KB cell lines by Comet Assay Method
| Test Sample | *CTC50 ± SEM (µg / ml)** | |
| Hela Cell | KB Cell | |
| n - Hexane Extract | 509.2 ± 6.3 | 385.2 ± 4.7 |
| Chloroform extract | 849.4 ± 7.7 | 088.6 ± 2.1 |
| Methanol extract | 875.3 ± 9.9 | 423.1 ± 1.4 |
| Ethyl acetate extract fraction | 965. 2 ± 8.9 | 586.2 ± 6.9 |
| Acetone fraction | 472.3 ± 4.8 | 311.5 ± 7.9 |
| Water fraction | 372.4 ± 5.1 | 200.1 ± 3.4 |
Average of three independent determinations three replicates values are mean ± SD.
CTC50 = concentration of the sample tolerated by 50% of the cultures exposed.
Thus it was fractioned on chromatoragphic column over silica gel 60 by elution with n - hexane - ethyl acetate (80:20), given 50 mg of compound A, 75 mg of compound B and 100 mg of compound C which were identified by spectroscopic data IR only. 1H, CNMR and MS have to be taken and analyzed for their structures.
Comet assay (partially modified procedure of Singh et al 1988) 12, 13
Unpolished microscopic slides were used. 1% solution of normal melting point agarose (NMP) was brought and pipette on the slide, covered by coverslip, cover slip removed and the agarose gel let to dry at normal room temperature. At the following step 0.6% NMP agarose was brought on top of the first layer, covered by coverslip and let to solidify in ice plate for 10 minutes. The third layer was a mixture of prepared cell in 0.5% LMP at 37%. About 50,000 cells were allowed to solidify for 10 minutes on ice and covered by the last layer of 0.5% LMP agarose. The coverslip was removed and the slides brought into lysis buffer for at least 1 hr at 4°C (0.03 M sodium hydroxide, 1.2 M sodium chloride, 0.5% lauryl sacrose, pH > 13; for spermatozoa lysing solution contained 1 % triton - X 100, too). The prepared agarose gels were brought into electrophoresis apparatus. Denaturation (40 minutes) and electrophoresis (20 minutes) occurred in the same buffer (0.03 M sodium hydroxide, 2 mM EDTA). The electrophoretic conditions were as following: 30V and 350 mA. Following electrophoresis the studies were washed three times for 5 minutes with the neutralization buffer (0.4 M Tris, pH 7.4), then the gels were stained with ethidium bromide (2 mcg/ml of dH2O) for 20 minutes, reused in distilled water and kept in humid environment and dark at 4°C. Epifluorescent microscope at 400 X magnifications was used for the examination of studies. Photographs were taken.
Rabbit Pyrogen Test14, 15, 16, 17, 18
A group of 5 experimental rabbits having average weight of 2.5 kg were taken on the day of the study, food was withheld and rabbit was acclimatized to the test condition for 2 hrs, the base line temperature was recorded initially half an hour prior to injection. Temperature was monitored by means of thermometer inserted at least 10 cm in to the rectum and recorded by the calibrated thermometers. An animal was excluded from the study if the baseline temperature was not within the range of 39.2° C to 39.8° C. 15 mg/kg of brewer's yeast solution (1ml/kg) was injected into the marginal ear vein of rabbit. Each dose was administered to the group of 5 rabbits. Temperature measurements were taken over an hour period at 50 minutes interval. The observations are tabulated in table 2.
Table 2.
The effect of BPEA and paracetamol on yeast - induced pyrexia in rabbits.
| Treatment | Rectal temperature (°C) a | |||||
| After yeast injection at | After drug administration | |||||
| 0h | 24h | 1h | 2h | 3h | 4h | |
| Control BPEA |
37.6±0.02 | 39.9±0.03 | 39.8±0.02 | 38.2±0.05 | 38.9±0.05 | 39.9±0.04 |
| 50 mg/kg BPEA |
38.7±0.04b | 37.8±0.03b | 37.5±0.02 | 38.7±0.02b | 39.4±0.04b | 38.1±0.06b |
| 100 mg/kg BPEA |
38.6±0.02b | 37.7±0.04 | 37.8±0.05b | 38.3±0.05 | 39.1±0.03b | 37.9±0.06b |
| 200 mg/kg | 37.7±0.07 | 39.9±0.06 | 37.7±0.02b | 39.9±0.03b | 38.7±0.05 | 37.9±0.04b |
| Paracetamol 150 mg/kg |
37.8±0.02b | 39.9±0.03b | 38.9±0.02 | 38.5±0.02b | 39.3±0.04b | 39.7±0.03 |
The results given are mean ± S.E.M.; number of animal used (n=6)
P < 0.01 Experimental groups were compared with control
Results and discussion
A rush to very specific, in vitro, robotic mechanism was cautiously carried out however, with mechanism based assays. The field of vision of such specific microscopes is very narrow; they must be assured that the scope of their bioassays can be wide enough to include diverse and unknown mechanism well as new chemical entities. In addition, in such specific bioassays the same extract, fractions or standard compounds have to be analyzed many times over and over again, and permits a large number of samples and dilutions within the shorter time than using the original test vials, before detecting activities.19, 20
These results suggest the presence of bioactive compounds, which would be helpful for further examinations, by using elaborated bioassays for detection of more specific pharmacological properties. It is important to separate active candidates and to identify the major active biomolecules that is present in plant, which can be suggested for further investigations to determine that any other components are responsible for the cytotoxic activity or the existence of synergic effect.
