Abstract
Background
A pre-packaged fixed-dose formulation of chloroquine (CQ) and sulfadoxine/pyrimethamine (S/P) combination (Homapak) is widely used for the treatment of falciparum malaria in Ugandan children. It is however a product whose pharmacokinetics and interactions have not been studied.
Objectives
To explore possible pharmacokinetic interactions between CQ and S/P during co-administration, and to determine their bioavailability in the locally made Homapak compared to the Good Manufacturing Practice (GMP) made formulations.
Methods
Thirty-two adult healthy volunteers were randomized into four groups and given single oral doses of fixed-dose CQ+S/P combination (Homapak), or GMP formulations of S/P (Fansidar), CQ (Pharco), or their combination. Plasma samples were followed for 21 days, analysed by HPLC-UV methods, with pharmacokinetic modeling using the WinNonlin software.
Results
Sulfadoxine in Homapak was more rapidly absorbed (ka = 0.55 h-1) than in Fansidar + CQ (ka = 0.27 h-1, p=0.004), but not more than S in Fansidar alone group (ka = 0.32 h-1, p=0.03). No significant differences were observed in the other pharmacokinetic parameters of S, P and CQ when given together or separately. The relative bioavailability of CQ and S in Homapak showed bioequivalence to reference formulations.
Conclusions
There were no pharmacokinetic interactions between CQ, S and P when the compounds were given together, however, more investigations would be needed to explore this further. Compared with GMP made drugs, both S and CQ are bioequivalent in Homapak, the Ugandan made fixed-dose formulation. Furthermore, the absorption of S was more rapid which could be advantageous in malaria treatment.
Keywords: sulfadoxine, pyrimethamine, chloroquine, bioequivalence, pharmacokinetic interactions
Introduction
In order to delay the development of resistance, combination therapy has been recommended for treatment of falciparum malaria1. Several antimalarial drug combinations are now in use, but not all are affordable and easily available2. Chloroquine (CQ) and sulfadoxine / pyrimethamine (S/P) are still the most affordable and easily available antimalarials, widely used in many resource poor nations despite the widespread resistance of P. falciparum to these drugs3.
The current antimalarial drug policy at health facilities in Uganda is Co-Artem, a co-formulated combination therapy containing artemether plus lumefantrine. However, the drug is not yet available4. A locally made pre-packaged fixed-dose generic formulation of chloroquine and sulfadoxine/pyrimethamine (locally known as Homapak)5 is still the recommended drug for the Home Based Management of Fevers (HBMF) program in the country6. The freely available Homapak has improved community access and compliance to prompt treatment of suspected malaria episodes in the under-five children, thereby reducing malaria related childhood mortality 7, 8.
While generic formulations are promoted to address accessibility and affordability of drugs, reports on the quality of medicines in African countries have generally portrayed generics as poor quality or fake products9–12. When the different products are either fake or substandard, the possibility of treatment failure and/or interactions is potentially increased2, 13. Co-administration of different products may also result in pharmacokinetic interactions14. Rengelshausen et al15 found that co-administration of methylene blue with chloroquine as treatment for malaria caused a 35% reduction in the area under the curve (AUC) of chloroquine. Grace et al16 have reported that chloroquine weakly inhibits the metabolism of arteether by inhibition of the enzyme arteether O-deethylase. Chloroquine has also been shown to decrease the bioavailability of ampicillin when they are co-administered17. The manufacturers of chloroquine advise that kaolin and antacids may reduce its absorption, and that cimetidine may inhibit chloroquine metabolism through the inhibition of CYP3A4, with the potential of increasing plasma CQ concentration. A Medline search did not yield any studies on sulfadoxine or pyrimethamine pharmacokinetic interactions.
The aim of this study was to explore possible pharmacokinetic interactions between CQ and S/P during co-administration in healthy adult Ugandan volunteers. Furthermore, we wanted to determine the bioequivalence of these compounds in the locally made Homapak compared to the Good Manufacturing Practice (GMP) made formulations.
