Abstract
Background
There are limited reports on HIV-1 RNA load, CD4+T-lymphocytes and antibody responses in relation to disease progression in HIV-1 infected untreated children in Africa.
Methods
To describe the relationships between these parameters, we conducted a longitudinal cohort study involving 51 perinatally HIV-1 infected children aged between 1 and 13 years. HIV status was determined by ELISA and confirmed by western blot and PCR. Antibodies were quantified by limiting dilution ELISA, plasma HIV-1 RNA load by RT-PCR and CD4+T-lymphocytes by FACSCount.
Results
Asymptomatic and symptomatic disease had, respectively, a rise in median HIV-1 RNA load from 1,195 to 132,543 and from 42,962 to 1,109,281 copies/ml in children below 6 years. The increase in viral load was 10-fold higher for asymptomatic compared to other categories and 2-fold faster for children less than 6 years than those above. Similarly, symptomatic children below 6 years had initial median CD4+T-lymphocyte counts of 647 (22%) cells/µL, declining to 378 (20%) while those above 6 years had initial values of below 335 (15%) but which increased to 428 (17%). Median viral load correlated significantly with median CD4+T-lymphocyte percentage in children above 6 years (p=0.026) but not below.
Conclusions
Viral load is lower in older than younger children and correlates significantly with percentage CD4+T-lymphocytes. Survival by HIV-1 infected children requires a competent immune response early in infection to counter the rapidly replicating virus. Interventions aimed at boosting the naïve immune system may prolong survival in these children.
Keywords: HIV, progression, immune response, threshold, untreated children, Africa
Running title: HIV load, CD4+ cells and antibodies in children
Introduction
Vertically HIV-1 infected infants fail to produce significant anti-HIV antibodies and develop profound immunodeficiency and AIDS.1 Although these infants may demonstrate increased total immunoglobulin early in the course of infection, 2 impaired B-cell function preceding severe hypogammaglobulinemia, 3 absence of enhancing antibodies, low T-helper cell profiles and increased viral load all affect AIDS survival4–6 and add to poor prognosis. Overall, these children exhibit different course of HIV progression, 7 with approximately 33% developing AIDS, 8, 9 and more than 90 % developing AIDS related symptoms10–12 during the first year of life. Consequently, long-term follow-up is hindered by associated high infant AIDS-mortality, relegating most pathogenesis studies to children 2 years or below.13, 14 Data from these studies suggest a rapid rise in plasma HIV-1 RNA load within the first two months of life, before declining slowly until the age of 24 months.10, 11 While this pattern correlates directly with viral load and CD4+ T-cell counts in some children, 15,16 others progress to disease without significant depletion of CD4+ T-cells.17,18 Here, we describe the effect overtime of clinical status and age on the course of viral load, CD4+ T-cell counts and antibody titres and how these outcomes relate to the risk of disease progression.
Methods
Study site and participants
This study was conducted at the Nyumbani Hospice for HIV-1 positive children in Nairobi. The institution housed a total of 51 children by the time of this study.
Based on the CDC criteria, 19 the children were grouped into the following categories; clinical categories N (asymptomatic), A (mildly symptomatic), B (moderately symptomatic) and C (severely symptomatic); Immunological categories 1 (no suppression), 2 (moderate suppression and 3 (severe suppression) and in two age groups, above and below 6 years. Clinical categorisation and age grouping were done before recruitment into the study. All the children were naïve to anti-retroviral treatment while none of the children's parents had a history for antiretroviral therapy.
Sample size
A difference in mean viral load overtime of between 0.7 and 1.1 log10 in different groups of children has been reported20. If we expect a mean difference of 1log10 to be significant at 5% level, 90% power and assuming a standard deviation of 1, then we needed to recruit 22 (16 for a power of 80) children in each of the two age-groups.
Inclusion Criteria
All children admitted to the Nyumbani children facility must have fulfilled a set of institutional criteria in that they must; (a) have tested seropositive for anti-HIV antibodies at the hospice or a referring institution, (b) be orphaned as a result of HIV-related death of a parent or both. Alternatively, children fulfilling condition (a) above but whose parentage could not be ascertained either due to abandonment or whose caretakers could not continue with their care all qualified for admission. To ensure that data accrued was available for routine clinical management of all children without unwarranted discrimination as well as factoring in losses to follow-up, we included all 51 children (aged between 1 and 13; median 6 years) duly admitted to, and living or present at the hospice during the study.
Clinical care and management
Clinical care was provided by a consultant paediatrician from the Medical school University of Nairobi in conjunction with institutional medical staff. Referrals were made where necessary. The majority of the children were diagnosed with multiple clinical conditions, mainly fungal or bacterial (ringworm, tineasis, oral candidiasis; pneumonia/recurrent, otitis media), inflammatory (Myositis/ Mucositis/ Dermatitis), gastrointestinal (giardiasis); nutritional (scabies, iron deficiency anaemia); viral (herpes zoster, chicken pox) and recurrent pyrexia (or fever) of unknown origin (PUO/FUO). Treatments were case-specific but included broad-spectrum antibiotics (bactrim, rifampicillin, augmentin), hydroxyurea, isoniazid and multivitamins. Trimethoprimsulfamethoxazole (TMP-SMX) was used for pneumonia (or PCP) treatment or prophylaxis. One child was admitted to the following anti-viral treatment during the follow-up; AZT (azidothymidine), 3TC (lamivudine) and efavirenz
Design
In order to determine the relationship over-time of viral load, CD4+ T-lymphocytes and anti-HIV antibody titres with age and clinical symptoms, we conducted a longitudinal cohort study of HIV-1 seropositive children between 1999 and 2003. Classification into clinical and immunological categories and age groups were conducted once only at the beginning of the study and maintained as such throughout follow-up. Each child was followed for a period of one year, with 3 repeat measurements taken every 4 months for the outcome variables of HIV-1 RNA load and CD4+ T-lymphocyte counts and percentages, and 2 measurements for antibody titres. These measurements were all accrued over a period of 3 years. All the laboratory analyses were conducted at the Nyumbani Diagnostic Laboratory in Karen, Nairobi except for PCR and western blots respectively done at the Departments of Biochemistry, University of Nairobi and the Virology Laboratory, Institute of Primate research, Nairobi.
