Abstract
Objective To compare the cost effectiveness of percutaneous transluminal coronary artery stenting with minimally invasive internal thoracic artery bypass for isolated lesions of the left anterior descending artery.
Design Cost effectiveness analysis.
Data sources Embase, Medline, Cochrane, Google Scholar, and Health Technology Assessment databases (1966-2005), and reference sources for utility values and economical variables.
Methods Decision analytical modelling and Markov simulation were used to model medium and long term costs, quality of life, and cost effectiveness after either intervention using data from referenced sources. Probabilistic sensitivity and alternative analyses were used to investigate the effect of uncertainty about the value of model variables and model structure.
Results Stenting was the dominant strategy in the first two years, being both more effective and less costly than bypass surgery. In the third year bypass surgery still remained more expensive but became marginally more effective. As the incremental cost effectiveness was £1 108 130.40 (€1 682 146.00; $2 179 194) per quality adjusted life year (QALY), the additional effectiveness could not be said to justify the additional cost at this stage. By five years, however, the incremental cost effectiveness ratio of £28 042.95 per QALY began to compare favourably with other interventions. At 10 years the additional effectiveness of 0.132 QALYs (range −0.166 to 0.430) probably justified the additional cost of £829.02 (range £205.56 to £1452.48), with an incremental cost effectiveness of £6274.02 per QALY. Sensitivity and alternative analysis showed the results were sensitive to the time horizon and stent type.
Conclusions Minimally invasive left internal thoracic artery bypass may be a more cost effective medium and long term alternative to percutaneous transluminal coronary artery stenting.
Introduction
Isolated disease of the left anterior descending coronary artery poses a therapeutic dilemma for cardiologists and cardiothoracic surgeons as affected patients are generally younger and have fewer comorbidities than those with multiple vessel disease.1 Current treatment options include percutaneous revascularisation with stenting or surgical bypass with a left internal thoracic coronary artery to left anterior descending artery anastomosis. With advances in minimally invasive direct coronary artery bypass, morbidity from surgical revascularisation has been noticeably reduced making it even more relevant to compare the cost effectiveness of stenting with that of surgical bypass.2 3
A recent meta-analysis of randomised trials comparing minimally invasive internal thoracic artery bypass with transluminal stenting suggested that surgical revascularisation for isolated lesions of the left anterior descending artery resulted in fewer complications in the mid-term.4 However, a real need remains to compare the cost effectiveness of the two procedures, which traditionally has not been possible because of a failure of the published literature to adequately tackle elements crucial to such evaluations.5 We used an evidence synthesis approach combining meta-analysis, decision analysis, and cost effectiveness analysis of comparative peer reviewed publications to compare percutaneous transluminal coronary artery stenting with minimally invasive direct coronary artery bypass with left internal thoracic artery for the management of isolated lesions of the left anterior descending artery.6 We also determined whether this translated into differences in quality of life and we carried out a sensitivity analysis to evaluate the robustness of the findings.
Methods
The evidence synthesis approach involves combining meta-analysis, decision analysis, and economic analysis.6 A meta-analysis of randomised trials comparing minimally invasive internal thoracic artery bypass with transluminal stenting for isolated lesions of the left anterior descending artery4 was carried out in line with Cochrane Collaboration recommendations and quality of reporting of meta-analyses guidelines.7 8 We calculated the incidences of clinical outcomes of interest from the meta-analysis weighted means data (odds ratios and 95% confidence intervals). To determine the least costly and most effective intervention we used decision analysis and Markov simulation to model long term outcomes of interventions in the absence of empirical long term follow-up data.9 We used quality adjusted life years (QALYs) and monetary cost as measures of effect and the incremental cost effectiveness ratio to assess whether improved efficacy justified increased cost. To investigate the uncertainty of our results we used sensitivity and alternative analysis.
