Abstract
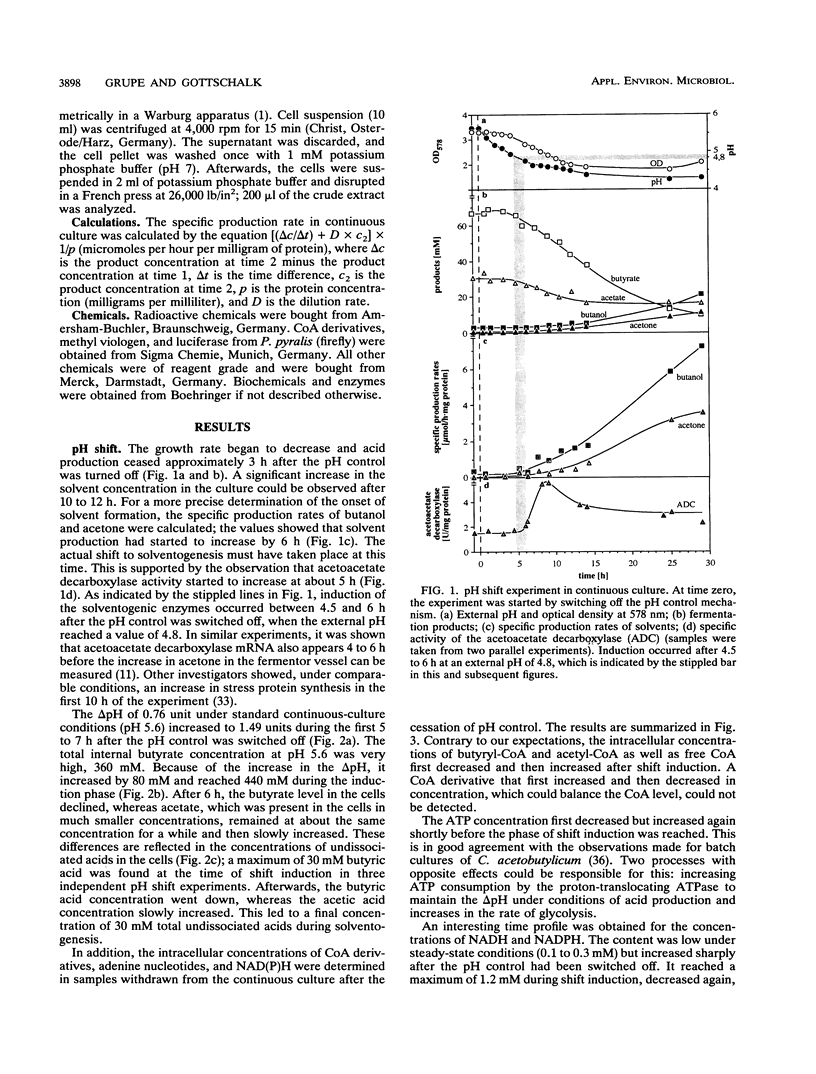
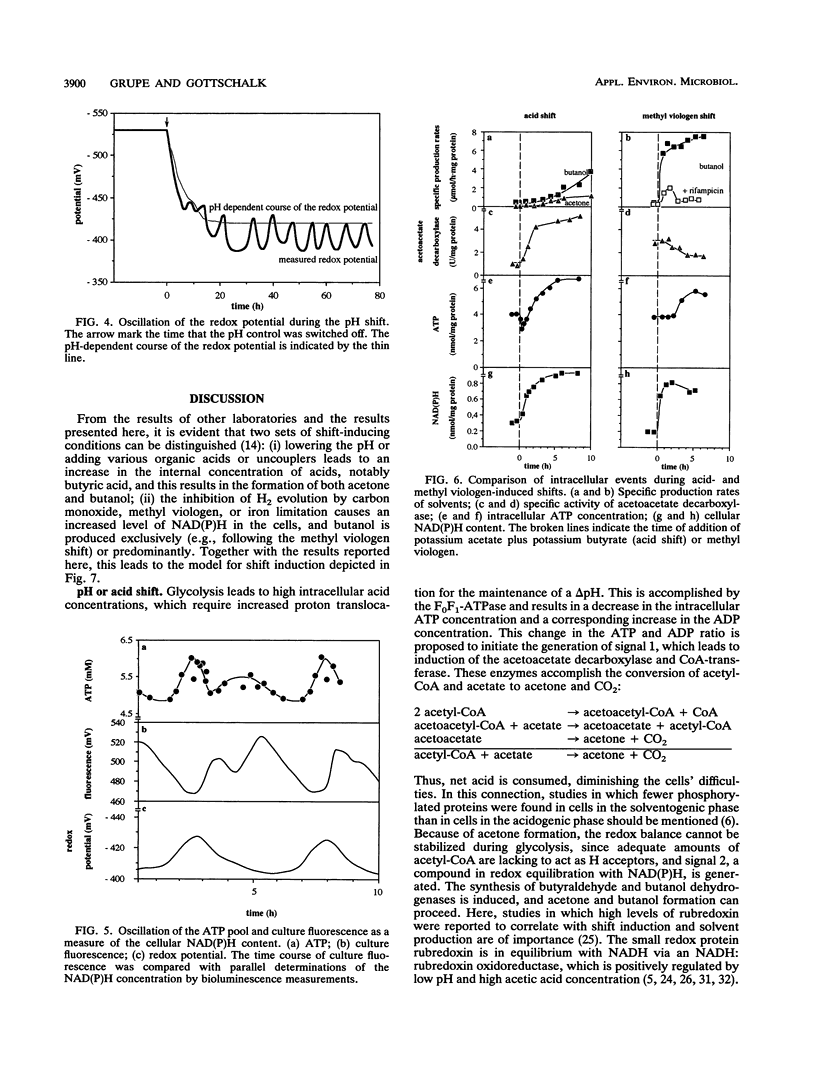
The pH of continuous cultures of Clostridium acetobutylicum growing at pH 5.6 was allowed to decrease to 4.3 after acid production and thereby to shift the cultures from acetate and butyrate to acetone and butanol formation. Several parameters were determined during the shift. An increase in the intracellular acid concentration to 440 mM was recorded. An excess of undissociated butyric acid but not of acetic acid just before the shift to solventogenesis was followed by a decline in acid production and subsequently by the uptake of acids. The intracellular ATP concentration reached a minimum before the onset of solventogenesis; this presumably reflects the ATP-consuming proton extrusion connected with the increase in the ΔpH from 0.7 to 1.4 units. The pool of NADH plus NADPH exhibited a drastic increase until solventogenesis was induced. The changes in the ATP and ADP and NADH plus NADPH pools during these pH shift experiments were the beginning of a stable metabolic oscillation which could also be recorded as an oscillation of the culture redox potential under steady-state solventogenic conditions. Similar changes were observed when the shift was induced by the addition of butyrate and acetate (50 mM each) to the continuous culture. However, when methyl viologen was added, important differences were found: ATP levels did not reach a minimum, acetoacetate decarboxylase activity could not be measured, and butanol but not acetone was produced. A model for the shift is proposed; it assumes the generation of two signals, one by the changed ATP and ADP levels and the other by the increased NAD(P)H level.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballongue J., Amine J., Masion E., Petitdemange H., Gay R. Rôle de l'acétate et du butyrate dans l'induction de la NADH: rubrédoxine oxydoréductase chez Clostridium acetobutylicum. Biochimie. 1986 Apr;68(4):575–580. doi: 10.1016/s0300-9084(86)80202-4. [DOI] [PubMed] [Google Scholar]
- Balodimos I. A., Rapaport E., Kashket E. R. Protein phosphorylation in response to stress in Clostridium acetobutylicum. Appl Environ Microbiol. 1990 Jul;56(7):2170–2173. doi: 10.1128/aem.56.7.2170-2173.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles L. K., Ellefson W. L. Effects of butanol on Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Nov;50(5):1165–1170. doi: 10.1128/aem.50.5.1165-1170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerischer U., Dürre P. mRNA analysis of the adc gene region of Clostridium acetobutylicum during the shift to solventogenesis. J Bacteriol. 