Methods
Materials
The drugs used in the study, Fansidar®, Batch No. 3054 (Hoffman-La Roche, Basel, Switzerland) containing 500mg/25mg per tablet of sulfadoxine/pyrimethamine (SP) respectively, and Chloroquine Phosphate (CQ) Batch No. 174 (Pharco Pharmaceuticals, Alexandria, Egypt) equivalent to 150mg of the base, were bought from the National Medical Stores, Uganda. Both Fansidar and CQ (Pharco) are made according to Good Manufacturing Practice (GMP). Homapak® Batch No. 0402 (Kampala Pharmaceutical Industries Ltd. Kampala, Uganda) was provided by the Ministry of Health. Homapak is available as blister packs containing one tablet of S/P (500mg/25mg of sulfadoxine/pyrimethamine respectively), and three tablets of CQ (each containing chloroquine phosphate equivalent to 150mg of the base) fixed-dose.
Study design
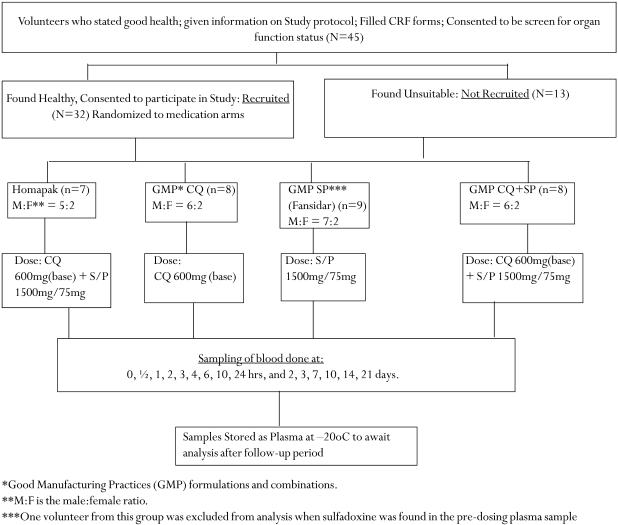
The study was conducted at the Department of Pharmacology and Therapeutics, Faculty of Medicine, Makerere University, Kampala, Uganda. The flow chart (Figure 1) shows the recruitment, grouping, dosing and sampling of the volunteers. Only volunteers of proven good health, with no history of intake of antimalarials in the last three weeks, were included in the study. This was an open label study, in which the volunteers were randomly assigned to Homapak, Fansidar only, Fansidar+CQ or CQ only formulations. A two-stage randomization process was carried out, firstly to assign the medications into four groups, and secondly to assign the volunteers to a particular group, using computer generated random numbers.
Figure 1.
Study design showing recruitment, grouping, dosing and sampling of volunteers.
In the morning of medication, each volunteer was given a single oral dose equivalent to sulfadoxine/pyrimethamine =1500mg/75mg or chloroquine=600mg, singly or combined on an empty stomach, swallowed with a glass of drinking water under medical supervision. In order to obtain the above dosage for Homapak, three blister packs were opened, from which three tablets of S/P and four tablets of CQ were administered to each volunteer in the group (Fig. 1). Venous blood samples (2 x 5 ml) were collected from each volunteer in heparinized vacutainer tubes at intervals indicated in the chart (Figure 1). The samples were immediately centrifuged at 3500 g for 10 min to obtain plasma, which was then stored at -20°C until assay. Volunteers were allowed light breakfast (a cup of tea and a toast of bread) after the 2-hour sample. Thereafter, water was allowed ad libitum. Lunch was taken between 5 to 6 hours after drug administration.