Model structure and variables
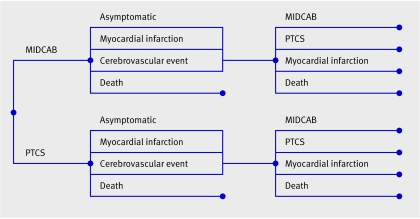
Figure 1 shows the structure of the model we used. The base case analysis was for a 61 year old male cohort, as this was the average age of patients in the included studies. We carried out the analysis for a 10 year time horizon, with one year Markov cycles. Costs and effects were discounted at 3.5%, and a range of 0-6% was used for sensitivity analysis, according to National Institute of Health and Clinical Excellence (NICE) guidelines on health technology assessment.10 We used decision analytical software (TreeAge-ProTM, TreeAge; Williamstown, MA, USA) for the cost effectiveness analysis.
Fig 1 Decision analytical model comparing outcome after percutaneous transluminal coronary artery stenting (PTCS) with minimally invasive direct internal thoracic coronary artery bypass (MIDCAB). Patients start Markov simulation depending on primary treatment. Branches immediately after Markov nodes show possible states that patients can enter. Model structure is the same after both interventions, but transition state probabilities, cost, and utility pay-offs associated with each intervention differ
Clinical variables
We converted the incidences of clinical outcomes obtained from the meta-analysis into transition probabilities11 with the exception of perioperative death and cerebrovascular event, which we assumed to always occur within the first cycle after the intervention. Transition probabilities for baseline mortality were obtained from the mortality tables of the UK government actuary's department.12 We obtained data on the likelihood of death after myocardial infarction and cerebrovascular event from the NICE report on coronary revascularisation13 and confidence intervals for these values from expert estimation. The table shows the variables used in the analysis.
Cost and utility variables, discount rate, and transition probabilities used in statistical model
| Variable | Minimum | Likeliest | Maximum |
|---|---|---|---|
| Cost variables (£)* | |||
| Cost of procedure: | |||
| MIDCAB | 2832.27 | 3146.97 | 3461.67 |
| PTCS | 1743.40 | 1937.11 | 2130.82 |
| Cost of follow-up: | |||
| MIDCAB | 454.66 | 505.18 | 555.70 |
| PTCS | 427.06 | 474.51 | 521.96 |
| Cost of myocardial infarction | 407.82 | 453.13 | 498.44 |
| Cost of stroke | 1427.40 | 1586.00 | 1744.60 |
| Travel expenses: | |||
| MIDCAB | 1.98 | 2.20 | 2.42 |
| PTCS | 2.48 | 2.76 | 3.04 |
| Out of pocket expenses for patient: | |||
| MIDCAB | 4.34 | 4.82 | 5.30 |
| PTCS | 4.91 | 5.46 | 6.01 |
| Cost to society for time off work: | |||
| Patient | |||
| MIDCAB | 1348.29 | 1498.10 | 1647.91 |
| PTCS | 1084.14 | 1204.60 | 1325.06 |
| Carer | |||
| MIDCAB | 29.12 | 32.36 | 35.60 |
| PTCS | 37.07 | 41.19 | 45.31 |
| Utility variables (QALYs)† | |||
| Asymptomatic utility | 0.774 | 0.860 | 0.946 |
| Utility of MIDCAB | 0.745 | 0.828 | 0.910 |
| Utility of PTCS | 0.772 | 0.857 | 0.943 |
| Utility of myocardial infarction | 0.752 | 0.835 | 0.919 |
| Utility after stroke | 0.504 | 0.560 | 0.616 |
| Utility of MIDCAB after stroke | 0.475 | 0.528 | 0.580 |
| Utility of PTCS after stroke | 0.502 | 0.557 | 0.613 |
| Utility of myocardial infarction after stroke | 0.482 | 0.535 | 0.589 |
| Utility after second stroke | 0.477 | 0.530 | 0.583 |
| Discount rate‡ | 0 | 0.035 | 0.06 |
| Transition probabilities§ | |||
| Probability of repeat revascularisation with PTCS: | |||
| MIDCAB | 0.0082 | 0.0174 | 0.0452 |
| PTCS | 0.0227 | 0.0583 | 0.1135 |
| Probability of repeat revascularisation with CABG: | |||
| MIDCAB | 0.0016 | 0.0073 | 0.0121 |
| PTCS | 0.0057 | 0.0094 | 0.0400 |
| Rate of myocardial infarction: | |||
| MIDCAB | 0.0083 | 0.0170 | 0.0419 |
| PTCS | 0.0096 | 0.0240 | 0.0482 |
| Rate of stroke: | |||
| MIDCAB | 0.0018 | 0.0044 | 0.0105 |
| PTCS | 0.0080 | 0.0192 | 0.0462 |
| Perioperative mortality: | |||
| MIDCAB | 0.0026 | 0.0224 | 0.0281 |
| PTCS | 0.0059 | 0.0075 | 0.0610 |
| Mortality†: | |||
| Myocardial infarction | 0.2 | 0.25 | 0.5 |
| Stroke | 0.1 | 0.2 | 0.3 |
| Baseline¶ | |||
£1.00 (€1.51; $1.96). MIDCAB=minimally invasive direct coronary artery bypass; PTCS=percutaneous transluminal coronary artery stenting; CABG=coronary artery bypass grafting.