1992 Jan;174(2):426–433. doi: 10.1128/jb.174.2.426-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. A., Stephens G. M., Morris J. G. Production of Solvents by Clostridium acetobutylicum Cultures Maintained at Neutral pH. Appl Environ Microbiol. 1984 Dec;48(6):1166–1170. doi: 10.1128/aem.48.6.1166-1170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Gibbins L. N., Forsberg C. W. Transmembrane pH gradient and membrane potential in Clostridium acetobutylicum during growth under acetogenic and solventogenic conditions. Appl Environ Microbiol. 1985 Oct;50(4):1043–1047. doi: 10.1128/aem.50.4.1043-1047.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. H., Bellows P., Datta R., Zeikus J. G. Control of Carbon and Electron Flow in Clostridium acetobutylicum Fermentations: Utilization of Carbon Monoxide to Inhibit Hydrogen Production and to Enhance Butanol Yields. Appl Environ Microbiol. 1984 Oct;48(4):764–770. doi: 10.1128/aem.48.4.764-770.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Marczak R., Ballongue J., Petitdemange H., Gay R. Differential levels of ferredoxin and rubredoxin in Clostridium acetobutylicum. Biochimie. 1985 Feb;67(2):241–248. doi: 10.1016/s0300-9084(85)80052-3. [DOI] [PubMed] [Google Scholar]
- Marczak R., Petitdemange H., Alimi F., Ballongue J., Gay R. Influence de la phase de croissance et de la composition du milieu sur le taux de biosynthèse de la NADH: rubrédoxine oxydoréductase chez Clostridium acetobutylicum. C R Seances Acad Sci III. 1983;296(10):469–474. [PubMed] [Google Scholar]
- Petitdemange H., Blusson H., Gay R. Detection of NAD(P)H--rubredoxin oxidoreductases in Clostridia. Anal Biochem. 1981 Sep 15;116(2):564–570. doi: 10.1016/0003-2697(81)90403-6. [DOI] [PubMed] [Google Scholar]
- Petitdemange H., Marczak R., Blusson H., Gay R. Isolation and properties of reduced nicotinamide adenine dinucleotiderubredoxin oxidoreductase of Clostridium acetobutylicum. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1258–1265. doi: 10.1016/0006-291x(79)91202-6. [DOI] [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Terracciano J. S., Kashket E. R. Intracellular Conditions Required for Initiation of Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):86–91. doi: 10.1128/aem.52.1.86-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