Blood collection and processing
Three millilitres of whole blood from each subject were collected in EDTA-coated tubes (Beckton-Dickinson, SA). Whole blood was used immediately for CD4+ T-lymphocytes enumeration. Blood plasma were processed and frozen in 1.8 ml screw-cap tubes at -85°C until needed for antibody and viral load quantitations.
Anti-HIV antibody screening and quantitation
For inclusion into the study, retrospective serological data for each child acquired before admission was adopted. This data showed that all children were positive for anti-HIV-1 antibodies. Consequently, all the children were prospectively followed up for HIV-1 specific IgG antibody titres using conventional ELISA. Briefly, plates were coated to a final concentration of 0.4µg per well with a 21 amino acid synthetic peptide derived from a conserved, immunodominant region of HIV-1-gp41 (Research Genetics, CA). After successive wash, enzyme and substrate reaction steps, the plate optical densities (O.D) were read at 420nm. Control and test plasma were assayed in duplicate and samples were considered positive if mean O.D was greater than mean O.D for the negative control plus 3 standard deviations. Antibody titres were recorded as reciprocal end point dilution.
Antibody confirmation by Western blot
Samples that had indeterminate or negative titres were analyzed by western blot (Bio-Rad, Sannoffi, Kenya) following manufacturer's instructions and results interpreted as before.21, 22 Briefly, nitrocellulose strips coated with HIV-1 antigen were incubated with wash/diluent buffer for 5 minutes before incubation with diluted patient and control plasma (30µl) diluted 1:100 in buffer. After washing and reaction with alkaline phosphatase conjugate, BCIP (5-bromo-4-chloro-3-indolyl-phosphate) and NBT (nitro blue tetrazolium) substrate solution was added. The reaction was stopped, strips blotted, air-dried and photographed.
Proviral DNA PCR
Western Blot analysed samples were further PCR analysed on Gene Amp 2400 (Perkin Elmer) using genomic DNA isolated from lysed peripheral blood mononuclear cells. HIV-1 gag and pol primers (GIBCO BRL- Biosystems, South Africa) (0.5µM) were used in the 50µl two-round PCR reaction containing 10µl (20ng) template and control DNA. Nested PCR included a 5 minutes hot-start at 95°C followed by 37 amplification cycles of denaturation, annealing and elongation at 94°C/45 sec, 55°C/60sec and 72°C/90 sec. respectively, and a final 10 min elongation at 72°C. HIV-1 DNA was detected on 2% agarose gel stained with 0.01% ethidium bromide. 23–26.
Quantitation of HIV-1 RNA Load
HIV-1 RNA loads were determined by reverse transcriptase polymerase chain reaction (Roche 1.5 Amplicor, Roche Diagnostics). Briefly, HIV-1 RNA was extracted from 200µl of frozen EDTA-treated plasma, reverse transcribed and PCR amplified on gene Amp 9600 thermocycler. Hybridization was done on micro-well plates coated with biotin labelled oligonucleotide probes before detection with avidin-horseradish peroxidase conjugate and 3,3′, 5,5′-tetramethylbenzidine substrate in the presence of hydrogen peroxide. After stopping reaction with 4.9% sulphuric acid, optical density was read at 450nm. Sample viral loads were compared against quantitation standard RNA, and calculated as HIV-1 RNA copies per millilitre of plasma. The detection limit was 50 RNA copies/mL plasma
Quantitation of T-lymphocyte phenotypes
T-lymphocytes were enumerated by fluorescence activated cell counter (FACSCount, Becton Dickinson) from freshly collected whole EDTA blood. Briefly, fifty microlitre blood was stained with fluorochrome labelled anti-CD4 and anti-CD8 monoclonal antibodies and incubated for 120 minutes at room temperature in the dark. The samples were fixed in 50µl of 5% formaldehyde in phosphate buffered saline and enumerated on a FACSCounter.
Data and statistical analysis
But for two exceptions, the study population was divided into groups and categories based on 1994 revised classification system for human immunodeficiency virus infected children under 13 years of age. First, all children separately classified as asymptomatic (CDC class N) and mildly symptomatic (CDC class A) were merged into a single clinical category referred to hereafter as asymptomatic (or CDC class N). The other two clinical categories were without alteration. Second was the grouping into only two age brackets namely; less than 6 years and six years to thirteen years. These minor modifications were to facilitate logical data analyses because only one child was above 12 years (13 years old) while the difference between clinical classes N and A was blurred. These categories or groups were defined as independent variables and used for statistical analyses of the three dependent outcome variables of viral (HIV-1 RNA) load, CD4+T-lymphocyte counts or percentages and antibody titres. Viral load and antibody titres were log-transformed while CD4 T-lymphocyte counts and percentage were based on absolute values. Non-parametric tests for 2 (Mann Whitney's) and multiple (Kruskal Walli's) independent variables were used to compare medians between groups while Friedman's test was used to compare difference between medians of repeated outcome measurements. Measures of association between dependent variables were achieved using Bivariate Correlation model. Analyses using immunological categories as independent variable are not included in this report. Statistical package for Social Scientists (SPSS) was used for data acquisition and analysis and graphical out-put by Microsoft excel. Only tables of significance are included.