Distributions for each variable were triangular.
*Reeves et al.17
†NICE.13
‡NICE.10
§Aziz et al.4
¶UK government actuary's department tables.12
Quality of life variables
All utility variables were obtained from the NICE assessment report on coronary revascularisation13 and were based on empirical data from the arterial revascularisation therapies study,14 which used the EQ-5D instrument15 to evaluate the utility of different health states. One year of good cardiac health was valued at 0.86 QALYs, consistent with other published estimates of age adjusted normal population values.16 Patients undergoing either transluminal stenting or minimally invasive internal thoracic artery bypass were assumed to have had angina for six weeks before the procedure, hence incurring a disutility of 0.02 QALYs for six weeks. Although we accept that variations in service provision mean that patients may have symptoms for longer, we thought it important to compare the efficacy and not the availability of the interventions. Patients incurred a disutility of 0.012 QALYs for 13 weeks when surviving coronary artery bypass grafting surgery, 0.0035 QALYs for six weeks when surviving transluminal stenting, and 0.1 QALYs for 13 weeks when surviving myocardial infarction. Patients surviving a cerebrovascular event incurred a permanent disutility of 0.3 QALYs, which was higher after a second stroke (0.33 QALYs). Modelling limitations meant that the disutility of subsequent strokes could not be accounted for, and although this has the potential to bias results to favour transluminal stenting, the number of patients with serial strokes was small. In the sensitivity analysis we sampled utility values from triangular distributions defined by limits of 10% either way of the original value.
Economic variables
We carried out the analysis from a UK health service perspective. Costs are reported in pounds sterling and incremental cost effectiveness ratios are reported in pounds sterling per QALY. Cost variables were based on NHS costs obtained from the health technology assessment report on stenting compared with minimally invasive direct coronary artery bypass grafting with left internal thoracic artery for proximal stenosis of the left anterior descending artery.17 To calculate the total cost of the initial procedure we included preoperative costs, cost of operation, cost of postoperative care, and follow-up costs for the first year (follow-up costs for subsequent years included primary care and drugs). This is consistent with the NICE assessment report, which accounted for follow-up in secondary care at 4, 6, 8, and 12 months only.13 The cost of a cerebrovascular event was set at £1586 (€2405; $3107; equivalent to a one week hospital stay) and the cost of a myocardial infarction was set at £453.13 (equivalent to a two day hospital stay).17 To reflect the uncertainty about cost variables we used default values of 10% either way in our analysis to define triangular distributions for sensitivity analysis.
Sensitivity analysis and uncertainty
An element of uncertainty is associated with attempts to consider the long term implications of healthcare interventions.9 We carried out a probabilistic analysis to examine the combined effect of uncertainty about variables in the model using second order Monte-Carlo simulation,9 with 1000 iterations in each loop. We sampled variables from distributions described in the table.