HPLC Analysis
Drug plasma levels were assayed using HPLC at the Division of Clinical Pharmacology, Karolinska Institutet at Karolinska University Hospital, Huddinge, Stockholm, Sweden. Sulfadoxine (limit of determination 29µmol/l) and pyrimethamine (limit of determination 270nmol/l) concentrations were determined using methods developed by Bergqvist et al18. Due to the huge differences in the plasma concentrations and the set limits of detection of sulfadoxine and pyrimethamine, separate runs with large volumes were used, leaving little plasma for pyrimethamine determination beyond the 24h period. Chloroquine concentrations (limit of determination 9.7nmol/l) were determined using the method described by Minzi et al19.
Statistical analysis and calculations
Pharmacokinetic calculations were performed by non-compartmental analysis using WinNonlin, version 4.1 (Pharsight Inc., Mountain View, CA, USA). Half-life (t1/2) was calculated from the terminal slope (ë) of the concentration-time profile, and AUC was calculated using the trapezoidal rule, according to standard methods. Clearances (CL) and distribution volumes (Vd) were normalized by body weight. One-compartment model was used to calculate the absorption rate constant (ka) of sulfadoxine and pyrimethamine, while a two-compartment model was applied for chloroquine. Relative bioavailability (AUCtest/AUCreference) was determined from the ratio of the bioavailability of the drugs in Homapak (test) to that in the GMP drugs (reference). Bioequivalence was assumed when the relative bioavailability of the test drug was between 0.80 – 1.25 within the 90% confidence interval20.
Data are presented as median values. All parameters were compared between treatment groups by the non-parametric Kruskal-Wallis test. To adjust for multiple comparisons, statistical significance was assumed only when p =0.01. Statistical calculations were performed using Statistica version 6.1 (Statsoft Inc., OK, USA). The Student's t-test was used to compare the volunteer characteristics.
Ethical clearance
The Institutional Review Board of Makerere University, Uganda, and the Ethics Committee of Karolinska Institute, Sweden, approved the study (No.270/03). The Uganda National Council for Science and Technology (UNCST) gave permission to conduct the study. A signed, informed consent was obtained form each of the volunteers, and the study was performed in accordance with ICH-GCP guidelines as well as the Helsinki declaration.
Results
One volunteer in the Fansidar+CQ group had sulfadoxine concentration of 18.6 ìmol/l in plasma before drug administration and was therefore excluded. None of the remaining 31 volunteers had detectable amounts of S/P or CQ in the pre-dosing sample. Analysis of the characteristics of the volunteers showed there were no significant differences in age, body weight, liver function tests, and haemoglobin or creatinine levels between the groups. The drugs were generally well tolerated and no serious adverse reactions were reported. All volunteers could be sampled as scheduled on all occasions during the 21-day study period (Figure 1).
Pharmacokinetics and bioavailability of sulfadoxine
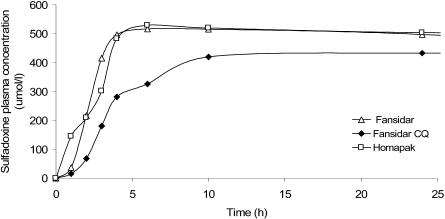
Sulfadoxine in Homapak was more rapidly absorbed (ka = 0.55 h-1) than in Fansidar + CQ, (ka = 0.27 h-1, p=0.004) but not more than S in Fansidar alone group (ka = 0.32 h-1, p=0.03). The median absorption time (tmax) were 4h, 6h, and 10h for Homapak, Fansidar alone and Fansidar+CQ groups respectively. These observations were not significantly different between the groups.
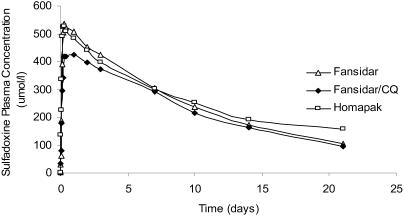
No significant differences were observed in the other phar macokinetic parameter s (Cmax, AUC, t½, CL and Vd) for S in all the groups (Table 1 and Figures 2 and 3). With Fansidar as reference, the relative bioavailablity of S in Homapak was 1.07 (90%CI: 0.94 – 1.12). The AUCrest (AUC0–8 – AUC0–21) for S was similar in both groups (21% and 22% respectively). These results show that there is bioequivalence for S when given as Homapak compared with GMP made S/P (Fansidar).