Ethical considerations
This study was cleared by the National Council for Science and Technology, Kenya and conducted in accordance with National guidelines for involving human subjects in biomedical research. Informed consent was obtained from the Board of Directors of the Children's home, acting in lieu of parents as legal guardians. Blood was obtained to coincide with routine clinical management, hence eliminating additional demand directly for the purpose of the study, while generating support data for patient care. There was no authoritative Governmental policy in Kenya concerning ARV therapy at the time of study and any treatments were based on patients' financial ability or child-specific donation. All deaths were reported to appropriate governmental authorities and post-mortem conducted at the Nairobi hospital. Publication of this manuscript has been approved by KEMRI.
Results
Participants
Of the 51 children recruited (median age of 6 years), 28 were males and 23 females. Out of this total, 3 children (2 females aged 1.1 and 1.24 and 1 male aged 8 years) were excluded from follow-up analyses after consistently demonstrating undetectable antibody titres as well as testing pro-viral DNA and viral antibody negative by PCR and western blots. Table 1 summarises subject distribution by age, sex and clinical category while table 2 shows the various immunological categories and corresponding median distribution of CD4+ T-lymphocytes at the first (baseline) measurement. Of the remaining 48 children, 26 had no evidence of suppression (median %CD4 T-cell, 42), 12 had moderate suppression (23% CD4+) while 10 were severely suppressed (8% CD4+).
Table 1. Summary subject distribution by age, clinical category and sex.
Table 1 details numerical and statistical distribution of individual 48 subjects successfully included in the follow-up under respective categories, excluding 3 who were dropped due to HIV-1 negative status confirmed during follow-up. Median, minimum and maximum refers to age in years while the rest of numerical entries refer to number of study subjects. Distribution is based on information collected at the beginning of the study. The bolded numbers are sub totals and totals within the categories.
| Age-group | Sex | Age (years) | CDC Clinical category (n, number of cases) |
Total | ||
| N | B | C | ||||
| 1-< 6 years | M | 2 | 3 | 8 | 13 | |
| F | 1 | 4 | 1 | 6 | ||
| 3 | 7 | 9 | 19 | |||
| Median | 2.4 | 3 | 4 | 3.75 | ||
| Minimum | 1.16 | 1 | 2 | 1 | ||
| Maximum | 5 | 4.5 | 5.4 | 5.4 | ||
| >= 6 years | M | 6 | 2 | 6 | 14 | |
| F | 6 | 2 | 7 | 15 | ||
| 12 | 4 | 13 | 29 | |||
| Median | 7 | 8.25 | 8 | 7 | ||
| Minimum | 6 | 6 | 6 | 6 | ||
| Maximum | 13 | 11 | 12 | 13 | ||
| Total (n=) | 15 | 11 | 22 | 48 | ||
< less than/below; >= greater than or equal to; = equal to; n, number of cases; N, asymptomatic; B, moderately symptomatic; C, severely symptomatic.
Table 2. Median CD4+ cell counts and percentages by age group of untreated HIV-1 infected children in Kenya.
Table 2 shows immunological classification based on age-specific CD4+ T-lymphocyte data obtained at the first of the three prospective time-points of measurements and defined by respective percentage values. CD4%, >=25 defined lack of suppression while CD4% of 15–24 and CD4%, <15 respectively defined moderate and severe suppression. 19
| Age <6 years | Age >= 6years | |||
| Category | CD4% (IQR) | CD4Counts per micro litre (IQR) |
CD4% (IQR) | CD4Counts per micro litre (IQR) |
| CD4%, >=25 | 39 (33–48) | 1362 (634–1677) | 42 (33–48) | 919 (665–1116) |
| CD4%, 15–24 | 24 (22–33) | 711 (459–857) | 22 (16–27) | 621 (385–766) |
| CD4%, <15 | 11.5 (2.2–16) | 245 (43–426) | 8 (4.6–9.3) | 108 (47–225) |
IQR Inter quartile range
Losses to follow-up
A total of 9 children did not have complete viral load and CD4+ T-lymphocyte measurements for the third time-point. These measurements constituted losses-to-follow up at specific time-points. Seven of these were as a result of death (median age 6 years; 3< & 4>=6 years) with median viral load measurements of 401,479 (minimum=61,629 and maximum= 2,849,578) copies per millilitre plasma and median CD4+ T-lymphocyte counts or percentage of 189 or 7% (minimum=2, maximum=586) cells per microlitre at time preceding death. The two remaining losses were due to critical illness (age= 2 years, viral load=796,009, CD4+ cells= 460) and anti-retroviral (ARV) treatment initiated before completion of study.
Confirmation of HIV-1 infection status
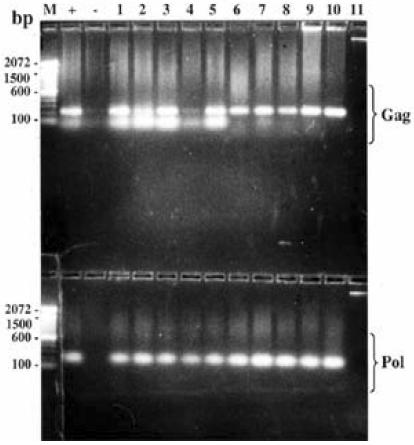
Further analyses by western blot (figure 1A) and PCR (figure 1B) on the children with negative or indeterminate ELISA titres failed to show detectable antibodies or proviral DNA (gag and pol) in 3 children (aged 1.1, 1.25 and 8 years old). The 3 were subsequently excluded from prospective analysis of viral load and CD4 T-lymphocytes and their antibody titre measurements have likewise been omitted from this report. Overall, pol appeared more specific than gag primers, which were however, highly sensitive.