We investigated uncertainty using a cost effectiveness acceptability curve.9 An intervention can be considered to be cost effective if it is both more effective and less costly than its comparator or if the additional cost justifies the increase in effectiveness. This seems to be the case if the incremental cost effectiveness ratio is less than the cost effectiveness threshold, which can be represented arbitrarily as a straight line on the cost effectiveness plane. The cost effectiveness acceptability curve shows how the certainty for an intervention being more cost effective varies as the amount a healthcare provider is prepared to pay for an improvement of 1 QALY increases. As this Bayesian interpretation of the data may be unfamiliar to clinicians who are more accustomed to dealing with uncertainty using standard methods, we calculated 95% confidence intervals for the incremental cost effectiveness ratio using Fieller's method, treating the results of each microsimulation as individual patient data. We opted not to use the bootstrapping method as we anticipated that a large proportion of the data on effectiveness would be clustered around zero.18 19 20
Alternative analysis
Demographic variables
To investigate the effect of demographics we carried out analyses with appropriate values for baseline mortality from tables produced by the UK government actuary's department12 for men aged 51, 61, and 71 and for women aged 61. We assumed that operative mortality, complications, and baseline morbidity remained constant.
Societal costing perspective
We investigated if the cost effectiveness of the interventions was sensitive to costing perspective. In addition to the cost of each of the procedures to the NHS we accounted for the cost to society of absence from work for patients and carers and the cost to the patient of travel and other out of pocket expenses.
Time horizon
To investigate the effect of variations in time horizon we carried out the analysis for different time horizons between 0 and 15 years. The authors accept that the validity of model outcomes weakens as the time horizon increases because of accumulated modelling error.
Pessimistic scenario
We carried out a pessimistic scenario, where the same reintervention rate was used after two years for both interventions. Reintervention rates probably continue to be higher after transluminal stenting; however as meta-analytical data were not available after three years for all outcomes we investigated the uncertainty about long term reintervention rates.
Utility values
To further investigate uncertainty about the quality of life associated with different health states we carried out an analysis using a utility of 0.86 QALYs for all states except death.
Drug eluting stents
To investigate the possible effect of drug eluting stents we carried out three further analyses. In the first analysis we extrapolated currently available data on the efficacy of drug eluting stents for one year follow-up from a meta-analysis by Roiron et al over a 10 year period.21 In this analysis it was assumed that reintervention rates were the same in both groups and that the incidence of the composite outcome of major adverse coronary and cerebral events with transluminal stenting was half that with bare metal stents (base model). Because there is some anecdotal evidence (case series) suggesting that occlusion rates with drug eluting stents are comparable to or higher than those with bare metal stents (particularly after stopping clopidogrel),22 we carried out a second analysis in which the cost of drug eluting stents was combined with the incidence of reintervention and major adverse coronary and cerebral events with bare metal stents (base case). We carried out a further analysis in which we assumed the rates of reintervention and major adverse coronary and cerebral events to be halfway between the previous two analyses. In all these analyses we assumed the incremental cost of drug eluting stents to be £200 compared with bare metal stents. Although NICE estimates the incremental cost of a drug eluting stent to be £520,13 we used a lower value reflecting the future cost reduction of technology related to drug eluting stents. The additional cost of 75 mg of clopidogrel daily in the first year after insertion of a drug eluting stent was calculated as £460.29.23
Results
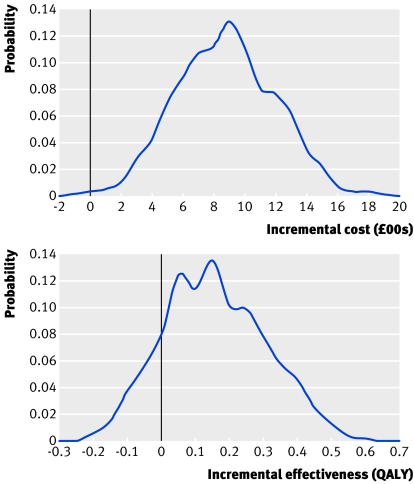
Overall, percutaneous transluminal coronary artery stenting cost £6317.07 per patient, yielding 6.718 QALYs per patient over 10 years, and minimally invasive direct internal thoracic coronary artery bypass grafting cost £7146.09 per patient, yielding 6.850 QALYs per patient over 10 years. This represents a gain of 0.132 QALYs (range −0.166 to 0.430) with minimally invasive internal thoracic artery bypass and an incremental cost of £829.02 (range £205.56 to £1452.48; fig 2), with an incremental cost effectiveness ratio of £6274.02 per QALY.