Table 1.
Pharmacokinetic parameters of sulfadoxine in the different formulations (median value and range).
| Cmax (mol/l) | AUC0–21 (mmolh/l) | tmax (h) | t1/2 (h) | Vd (l/kg) | CL (ml/h/kg) | ka (h-1) | Relative Bioavailabilitya (90%CI) | AUCrest %d | |
| Formulation | |||||||||
| Fansidar | 532 | 122 | 6 | 229 | 0.15 | 0.39 | 0.32 | 1.00 | 22 |
| n=9 | (455–649) | (102–159) | (1–6) | (136–272) | (0.12–0.18) | (0.30–0.56) | (0.29–0.62) | (Reference) | (7.6 – 26) |
| Fansidar+CQ | 463 | 118 | 10 | 221 | 0.16 | 0.54 | 0.27 | 0.97 | 16 |
| n=8 | (332–546) | (99–140) | (6–24) | (154–347) | 0.11–0.33) | (0.45–0.58) | (0.17–0.53) | (0.88–1.06) | (11 – 32) |
| Homapak | 530 | 131b | 4 | 224 | 0.15 | 0.43 | 0.55 | 1.07 | 21 |
| n=7 | (495–617) | (110–169) | (2–10) | (187–267) | (0.14–0.20) | (0.33–0.62) | 0.42–2.6)c | (0.94–1.12) | (12 – 27) |
The acceptable value should be within 0.85 – 1.25 at 90% Confidence Interval
p=0.03 compared to Fansidar+CQ
p = 0.004, compared to Fansidar+CQ
AUCrest is derived from AUC0–8 – AUC0–21 as a percentage of the observed AUC0–8
Figure 2.
Median plasma concentrations of sulfadoxine during 0–21 days after single dose of Homapak, Fansidar and Fansidar+CQ.
Figure 3.
Median plasma concentrations of sulfadoxine during 0–24 hours after single dose of Homapak, Fansidar and Fansidar+CQ.
Pharmacokinetics of pyrimethamine
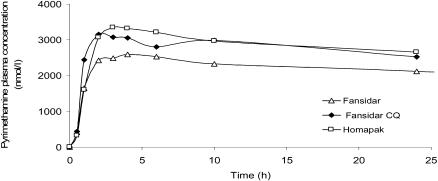
Pyrimethamine plasma concentrations could only be obtained during the absorption and early elimination phase. There were, however, no differences in the pharmacokinetic parameters of P (Cmax, tmax and AUC0–24h) in the first 24 hours between the groups (Table 2 and Figure 4).
Table 2.
Pharmacokinetic parameters of pyrimethamine in the different formulations (median value and range).
| Cmax ìmol/l | AUC24h ìmolh/l | tmax h | |
| Formulation | |||
| Fansidar n=9 | 2.9 (2.0–3.9) | 55 (43–69) | 4 (2–6) |
| Homapak n=7 | 3.6 (2.6–4.8) | 66 (54–80) | 2 (2–4) |
| Fansidar+CQ n=8 | 3.3 (2.4–4.3) | 63 (43–82) | 4 (1–10) |
Figure 4.
Median plasma concentrations of pyrimethamine during 0 – 24 hours after single dose of Homapak, Fansidar and Fansidar+CQ.