Figure IA. Western Blot.
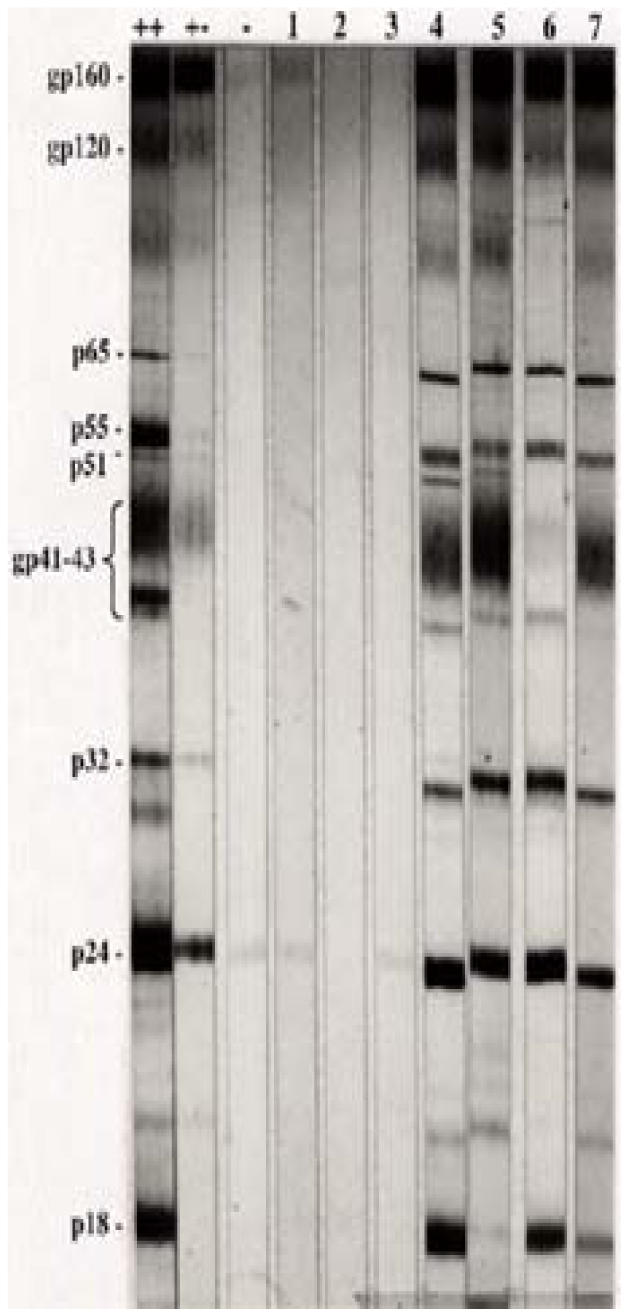
Figure 1A shows Western blot profile for 3 initially antibody positive but later negative (lanes 1–3) and some selected antibody indeterminate (lanes 4–7) children. (++) strong positive; (+-) weak positive and (-) negative control serum
Figure IB.
Figure 1B shows gag (upper panel) and pol (lower panel) proviral HIV-1 DNA nested PCR for children with indeterminate anti-HIV-1 antibodies and typical positive (lanes 1–10) or negative (lane 11) results. Column M shows the separation of a 100 base-pairs DNA ladder while + and - represents positive and negative control human DNA respectively.
Antibody titres
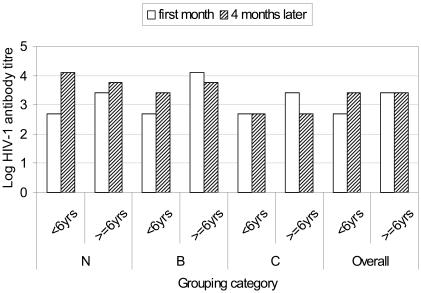
Antibody titres for individual subjects were considered undetectable if less than 100 reciprocal endpoint units and indeterminate if undetectable at one or the other time point. Of the 48 children followed, 24 (50%) had indeterminate titres, 16 of them below and 8 above 6 years. Descriptive statistics as well as frequency distribution for antibody titres are shown in table 3. Overall, median antibody titres were stable over the period of measurement for older children but increased reasonably in younger children, attaining same values as in older ones (figure 2). The differences in median antibody titres between the two age groups and the 3 clinical categories were not statistically significant (p> 0.15) as shown in table 4.
Table 3. Frequency distribution of Median Viral load, CD4T-lymphocyte counts and percentage and antibody titres by immunological, clinical and age categories in HIV-1 infected children.
Table 3 shows descriptive statistics for the three outcome variables as defined by respective grouping variables. Logarithmic transformed viral load, absolute CD4+ counts and percent and antibody titres are given as median of the three time points (2 for antibody) measured after averaging all the time-points. Entries with missing cases are described elsewhere as losses-to-follow at the third time-point. This table was generated using the Statistical Package for Social Scientists (SPSS) software.