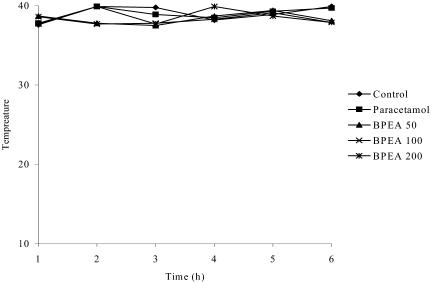
On the other hand, based on the possible relationship between comet assay and plant bioactivity21, 22 this work encouraged the research on biological assays in vivo of this plant. The antipyretic effects were produced after intraperitoneal administration of extracts to fever induced rabbits. In our tests, the intraperitoneal administration of methanolic extracts from whole part of Bidens pilosa (500mg/kg body weight) to normal rabbits produced a significant reduction in body temperature. Compared with the control and experimental groups (Fig. 1) whose body temperature decreased after a 4 hrs treatment. Studies are currently in progress in our laboratories to determine the active principles of Bidens pilosa.
Fig. 1.
Effect of the ethyl acetate extract of Bidens pilosa (500 mg/kg) on body temperature in normal rabbits treated during 6 hrs all t values are mean ± SEM, n=6(**), significant differences between treated and control group were evaluated by co-efficient correlation.
Conclusion
We have adopted a microtitter assay based on metabolic reduction of MTT to evaluate the cytotoxic effect on different cells and on the basis of rabbit pyrogen test lowering the body temperature of fever therapeutic index to evaluate the body temperature. This technique allows evaluating dose dependent effect, by linear regression analysis shows acceptable R2 values and correlation coefficients.
Table 1 and 3 shows concentration of extract to produce a reduction viability of cell cultures of 50% (CC50 values) as determined by comet assay and it produces a lower body temperature of rabbit as determined by rabbit pyrogen test. The ethyl acetate fractions of methanolic extract from Bidens pilosa on Hela and KB cells present in CC50 values of 965.2 and 586.2 at 24 h respectively23. The methanolic extract of Biden pilosa on rabbit presented lower body temperature of 1° C at 4 h. The comparison of the CC50 values of the methanolic extract from this plant proves to conclude that this extract would be helpful for further studies of activity monitored fractions to identify the active principles. The extract examined showed an important cytotoxicity and antipyretic activity.
Table 3.
Antipyretic activity of Bidens pilosa extracts and fractions on Rabbits
| Test Sample | MFI ± SEM (° C)* |
| BPnHE | 0.05 |
| BPCE | 0.04 |
| BPME | 0.03 |
| BPEAE | 0.03 |
| BPAF | 0.04 |
| BPWF | 0.02 |
| Paracetamol | 0.09 |
Average of three independent determinations three
In our test, the intraperitoneal administration of Methanolic extracts from whole plant of Bidens pilosa (500mg / kg body weight) to normal rabbits produced a significant reduction in body temperature. Compared with control and experimental groups of rabbits (Fig. 1) there is a reduction in the body temperature after 4 treatments with Methanolic extract. Studies are currently in progress in our laboratories to determine the active principles of Bidens pilosa, which is responsible for the antipyretic activity.
Table 1 and 3 compound whose anticancer and antipyretic activity has been proved, with the values of Bidens pilosa extract and it concludes that they have an acceptable anticancer and antipyretic activity.