Pharmacokinetics and bioavailability of chloroquine
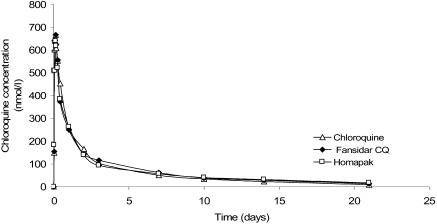
The median concentration-time profiles of chloroquine in the three groups were similar (Figure 5). No significant differences were found between the three groups for any of the pharmacokinetic parameters (Table 3). With GMP made CQ (Pharco) as reference, the relative bioavailability of CQ in Homapak was 0.97 (90%CI: 0.89 – 1.20). The AUCrest for CQ in Homapak and CQ alone (Pharco) groups were similar (7.3 % and 6.8% respectively). These findings also show CQ in Homapak bioequivalent to GMP made CQ.
Figure 5.
Median plasma concentrations of chloroquine during 0–21 days after single dose of Homapak, Chloroquine (GMP CQ) and Fansidar+CQ.
Table 3.
Pharmacokinetic parameters of chloroquine in the different formulations (median values and range).
| Cmax (mol/l) | AUC0–21 (mmolh/l) | tmax (h) | t1/2 (h) | Vd (l/kg) | CL (ml/h/kg) | ka (h-1) | Relative Bioavailabilitya (90%CI) | AUCrest %d | |
| Formulation | |||||||||
| CQa | 760 | 34 | 2 | 155 | 113 | 0.50 | 1.8 | 1.00 (Reference) | 7.3 |
| n=8 | (466–1186) | (19–54) | (1–4) | (87–232) | (55–257) | (0.39–0.77) | (0.27–3.4) | (1.9–21) | |
| Fansidar+CQ | 731 | 43 | 3 | 162 | 105 | 0.44 | 0.47 | 1.26 | 7.9 |
| n=8 | (449–1194) | (26–70) | (1–3) | (102–395) | (79–203) | (0.28–0.72) | (0.24–3.3) | (1.03 – 1.36) | (2.9 – 30) |
| Homapak | 700 | 33 | 2 | 151 | 117 | 0.55 | 1.2 | 0.97 | 6.8 |
| n=7 | (495–813) | (17–45) | (1–4) | (93–325) | (69–548) | (0.38–1.2) | (0.19–7.8) | (0.89 – 1.20) | (1.7 – 24) |
The acceptable value should be within 0.85 – 1.25 at 90% Confidence Interval
AUCrest is derived from AUC0–8 – AUC0–21 as a percentage of the observed AUC0–8
Discussion
In the present study there were no clinically important pharmacokinetic interactions between chloroquine and sulfadoxine/pyrimethamine. This is an important finding, as the three compounds are used together as first line treatment of falciparum malaria in several resource poor nations3, 4, even as more effective antimalarials are advocated21, 22. A significant pharmacokinetic interaction between the compounds would have led to suppression or elevation of the bioavailability of any of the components, which was not the case in this study.
When administered as Homapak, S was more rapidly absorbed than as Fansidar, however, its bioavailability was found to be similar in the different formulations. This could probably have been due to different pharmaceutical preparations of the tablets13, 14.
It is well known13 that drugs in tablets forms with more rapid dissolution in the gastro-intestinal tract usually have more rapid systemic absorption, as measured by the absorption rate constant or time to maximal concentrations. Therefore, a rapid absorption, as was seen for S in Homapak, is desired for an antimalarial formulation for rapid parasite clearance, while slow absorption may lead to delayed parasite clearance and slower clinical response23.
Impaired absorption may occur when different formulations are given simultaneously18. However, there was no difference in ka when S was given as standard Fansidar with or without simultaneous intake of CQ. Although a reduction in absorption did not occur between Fansidar and CQ (Pharco) there is no guarantee that it cannot be seen when other preparations from other manufacturers are used, and as such more investigations need to be done in this area.