| Median | ||||||
| Age Group |
*Clinical category |
Log viral load |
CD4+ counts (cells/µL) |
Per-cent (%) CD4+ cells |
Antibody titres |
|
| Immunologic category | ||||||
| Category 1: (No suppression) |
||||||
| <6yrs | N or A (n=2) | 4.56 | 1075 | 33.5 | 3400 | |
| B (n=4) | 5.02 | 756 | 30.5 | 800 | ||
| C (n=3) | 4.94 | 959 | 32 | 1500 | ||
| Total (n=9) | 5.01 | 904 | 31 | 1300 | ||
| >=6yrs | N or A (n=11) | 4.06 | 903 | 42 | 2500 | |
| B (n=2) | 4.27 | 766 | 36 | 22500 | ||
| C (n=4) | 4.75 | 872 | 36 | 1500 | ||
| Overall (n=17) | 4.29 | 868 | 39 | 2500 | ||
| Overall (n=26) | 4.68 | 872 | 36 | 1500 | ||
| Category 2: (Moderate suppression) |
||||||
| <6 years | N or A (n=1) | 3.51 | 552 | 19 | 7500 | |
| B (n=2) | 5.26 | 585 | 19 | 3750 | ||
| C (n=3) | 5.37 | 554 | 20 | 250 | ||
| Overall (n=6) | 5.11 | 553 | 20 | 3875 | ||
| >=6yrs | C (n=3) | 4.26 | 517 | 18 | 1250 | |
| B (n=2) | 4.42 | 406 | 18 | 6250 | ||
| N or A (n=1) | 3.68 | 679 | 21 | 7500 | ||
| Overall (n=6) | 4.16 | 464 | 19 | 1875 | ||
| Overall (n=12) | 4.43 | 535 | 20 | 1875 | ||
| Category 3: Severe suppression |
||||||
| <6yrs | C (n=3) | 5.37 | 117 | 6 | 1300 | |
| N or A (n=1) | 5.13 | 246 | 10 | 2500 | ||
| Overall (n=4) | 5.25 | 182 | 8 | 1900 | ||
| >=6yrs | C (n=6) | 4.95 | 116 | 6.5 | 1500 | |
| Overall (n=10) | 5.06 | 140 | 6.5 | 1500 | ||
| Overall (N=48) | 4.73 | 644 | 26 | 1500 | ||
Based on 1994-revised Human Immunodeficiency Virus Paediatric Classification System; Centre for Disease Control and Prevention.19
Figure 2.
Figure 2 is a bar graph showing median anti HIV-1 antibody titres against respective grouping categories. Antibody titres were measured over a 2-time point period separated by a 4-months interval and reported as reciprocal end-point titres. Data was acquired using Statistical Package for Social Scientists (SPSS) and graphs generated using Microsoft Excel. Log-transformed medians of grouped data were analysed as described under statistical methods' section.
Table 4. Test of significance for viral load, CD4+T-lymphocyte and antibody profiles using age group (a) and clinical category (b) as independent or analysis variables.
Table 4: Analyses were based on entries in table 3 and compare the differences between age groups (a) or clinical categories (b) using non-parametric tests for 2 or multiple independent variables respectively. Data was acquired and analysed using the Statistical Package for Social Scientists (SPSS). Significance level is considered for a ñ-value of less than 0.05
| Test Statistics | Median viral load (log) |
Median CD4+ T-cells/mL |
Median CD4+ T-cell percent |
Median antibody titre (log) |
|
| (a) | Mann-Whitney U | 115.500 | 259.000 | 216.500 | 209.000 |
| Wilcoxon W | 550.500 | 449.000 | 406.500 | 399.000 | |
| Z | 3.374 | -.348 | -1.245 | -1.413 | |
| Asymp. Sig. (2-tailed) | 0.001 | 0.728 | 0.213 | .158 | |
| (b) | Kruskal Wallis Test | ||||
| Chi-Square | 13.054 | 9.182 | 14.805 | 3.760 | |
| df | 2 | 2 | 2 | 2 | |
| Asymp. Sig. | 0.001 | 0.010 | 0.001 | 0.153 |
a Grouping Variable: age groups, significance level, 0.05
b Grouping Variable: clinical category, significance level, 0.05
n (total number of cases) =48
HIV RNA (viral) load and CD4+ T-lymphocytes
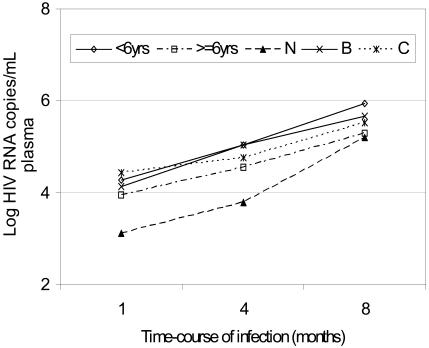
All computational analyses for significance levels entirely excluded subjects whose third time-point entries were missing (losses to follow-up described elsewhere). Figures 3 & 4 show profiles in medians while table 3 details the frequency distribution for viral load and CD4+ T-lymphocyte counts and percentages. Table 4 shows non-parametric test statistics for these variables. Although one child had a 3-time point undetectable viral load (<50 HIV-1 RNA copies/millilitre plasma), the lowest detectable viral load for children in the asymptomatic category (Class N) was 2.02 (median, 3.11) log10 copies at first time point. This increased to median of 5.2 log10 copies at the third time-point, representing a significant 2.09 logarithmic increase (p<0.0001) over the three time-points. On the other hand, severely symptomatic (Class C) children had initial viral load between 3.23 and 6.06 (median, 4.44) log10 copies, rising to between 4.39 and 6.32 (Median, 5.53) log10, representing a median increase of 1.09 log10 copies (p<0.0001) by the end of the study. Compared, the rise in viral load overtime was 10-fold faster for asymptomatic than symptomatic category. These viral loads were significantly higher for children below 6 than those above 6 years (p=0.001) as did for severely symptomatic compared to either asymptomatic or moderately symptomatic category (p=0.001). Similarly, severe immunological suppression was associated with comparably raised viral loads in the respective categories. Figure 3 shows a graphical trend of the viral load over time in each category.