Fig 2 Distribution of incremental costs and incremental effectiveness of minimally invasive internal thoracic artery bypass compared with transluminal stenting
Uncertainty about variables
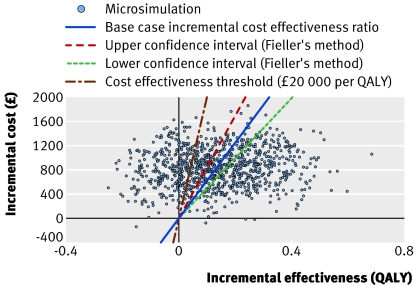
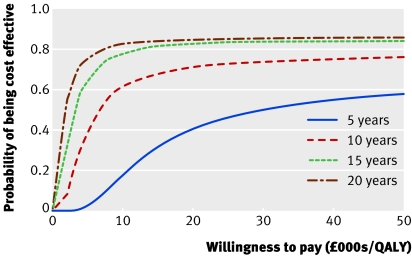
Figure 3 shows the incremental cost plotted against incremental effectiveness for the results of the Markov simulation for the base case (solid line is incremental cost effectiveness ratio for this). The dashed lines represent 95% confidence intervals, calculated using Fieller's method, at £5007.67 per QALY and £8505.13 per QALY respectively, suggesting no significant uncertainty associated with the incremental cost effectiveness ratio. Figure 4 shows the cost effectiveness acceptability curve, with the proportion of cases generated by Markov simulation that are cost effective for each cost effectiveness threshold, plotted against the cost effectiveness threshold for minimally invasive internal thoracic artery bypass at horizons of 5, 10, 15, and 20 years.
Fig 3 Scatter plot of incremental cost effectiveness ratio. Incremental cost effectiveness ratio for base case is plotted at £6274.02 per QALY and 95% confidence intervals (Fieller's method) are plotted at £8505.13 per QALY and £5007.67 per QALY. 71.1% of the points lie under the cost effectiveness thresholds, arbitrarily plotted at £20 000 per QALY
Fig 4 Cost effectiveness acceptability curves for different time horizons, showing certainty with which minimally invasive internal thoracic artery bypass is the most cost effective intervention as amount healthcare providers are prepared to pay for one QALY varies
Alternative analysis
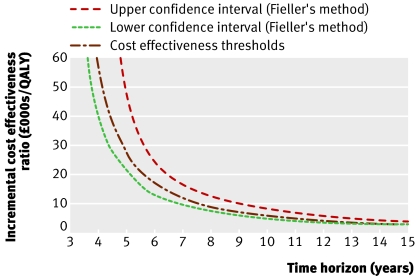
Figure 5 shows that as the time horizon increased minimally invasive internal thoracic artery bypass became more effective, overtaking transluminal stenting after three years. Beyond this, minimally invasive internal thoracic artery bypass became more effective but was more expensive than transluminal stenting. The incremental cost effectiveness ratio was £28 042.95 per QALY at five years, £17 474.26 per QALY at six years, £6274.02 per QALY at 10 years, and £2878.98 per QALY at 15 years. The incremental cost effectiveness ratio of £10 354.79 per QALY at 10 years in the pessimistic analysis suggests that although the results may be sensitive to uncertainty about the long term reintervention rates, minimally invasive internal thoracic artery bypass remained the most cost effective alternative at 10 years even when the reintervention rates and incidence of major adverse coronary and cerebral events were considered to be the same after two years. Uncertainty about utility variables had little effect on the results, as shown by the incremental cost effectiveness ratio of £6394.81 per QALY in the “same utility analysis.” The results were shown to be insensitive to demographics (incremental cost effectiveness ratio was £5808.22 per QALY in women, £6435.37 per QALY in 71 year olds, and £5723.57 per QALY in 51 year olds). The additional cost of absence from work after minimally invasive internal thoracic artery bypass was offset by the societal cost of more frequent reintervention after transluminal stenting, resulting in a lower incremental cost (£776.65) and incremental cost effectiveness ratio (£5499.45 per QALY) in the societal perspective analysis compared with the base case.