References
- 1.Nelms Benjamin E. Measuring apoptis in individual cells with the comet assay promegen notes. 1997;(64):13. [Google Scholar]
- 2.Rubinstein LV, Shoemaher RH, Paull KD, Simon RM, Tosmi S, Skehau P, Scudievo DA, Monks A, Boyd MR. Comparison of in vitro anticancer - drug - screening data queerated with tetrazolium assay - screening assay versus a protein assay against a diverse paued of human tumor cell tissues. JNCI. 2003;82(13):113–118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 3.Amole OO, Onbanjo AO. Antipyretic effect of Rawolfia vomitoria in rabbits. Nig Jr Nat Prod and Med. 1999;(3):77–78. [Google Scholar]
- 4.Colegate Steven M, Molyneux Russell J. Bioactive natural products: detection, isolation and structural determination. CRC Press; 1993. Sep, [Google Scholar]
- 5.David Medicine at your Feet: Healing Plants of Hawaiian Kingdom. 2004 [Serial online] Available from: URL: Internet address: http://www.medicineatyourfeet.com/
- 6.Geissberger P, Sequin U. Constituents of Bidens pilosa L.: do the components found so far explain the use of this plant in traditional medicine? Acta Tropica. 1991 Feb;48(4):251–261. doi: 10.1016/0001-706x(91)90013-a. [DOI] [PubMed] [Google Scholar]
- 7.Taylor Leslie. The Healing Power of Rainforest Herbs. 2004 Sep – Oct; [Serial online] Available from: URL: http://rain-tree.com/picaopreto.htm.
- 8.Cordett GA. Changing strategies in natural products chemistry. Phytochem. 1955;(40):1585–1612. [Google Scholar]
- 9.Itami T, Ema M, Kanoh S. Antipyretic mechanism of indomethacin in rabbits. J Pharmacobiodyn. 1986 Mar;9(3):271–275. doi: 10.1248/bpb1978.9.271. [DOI] [PubMed] [Google Scholar]
- 10.Lengsfeld AM, Dietrich J, Schultze - Maurer B. Accumulation and release of vinblastine and vincristine by HeLa cells: light microscopic, cinematographic and biochemical study. Cancer Res. 1982 Sep;42(9):3798–3805. [PubMed] [Google Scholar]
- 11.Singh NP, McLog MT, Tice RR, Scheider EL. A Sample technology for quantification of low levels of DNA damage in individual cells. Exp Clin Res. 1988;(175):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 12.Krause G, Wolz L, Scherer G. Performing the comet assay for genetic toxicology applications. Life Science News. 2001;(7):1–3. [Google Scholar]
- 13.Issued by funding / sponsoring agency: CRP. Romana ML, author. Introduction of the comet assay for the evaluation of oxidative stress, antioxidant effects and environmental pollutants effects on animal cells. Final report. Feeding influences on the quality of food of animal origin and the nutritional complementary value of the foods of plant origin: Dept. of Zootechnical (Groblje), Univ. of Ljubljana. 1999 Feb; Report No.: V4 - 9118 - 0402 - 98.
- 14.Hajare SW, Suresh Chandra SK, Tandan J, Lal Sarma J, Telang AG. Analgesic and antipyretic activities of Dalbergia sissoo leaves. Ind Jr Pcology. 2000;(32):357–360. [Google Scholar]
- 15.Michael JA, Daniel SL, Dana MG. Parenteral Quality Control Sterility, Pyrogen, Particulate, and Package Integrity Testing. 3rd ed. Culinary and Hospitality Industry Publications Services; 1998. [Google Scholar]
- 16.British Pharmacopoeia. 1996. [Google Scholar]
- 17.Indian Pharmacopoeia. 1996. [Google Scholar]
- 18.United States of Pharmacopoeia. 1995. [Google Scholar]
- 19.Mclaughlu Jerry L, Linglurg L, Rogers MS, Anderson Jon E. The use of biological assays to evaluate botanicals. Drug Information Journal. 1988;(32):513–524. [Google Scholar]
- 20.Márcia K, Jean CB, Rozangela CP, Rosendo AY, Valdir CF, Alcíbia AM, et al. Cytotoxic, hypoglycemia activity and phytochemical analysis of Rubus imperialis (Roseaceae) Z Naturforsch. 2002;57(c):272–276. doi: 10.1515/znc-2002-3-412. [DOI] [PubMed] [Google Scholar]
- 21.Avkobahi A, Bathown R, Owais W, Najib N. Biological activity of some fordnion medicinal plant extracts. Fitoterapia. 1996;(67):435–442. [Google Scholar]
- 22.Shimada H, Tyler VE, Mclanghlin JL. Biologically active acylglycerides from the berries of saw palmetto (serenoarepent) S Nat Prod. 1997;(60):417–418. doi: 10.1021/np960552o. [DOI] [PubMed] [Google Scholar]
- 23.Betancur Gelvis LF, Saez J, Granados H, Salazar A, Ossa JE. Antitumor and antiviral activity of colombian medicinal plant extracts. Mem Inst Oswaldo Cruz. 1999;94(4):531–535. doi: 10.1590/s0074-02761999000400019. [DOI] [PubMed] [Google Scholar]