Figure 3.
Figure 3 is a profile (line) plot showing trends in median log transformed HIV-1 RNA (viral) load during infection for different categories or groups of children. Viral loads were measured over a 3-time point period separated by a 4-months interval and reported as medians of log-transformed HIV-1 RNA copies per millilitre plasma. Data was acquired using Statistical Package for Social Scientists (SPSS) and graphs generated using Microsoft Excel. Log-transformed medians of grouped data were analyzed as described under statistical methods' section.
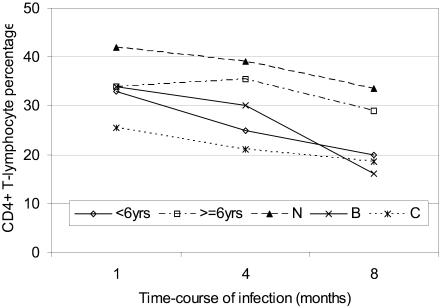
Figure 4.
Figure 4 is a profile (line) plot showing progression of CD4+T-lymphocyte percentage during infection. CD4+T-lymphocytes were measured over a 3-time point period separated by a 4-months interval and reported as median counts per microlitre blood or as percentage of total (CD3+) T-Lymphocytes for different categories. Data was acquired using Statistical Package for Social Scientists (SPSS) and graphs generated using Microsoft Excel. Analyses have been described under statistical methods' section.
Although there was no clear difference in initial CD4+T-lymphocyte counts or percentages between the two age groups, the subsequent decline from 33% to 20% for children less than 6 years was significant (p<0.002, n=15) but not for those above 6 years (p=0.086, n=24) (table not shown). At 42%, median CD4+ T-cells were highest for asymptomatic compared to moderately symptomatic (34%) or severely symptomatic (25.5%) children. These values were lowest for severely suppressed children in any category. Immunological progression saw median CD4% decline 6-fold for severely suppressed children compared to those without evident suppression (table 2). Although CD4% seemed to be a better marker of difference than absolute counts, these differences were not statistically significant between groups, when clinical and immunological categories or age group were the independent factors of analysis.
Bivariate Correlation Model
Non-parametric correlations (table 5) were computed to evaluate association. For children above 6 years, Spearman's rho revealed a significant association between viral load and CD4+% (p=0.026). There was no significant correlation between the rest of the outcome variables, neither were there any deviations from this pattern of association when Pearson correlation was used.
Table 5.
Bivariate Correlations
| Spearman's rho | Median viral load |
Median CD4+ % |
Median antibody |
||
| Median viral load | |||||
| >= 6 years | Correlation Coefficient | 1.000 | -.414(*) | -.210 | |
| Sig. (2-tailed) | . | .026 | .273 | ||
| N | 29 | 29 | 29 | ||
| < 6 years | Correlation Coefficient | 1.000 | -.207 | .017 | |
| Sig. (2-tailed) | . | .395 | .945 | ||
| N | 19 | 19 | 19 | ||
| Median CD4+ % | |||||
| >= 6 years | Correlation Coefficient | -.414(*) | 1.000 | .040 | |
| Sig. (2-tailed) | .026 | . | .835 | ||
| N | 29 | 29 | 29 | ||
| < 6 years | Correlation Coefficient | -.207 | 1.000 | -.165 | |
| Sig. (2-tailed) | .395 | . | .499 | ||
| N | 19 | 19 | 19 | ||
| Median antibody | |||||
| >= 6 years | Correlation Coefficient | -.210 | .040 | 1.000 | |
| Sig. (2-tailed) | .273 | .835 | . | ||
| N | 29 | 29 | 29 | ||
| < 6 years | Correlation Coefficient | .017 | -.165 | 1.000 | |
| Sig. (2-tailed) | .945 | .499 | . | ||
| N | 19 | 19 | 19 |
Correlation is significant at the 0.05 level (2-tailed).
Discussions
We report in this paper, a relationship between HIV-1 RNA loads, CD4+ T-lymphocytes and anti- HIV-1 antibodies with age group and clinical category of perinatally HIV-1 infected African children.
Viral loads were higher for children below 6 than those above 6 years as did those with severe symptoms compared to other clinical categories. Both age group and clinical category of children significantly affected viral load outcome. However, a 100-fold logarithmic increase in viral loads overtime recorded for the asymptomatic compared to a 10-fold rise in the symptomatic category shows that although initial HIV-1 RNA load is low in these children, a rapid and logarithmic rise during the course of infection may increase the risk of developing AIDS in initially asymptomatic children. While previous studies have reported an unlikelihood of survival beyond 2 years of age at >750,000, 14 or increased risk of death at >250,000 27 HIV-1 RNA copies, 7 children (median age 6 years) who died during our follow-up had a median viral load of 5.6 log10 (401,479) and a maximum of 6.45 log10 copies/millilitre at time of death. This compared favourably with our final time-point median viral load of 5.37 (max.5.68) and 6.05 (max.6.31) log10 copies respectively for surviving children above (n=13) and below (n=9) 6 years in the symptomatic clinical category, suggesting that these children may have reduced chances of survival long after attainment symptomatic status. With a median viral load greater than 1000,000, younger children (median age=3.5 years) in this study were at a greater risk of rapid progression to disease or death, progressing at viral loads greater than previously reported.14, 28 Our observation conforms with recent findings, 20 and underscores the usefulness of viral load measurements in defining the course of disease in African children.