Fig 5 Effect of time horizon on incremental cost effectiveness ratio for minimally invasive internal thoracic artery bypass compared with transluminal stenting
Drug eluting stents
The results of the alternative analysis for drug eluting stents differed most from the base case. In the first analysis, transluminal stenting was £686.04 cheaper and 0.042 QALYs more effective. In the second analysis, minimally invasive internal thoracic artery bypass was more expensive but more effective, with an incremental cost effectiveness ratio of £6970.58 per QALY. In the third analysis, minimally invasive internal thoracic artery bypass was most cost effective, with an incremental cost effectiveness analysis of £302.53 per QALY.
Discussion
Although percutaneous transluminal coronary artery stenting for patients with lesions of the left anterior descending artery is initially cheaper and more effective than minimally invasive internal thoracic artery bypass, the latter is more cost effective long term, with an incremental cost effectiveness ratio at 10 years of £6274.02 per QALY. The absolute difference in effect between the interventions is small at 10 years, but probabilistic sensitivity analysis suggests with 71.1% certainty that minimally invasive internal thoracic artery bypass is the most cost effective alternative at a cost effectiveness threshold of £20 000 per QALY and 73.4% at £30 000 per QALY. These findings have important implications for the choice of primary revascularisation strategy in patients with isolated lesions of the left anterior descending artery.
Several other factors should also be considered. Firstly, beyond six years the incremental cost effectiveness ratio of minimally invasive internal thoracic artery bypass compares favourably with other healthcare interventions and becomes even more effective the longer a patient lives. Despite accumulated modelling error, the cost of minimally invasive internal thoracic artery bypass seems justifiable. Secondly, the finding that results were sensitive to uncertainty of the variables in the model could be due largely to the uncertainty about the complication rate after intervention. Thirdly, although there is good quality evidence on the incidence of composite outcomes such as major adverse coronary and cerebral events,4 myocardial infarction and stroke are poorly reported. Fourthly, alternative analysis suggests that these results are not sensitive to demographics, uncertainty about long term reintervention rates, or the utility of different health states. Finally, many of the assumptions made in this analysis were biased towards transluminal stenting, increasing the robustness of the finding that minimally invasive internal thoracic artery bypass is more cost effective. The effect of myocardial infarction and cerebrovascular event on cost was underestimated in the base case and in the societal analysis, both of which are more common after transluminal stenting.
The findings of this study are less equivocal than previously published economic data on transluminal stenting compared with coronary artery bypass grafting in multiple vessel disease.24 This may be a reflection of varying population characteristics of patients with single and multiple vessel disease,1 but also may be due to other methodological limitations. Model variables in this study were obtained from meta-analysed data, and the associated confidence intervals reflect the degree of uncertainty. The event rates used by Yock et al24 were based on modifications to the data from the bypass angioplasty revascularisation investigation25 and the Emory angioplasty versus surgery trial,26 and used values that did not directly compare stenting with bypass grafting.27 28 Despite the high degree of uncertainty associated with many of the variables in our model the values provide more contemporary estimates of the event rates after these quickly evolving interventions.2 3 29 Similarly, the utility (based on the arterial revascularisation therapies study13 14) and cost estimates (based on the health technology assessment report17) used in our analysis may represent truer estimates of the utility and costs, measured from an NHS perspective in a contemporary UK population.
Study limitations
The decision analytical modelling techniques used in this study have several limitations. Firstly, results were limited by the accuracy of the model structure and estimates of the model variables. Secondly, transition probabilities were based on meta-analytical data,4 which for variables with low event rates resulted in a higher degree of uncertainty. Thirdly, utility estimates were based on validated empirical data.13 14 The alternative analysis did, however, show that minimally invasive internal thoracic artery bypass was still cost effective when all states were considered to have equal utility. Fourthly, without long term follow-up data the model structure could not be validated; however, we investigated sources of potential bias towards minimally invasive internal thoracic artery bypass using alternative analysis. Finally, we did not carry out an analysis of the effects on budgets. Considering that about 9%17 of the 23 032 patients undergoing transluminal stenting in the United Kingdom annually30 have isolated lesions of the left anterior descending artery, the additional cost to the NHS of using minimally invasive internal thoracic artery bypass rather than transluminal stenting could be in the region of £1.7m (£829.02 per patient).