In poor resource countries locally made generic drugs are often used24, 25. It is of importance to establish that they are not of substandard quality or even faked9–12. Fortunately, in this study, Homapak has been found to be of good quality. Compared with GMP made formulations, both CQ and S in Homapak showed bioequivalence. Due to technical reasons, this could not be fully studied for P. There were, however, no differences in absorption of P during the first 24 hours. Based on these findings, we believe that there is probably good bioavailability of P in Homapak as well. However, in the absence of complete pharmacokinetic data on pyrimethamine, we cannot unequivocally conclude that Homapak is bioequivalent to GMP made drugs.
Conclusions
There were no pharmacokinetic interactions between CQ, S and P when the compounds were given together, however, more investigations would be needed to explore this further. Compared with GMP made drugs, both S and CQ are bioequivalent in Homapak, the Ugandan made fixed-dose formulation. Furthermore, the absorption of S was more rapid which could be advantageous in malaria treatment.
Acknowledgments
We are grateful to Juliet Eyotaru, John Barugahare of Mulago Hospital, Uganda, and Judith Ayugi of Makerere University, Rajaa A. Mirghani of Karolinska University Hospital, Huddinge, Karolinska Institute for their invaluable assistance during the conduct of this study. This study was funded by SIDA/SAREC - Makerere University/Karolinska Institute Research Collaboration.
Contribution by the different authors
Obua, C. (Corresponding author) - was responsible for the conceptualization and design of the project, development of the project, execution of the experiments, data collection and analysis, and overall responsibility for the production of the manuscript.
Ntale M. - was responsible for the pharmacokinetic analysis and scientific interpretation of results and input in the manuscript.
Lundblad, M.S. - was responsible for the WinNonlin pharmacokinetic modeling, the scientific interpretation of the results, and input in the manuscript write-up.
Mahindi M. - participated in the experimental design, laboratory analysis and gave critical comments on the manuscript.
Gustafsson, L.L. - Participated in the conceptualization and development of the project, and critical inputs during manuscript preparation.
Ogwal-Okeng, J.W. - participated in the conceptualization, laboratory and project development, design of the experiment and manuscript preparation.
Anokbonggo, W.W. - participated in experimental design, safety monitoring, critical revising of the manuscript.
Hellgren, U. - together with the corresponding author, was responsible for the conceptualization and study design, overall ethical and scientific review of the project and manuscript preparation.
References
- 1.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giao P T, de Vries PJ. Pharmacokinetic interactions of antimalarial agents. Clin Pharmacokinet. 2001;40:343–373. doi: 10.2165/00003088-200140050-00003. [DOI] [PubMed] [Google Scholar]
- 3.Maitland K, Makanga M, Williams TN. Falciparum malaria: Current therapeutic challenges. Curr Opin Infect Dis. 2004;17:405–412. doi: 10.1097/00001432-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 4.GlobalHealthReporting.org Malaria: WHO announces shortage of “most effective” malaria drug through March 2005. [ http://www.kaisernetwork.org/daily_reports/rep_index.cfm]
- 5.Implementation Guidelines for the Home Based Management of Fever Strategy. 1st Ed. Kampala: Ministry of Health, Uganda; 2002. [Google Scholar]
- 6.Africa Malaria Report. [ http://www.rbm.who.int/amd2003/amr2003/ch1-3.htm]