On the other hand, CD4+ T lymphocyte counts and percentages declined inversely to viral load but gradually and significantly (p<0.0001) over the period of measurement. Like in viral load, both age group and clinical category significantly affected these differences. In spite of a few symptomatic children, majority of them below 6 years recording higher individual CD4+ T-lymphocyte counts and percentages than asymptomatic children, the symptomatic category had comparably low CD4+ T lymphocyte counts and percentages overall. The afore mentioned exception may be linked to functional abnormality of cellular immunity, 22 or inherently high total lymphocytes in younger children.17 These levels were generally stable over-time for individual children, but started from similar level for the two age-groups before declining rapidly for the children less than 6 years. Median CD4+ T-lymphocyte counts of 554 (20%) for children below 6 years and 335 (16%) per microlitre blood for children above 6 years characterised symptomatic clinical status. These values may be useful in initiation and management of antiretroviral therapy alongside specific viral load thresholds.
CD4+ T-lymphocyte counts and viral loads correlated significantly with % CD4+ T-cells for children above but not below 6 years. Although not significant, antibody levels were lowest overall for children with symptomatic disease but generally attained a constant level for the two age-groups during the period of study despite starting low in the under 6 years category. This profile reflects a pattern in which younger children gradually develop specific anti-HIV-1 antibody responses similar to that seen in older children. However, they may be initially exposed to an early risk of immunological suppression than older ones. Coupled with maternally acquired but immunologically defective antibodies, 29, 30 early immune suppression may lead to unrestricted viral replication further compounding progression to AIDS. This observation draws credence from our data, in which a significant correlation between viral load and CD4+ T-lymphocytes was observed. A possible deviation from this pattern may exist as we have already observed, with some severely symptomatic children demonstrating indeterminate, undetectable or reduced antibody titres possibly due to severe immunosuppression or impaired B-cell function.31, 32 With 3 children (median age=2 years) persistently testing PCR and western blot negative, the possibility that maternally acquired antibodies may persist beyond the previously reported 15 months22 can not be precluded. Thus, a combination of these two methods offers reliable approach for definitive diagnosis of infection in younger children.
We conclude that HIV-1 RNA load is higher in younger than older untreated perinatally HIV-1 infected children. Although highest in symptomatic children, these viral loads rise 10-fold faster and more logarithmically during the course of infection in asymptomatic than symptomatic children, suggesting the symptom-free phase may be short in untreated children. This pattern is accompanied by gradual and significant depletion of CD4+ T-cells. Early interventions targeting the developing neonatal immune system may be useful in prolonging survival in these children. Such intervention should take into account age as a variable.
Acknowledgments
We thank all both the Children of God Relief Fund, USA and the Nigel Montgomery, Treasurer Nyumbani (UK) LTD for financial donations used for the better part of this work. In same regard, special thanks to Father (Dr) Angelo D'Agostino, the Director of Nyumbani Children's Home in Kenya for facilitating these funding and for logistic support. Many thanks to the management of the orphanage, especially to Protus Lumiti and Sister Teresa Palakudy for administrative support. Unequalled gratitude goes to the orphaned children of Nyumbani who participated in this work. Their life and vigour was a challenge. Finally, we appreciate the Director, Kenya Medical Research Institute (KEMRI) and the University of Nairobi for institutional facilitation preceding submission and publication of this manuscript.
References
- 1.Goudsmit J, de Wolf F. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet. 1986;2:177–180. doi: 10.1016/s0140-6736(86)92485-2. [DOI] [PubMed] [Google Scholar]
- 2.Scott GB, Buck BE, Leterman JG, Bloom FL, Parks WP. Acquired immune deficiency syndrome in infants. N Engl J Med. 1984;310:76–81. doi: 10.1056/NEJM198401123100202. [DOI] [PubMed] [Google Scholar]
- 3.Falloon JJ, Eddy L, Weiner L, Pizzo PA. Human immunodeficiency virus infection in children. J Pediatr. 1989;114:1–30. doi: 10.1016/s0022-3476(89)80596-7. [DOI] [PubMed] [Google Scholar]
- 4.Buchbinder SP, Katz MH, Hessol NA, O'Malley PM, Holmberg SD. Long-term HIV-1 infection without immunologic progression. AIDS. 1994;8:1123–1128. doi: 10.1097/00002030-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Haynes BF, Pantaleo G, Fauci AS. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 6.Saye HK, Lynne P, Neil S, et al. Tumour necrosis factor c2 microsatellite allele is associated with the rate of HIV disease progression. AIDS. 1997;11:423–428. doi: 10.1097/00002030-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Melvin AJ, Rodrigo AG, Mohan KM, Lewis PA, Manns-Acruino L, Coombs RW, Mullins JI, Frenkel LM. HIV-1 dynamics in children. J Acquir Immune Defic Syndr & Hum Retrovirol. 1999;20:468–473. doi: 10.1097/00042560-199904150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control: HIV/AIDS Surveillance. Atlanta: Department of Health and Human Services; 1992. Jul, [Google Scholar]