Implications for practice
The finding that minimally invasive internal thoracic artery bypass could be a more cost effective long term intervention than transluminal stenting has important implications for patients, clinicians, health service planning provision, and training. These results do not, however, account for the increased use of drug eluting stents over bare metal stents.21 As data on outcomes comparing transluminal stenting using drug eluting stents to bare metal stents do not currently extend beyond one year and no data compare transluminal stenting using drug eluting stents with minimally invasive internal thoracic artery bypass, rigorous comparison of the two interventions is at present not possible. Similarly the impact of robot assisted totally endoscopic coronary artery bypass grafting has not yet been evaluated and could facilitate even less invasive bypass grafting than minimally invasive internal thoracic artery bypass.31 32
What is already known on this topic
Surgical bypass may offer a more favourable long term outcome for multiple vessel coronary disease compared with percutaneous interventions
Minimally invasive left internal thoracic artery bypass results in fewer mid-term complications than transluminal stenting
What this study adds
Minimally invasive left internal thoracic artery bypass is more effective in the long term, justifying its initial additional cost
These findings do not take into account the effect of drug eluting stents, for which data on long term effectiveness are awaited
Contributors: CR was responsible for the study design, statistical analysis, data interpretation, and drafting of the manuscript. OA was responsible for drafting the manuscript, critical editing and manuscript direction, and revision for important intellectual content. SSP and CJ were responsible for data collection, data extraction analysis, and interpretation. SM was responsible for data extraction analysis, critical statistical analysis, and the study design. AD provided important intellectual content. TA is guarantor. His involvement was critical to every phase of this work and he had access to the data and controlled the decision to publish.
Funding: This study was undertaken as part of ongoing research at the Department of Biosurgery and Surgical Technology, Imperial College London, and did not receive separate funding.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Zimmerman FH, Cameron A, Fisher LD, Grace NG. Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (Coronary Artery Surgery Study Registry). J Am Coll Cardiol 1995;26:654-61. [DOI] [PubMed] [Google Scholar]
- 2.Niinami H, Takeuchi Y, Ichikawa S, Suda Y. Partial median sternotomy as a minimal access for off-pump coronary artery bypass grafting: feasibility of the lower-end sternal splitting approach. Ann Thorac Surg 2001;72(3):S1041-5. [DOI] [PubMed] [Google Scholar]
- 3.Casula R, Athanasiou T, Foale R. Recent advances in minimal-access cardiac surgery using robotic-enhanced surgical systems. Expert Rev Cardiovasc Ther 2004;2:589-600. [DOI] [PubMed] [Google Scholar]
- 4.Aziz O, Rao C, Panesar SS, Jones C, Morris S, Darzi A, et al. Meta-analysis of minimally invasive internal thoracic artery bypass versus percutaneous revascularisation for isolated lesions of the left anterior descending artery. BMJ 2007;doi=10.1136/bmj.39106.476215.BE. [DOI] [PMC free article] [PubMed]
- 5.Gray DT, Veenstra DL. Comparative economic analyses of minimally invasive direct coronary artery bypass surgery. J Thorac Cardiovasc Surg 2003;125(3):618-24. [DOI] [PubMed] [Google Scholar]
- 6.Ades AE, Claxton K, Sculpher M. Evidence synthesis, parameter correlation and probabilistic sensitivity analysis. Health Econ 2006;15:373-81. [DOI] [PubMed] [Google Scholar]
- 7.Clarke M, Oxman AD, eds. Cochrane reviewer's handbook 4.1.3 Cochrane Library [updated June 2001]. In:
- 8.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Onkologie 2000;23:597-602. [DOI] [PubMed] [Google Scholar]
- 9.Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddard GL. Methods for the economic evaluation of health care programmes 3rd ed. Oxford: Oxford University Press, 2004
- 10.National Institute for Clinical Excellence. Guide to the methods of technology appraisal. London: NICE, 2004 [PubMed]
- 11.Petitti DB. Meta-analysis, decision analysis and cost-effectiveness analysis: methods for quantitative synthesis in medicine 2nd ed. New York: Oxford University Press, 2000
- 12.UK Government Actuary's Department. Interim life tables 2005 London: GAD, 2006
- 13.National Institute for Clinical Excellence. Coronary artery stents: rapid review and economic evaluation. assessment report. London: NICE, 2003
- 14.Serruys PW, Unger F, van Hout BA, van den Brand MJ, van Herwerden LA, van Es GA, et al. The ARTS study (arterial revascularization therapies study). Semin Interv Cardiol 1999;4:209-19. [DOI] [PubMed] [Google Scholar]
- 15.Kind P, Brooks R, Rabin R. EQ-5D concepts and methods: a developmental history New York: Springer Science, 2005.