- 7.Kilian AH, Tindyebwa D, Gulck T, Byamukama W, Rubaale T, Kabagambe G, Korte R. Attitude of women in western Uganda towards pre-packed, unit-dosed malaria treatment for children. Trop Med Int Health. 2003;8:431–438. doi: 10.1046/j.1365-3156.2003.01044.x. [DOI] [PubMed] [Google Scholar]
- 8.Nsungwa-Sabiiti J, Tomson G, Pariyo G, Ogwal-Okeng J, Peterson S. Community effectiveness of malaria treatment-a long way from Abuja targets. Annals of Tropical Paediatrics. 2005;25:91–100. doi: 10.1179/146532805X45683. [DOI] [PubMed] [Google Scholar]
- 9.McGregor A. Counterfeit drugs flood developing world. Lancet. 1997;350:1690. [Google Scholar]
- 10.Rozendaal J. Fake antimalaria drugs in Cambodia, correspondence. Lancet. 2001;357:890. doi: 10.1016/s0140-6736(05)71830-4. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RB, Shakoor O, Behrens RH, et al. Pharmacopoeial quality of drugs supplied by Nigerian pharmacies. Lancet. 2001;357:1933–1936. doi: 10.1016/s0140-6736(00)05065-0. [DOI] [PubMed] [Google Scholar]
- 12.Behrens RH, Awad AI, Taylor RB. Substandard and counterfeit drugs in developing countries. Tropical Doctor. 2002;32:1–2. doi: 10.1177/004947550203200102. [DOI] [PubMed] [Google Scholar]
- 13.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 1995. [Google Scholar]
- 14.Handbook of Pharmaceutical Excipients. 2nd Ed. London: The Pharmaceutical Press; 1994. [Google Scholar]
- 15.Rengelshausen J, Burhenne J, Fröhlich M, et al. Pharmacokinetic interaction of chloroquine and methylene blue combination against malaria. Eur J Clin Pharmacol. 2004;60:709–715. doi: 10.1007/s00228-004-0818-0. [DOI] [PubMed] [Google Scholar]
- 16.Grace JM, Aguilar AJ, Trotman KM, Peggins JO, Brewer TG. Metabolism of â-Arteether to Dihyroqinghaosu by Human Liver Microsomes and Recombinnant Cytochrome P450. Drug Metab Dispos. 1998;26:313–317. [PubMed] [Google Scholar]
- 17.Ali HM. Reduced ampicillin bioavailability following oral coadministration with chloroquine. J Antimicrob Chemother. 1985;15:781–784. doi: 10.1093/jac/15.6.781. [DOI] [PubMed] [Google Scholar]
- 18.Bergqvist Y, Eriksson M. Simultaneous determination of pyrimethamine and sulfadoxine in human plasma by high-performance liquid chromatography. Trans R Soc Trop Med Hyg. 1985;79:297–301. doi: 10.1016/0035-9203(85)90365-7. [DOI] [PubMed] [Google Scholar]
- 19.Minzi OMS, Rais M, Svensson JO, Gustafsson LL, Ericsson O. High-performance liquid chromatographic method for the determination of amodiaquine, chloroquine and their metabolites in biological samples. J Chromatogr B. 2003;783:473–840. doi: 10.1016/s1570-0232(02)00727-4. [DOI] [PubMed] [Google Scholar]
- 20.EMEA, author. Evaluation of Medicines for Human Use: Committee for Proprietory Medicinal Products-Note for Guidance on the Investigation of Bioavailability and Bioequivalence. CPMP/EWP/QWP/1401/98. 2001
- 21.Attaran A, Barnes KI, Curtis C, d'Alessandro U, Fanello CI, Galinski MR, Kokwaro G, Looareesuwan S, Makanga M, Mutabingwa TK, Talisuna A, Trape JF, Watkins WM. WHO, the Global Fund, and medical malpractice in malaria treatment. Lancet. 2004;363:237–240. doi: 10.1016/S0140-6736(03)15330-5. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization, author. WHO/HTM/MAL/2006.1108. Geneva: WHO; 2006. WHO Guidelines for treatment of malaria. [Google Scholar]
- 23.Stockley's Drug Interactions. 6th Ed. London: Pharmaceutical Press; 2002. pp. 1–14. [Google Scholar]
- 24.Brundtland GH. Essential Medicines: 25 years of better health. JAMA. 2002;288:3102. doi: 10.1001/jama.288.24.3102. [DOI] [PubMed] [Google Scholar]
- 25.Quick JD, Hogerzeil H V, Velásquez G, Rago L. Twenty-five years of essential medicines. BullWHO. 2002;80:913–914. [PMC free article] [PubMed] [Google Scholar]