- 9.De Rossi A, Ometto L, Masiero S, Zanchetta M, Chieco-Bianchi L. Viral phenotype in mother-to-child HIV-1 transmission and disease progression of vertically acquired HIV-1 infection. Acta Paediatr Suppl. 1997;421:22–28. doi: 10.1111/j.1651-2227.1997.tb18315.x. [DOI] [PubMed] [Google Scholar]
- 10.Paediatric European Network For The Treatment of AIDS (PENTA), author HIV-1 viral load and CD4+ cell counts in untreated children with vertically acquired asymptomatic or mild disease. AIDS. 1998;12:F1–F8. [PubMed] [Google Scholar]
- 11.Shearer WT, Thomas CQ, Larussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type-1. N Engl J Med. 1997;19:1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 12.Tovo PA, de Martino M, Gabiano Cappello NC, et al. Prognostic factors and survival in children with perinatal HIV infection. Lancet. 1992;339:1249–1253. doi: 10.1016/0140-6736(92)91592-v. [DOI] [PubMed] [Google Scholar]
- 13.Abrams EJ, Weedon J, Steketee RW, et al. Association of human immunodeficiency virus (HIV) load early in life with disease progression among HIV-infected infants. J Infect Dis. 1998;178:101–108. doi: 10.1086/515596. [DOI] [PubMed] [Google Scholar]
- 14.Tetali S, Bakshi S, Than S, et al. Plasma virus load evaluation in relation to disease progression in HIV-infected children. AIDS Res Hum Retroviruses. 1998;14:571–577. doi: 10.1089/aid.1998.14.571. [DOI] [PubMed] [Google Scholar]
- 15.Romiti ML, Colognesi C, Cancrini C, et al. Prognostic value of a CCR5 defective allele in paediatric HIV infection. Mol Med. 2000;6:28–36. [PMC free article] [PubMed] [Google Scholar]
- 16.Rich KC, Fowler MG, Mofenson LM, et al. Maternal and infant factors predicting disease progression in human immunodeficiency virus type 1-infected infants. Pediatrics. 2000;105(1):e8. doi: 10.1542/peds.105.1.e8. [DOI] [PubMed] [Google Scholar]
- 17.Denny T, Yogev R, Gelman R, et al. Lymphocyte subsets in healthy children during the first 5 years of life. JAMA. 1996;267:1484–1488. [PubMed] [Google Scholar]
- 18.McKinney RE, Wilfert CM. Lymphocyte subsets in children younger than 2 years old. Normal values in populations at risk of Human Immunodeficiency Virus infection and diagnostic and prognostic applications to infected children. Pediatr Infect Dis J. 1992;11:639–644. [PubMed] [Google Scholar]
- 19.Centers for Disease Control, author. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. 1994. Official authorized addenda: human immunodeficiency virus infection codes and official guidelines for coding and reporting ICD-9-CM. MMWR. 1994;43(RR-12):1–19. [Google Scholar]
- 20.Rouet F, Sakarovitch C, Msellati P, et al. Pediatric Viral Human Immunodeficiency Virus Type 1 RNA Levels, Timing of Infection, and Disease Progression in African HIV-1-Infected Children. Pediatrics. 2003;112(4):e289–e289. doi: 10.1542/peds.112.4.e289. [DOI] [PubMed] [Google Scholar]
- 21.Celum CL, Coombs RW, Lafferty W, Inui TS. Indeterminate human immunodeficiency virus type-1 western blots: seroconversion risks, specificity of supplemental tests and an algorithm for evaluation. J Infect Dis. 1991;164:656–664. doi: 10.1093/infdis/164.4.656. [DOI] [PubMed] [Google Scholar]
- 22.Antonio VS, Campos JM. Laboratory methods for early detection of human immunodeficiency virus type 1 in newborns and infants. Clin Microbiol Rev. 1992;5:238–247. doi: 10.1128/cmr.5.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning - a Laboratory Manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 1989. [Google Scholar]
- 24.Lynch CE, Roberta M, Louie PH, Rodgers G. Detection of HIV-1 DNA by PCR: evaluation of primer pair concordance and sensitivity of a single primer pair. AIDS. 1992;5:433–440. [PubMed] [Google Scholar]
- 25.Vandamme AM, Fransen K, Debaisieux L, et al. Standardisation of primers and an algorithm for HIV-1 diagnostic PCR evaluated in patients harbouring strains of diverse geographical origin. J Virol Methods. 1995;51:305–316. doi: 10.1016/0166-0934(94)00126-2. [DOI] [PubMed] [Google Scholar]
- 26.Loche M, Mach B. Identification of HIV infected seronegative individuals by direct diagnostic test based on hybridisation to amplified viral DNA. Lancet. 1988;(ii):418–421. doi: 10.1016/s0140-6736(88)90412-6. [DOI] [PubMed] [Google Scholar]
- 27.Taha TE, Kumwenda NI, Hoover DR, et al. Association of HIV-1 load and CD4+ lymphocyte counts with mortality among untreated African children over 1 year of age. AIDS. 2000;14:453–459. doi: 10.1097/00002030-200003100-00021. [DOI] [PubMed] [Google Scholar]
- 28.Valentine ME, Jackson CR, et al. Evaluation of surrogate markers and clinical outcomes in two-year follow-up of eighty-six human immunodeficiency virus infected paediatric patients. Pediatr Infect Dis J. 1998;17:18–23. doi: 10.1097/00006454-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Krilov LR, Kamani N, Hendry RM. Longitudinal serologic evaluation of an infant with acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1987;6:1066–1067. [PubMed] [Google Scholar]
- 30.Ragni MV, Urbach AH, Tailor S. Isolation of human immunodeficiency virus and detection of HIV DNA sequences in the brain of an ELISA antibody-negative child with acquired immune deficiency syndrome and progressive encephalopathy. J Pediatr. 1987;110:892–894. doi: 10.1016/s0022-3476(87)80404-3. [DOI] [PubMed] [Google Scholar]
- 31.Sison AV, Sever JL, Brandt CD, et al. Annual meeting of Laboratory Tumor and Cell Biology. Bethesda, Maryland: National Cancer Institute; 1991. Virological and immunological factors in perinatal HIV-1 transmission. [Google Scholar]
- 32.Peters VB, Mayer L, Sperber KE. Correlation of clinical parameters and immunological function with human immunodeficiency virus plasma viremia in children. Viral Immunol. 1999;12(2):139–148. doi: 10.1089/vim.1999.12.139. [DOI] [PubMed] [Google Scholar]