- 16.Szende A, Williams A. Measuring self reported population health: an international perspective based on EQ-5D Rotterdam: Euroqol Group, 2004 [PubMed]
- 17.Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al. A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery. Health Technol Assess 2004;8(16):1-43. [DOI] [PubMed] [Google Scholar]
- 18.Campbell MK, Torgerson DJ. Bootstrapping: estimating confidence intervals for cost effectiveness ratios. Q J Med 1999;92:177-82. [DOI] [PubMed] [Google Scholar]
- 19.Heitjan DF. Fieller's method and net health benefits. Health Econ 2000;9:327-35. [DOI] [PubMed] [Google Scholar]
- 20.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. [DOI] [PubMed] [Google Scholar]
- 21.Roiron C, Sanchez P, Bouzamondo A, Lechat P, Montalescot G. Drug eluting stents: a meta-analysis of randomised controlled trials. Heart 2006;92(5):641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessely R, Kastrati A, Schomig A. Late restenosis in patients receiving a polymer-coated sirolimus-eluting stent. Ann Intern Med 2005;143(5):392-4. [DOI] [PubMed] [Google Scholar]
- 23.British Medical Association, Royal Pharmaceutical Society of Great Britain. British national formulary London: BMA, RPS, 2006. (No 51.)
- 24.Yock CA, Boothroyd DB, Owens DK, Garber AM, Hlatky MA. Cost-effectiveness of bypass surgery versus stenting in patients with multivessel coronary artery disease. Am J Med 2003;115(5):382-9. [DOI] [PubMed] [Google Scholar]
- 25.Bypass Angioplasty Revascularization Investigators. Seven year outcome in the bypass angioplasty revascularization investigation (BARI) by treatment and diabetic status. J Am Coll Cardiol 2000;35:1122-9. [DOI] [PubMed] [Google Scholar]
- 26.King SB III, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eight-year mortality in the Emory angioplasty versus surgery trial (EAST). J Am Coll Cardiol 2000;35:1116-21. [DOI] [PubMed] [Google Scholar]
- 27.George CJ, Baim DS, Brinker JA, Fischman DL, Goldberg S, Holubkov R, et al. One-year follow-up of the stent restenosis (STRESS I) study. Am J Cardiol 1998;81:860-5. [DOI] [PubMed] [Google Scholar]
- 28.Macaya C, Serruys PW, Ruygrok P, Suryapranata H, Mast G, Klugmann S, et al. Continued benefit of coronary stenting versus balloon angioplasty: one-year clinical follow-up of Benestent trial. Benestent Study Group. J Am Coll Cardiol 1996;27:255-61. [DOI] [PubMed] [Google Scholar]
- 29.Popma JJ, Kantz RE, Bairn DS. Editorial: a decade of improvement in the clinical outcomes of percutaneous coronary interventions for multi-vessel coronary artery disease. Circulation 2002;106:1592-4. [DOI] [PubMed] [Google Scholar]
- 30.Department of Health. NHS reference costs 2005 London: DoH, 2006
- 31.Kappert U, Cichon R, Schneider J, Gulielmos V, Ahmadzade T, Nicolai J, et al. Technique of closed chest coronary artery surgery on the beating heart. Eur J Cardiothorac Surg 2001;20(4):765-9. [DOI] [PubMed] [Google Scholar]
- 32.Argenziano M, Katz M, Bonatti J, Srivastava S, Murphy D, Poirier R, et al. Results of the prospective multicenter trial of robotically assisted totally endoscopic coronary artery bypass grafting. Ann Thorac Surg 2006;81:1666-75. [DOI] [PubMed] [Google Scholar]