Abstract
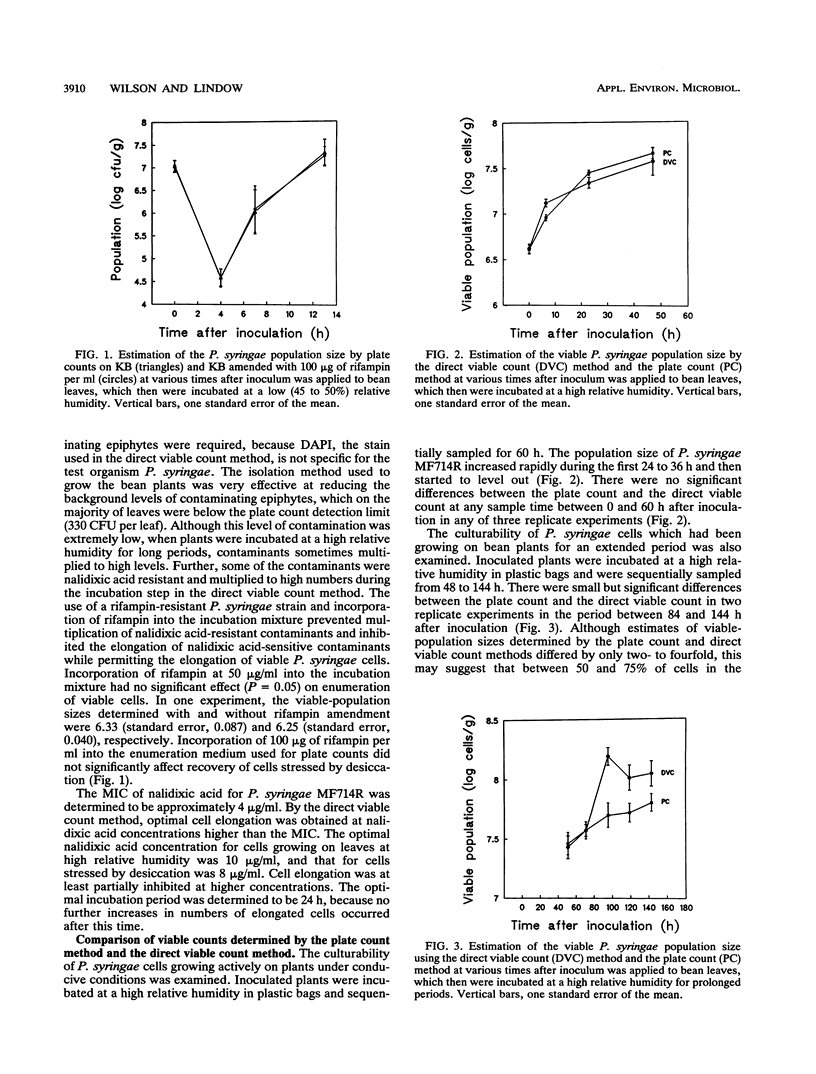
The direct viable count method, used to detect viable but nonculturable bacteria in aquatic systems, was modified to examine epiphytic populations of Pseudomonas syringae. Viable-population sizes determined from the number of cells that elongated when incubated with yeast extract and nalidixic acid were compared with those determined by the conventional plate count method. The plate count method accurately determined the number of viable cells in epiphytic P. syringae populations in a state of active growth under conditions of high relative humidity. The plate count method also accurately determined the number of viable cells in P. syringae inoculum, or a growing P. syringae population, subject to desiccation stress under conditions of low relative humidity. In epiphytic populations of P. syringae older than 80 h, however, the plate count underestimated the viable-population size by about two- to fourfold, suggesting that up to 75% of the P. syringae population was nonculturable. These nonculturable cells may have entered a starvation-survival state, induced by low nutrient availability in the phyllosphere environment. Epiphytic P. syringae populations undergoing rapid size changes due to growth and death under fluctuating environmental conditions in the field should be accurately enumerated by the plate count method. However, the possible underestimation of viable-population size under some circumstances should be considered in epidemiological studies of phytopathogenic bacteria and when genetically engineered microorganisms in terrestrial ecosystems are monitored.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström I., Heinänen A., Salonen K. Comparison of acridine orange, acriflavine, and bisbenzimide stains for enumeration of bacteria in clear and humic waters. Appl Environ Microbiol. 1986 Mar;51(3):664–667. doi: 10.1128/aem.51.3.664-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Influence of environmental stress on enumeration of indicator bacteria from natural waters. Appl Microbiol. 1975 Feb;29(2):186–194. doi: 10.1128/am.29.2.186-194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. J., Xu H. S., Colwell R. R. Viable but nonculturable bacteria in drinking water. Appl Environ Microbiol. 1991 Mar;57(3):875–878. doi: 10.1128/aem.57.3.875-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan K., Fieland V., Fowles N., Ganio L., Seidler R. Efficacy of burning, tillage, and biocides in controlling bacteria released at field sites and effects on indigenous bacteria and fungi. Appl Environ Microbiol. 1992 Apr;58(4):1207–1214. doi: 10.1128/aem.58.4.1207-1214.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco D. E., Mah R. A., Rabin A. C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans Am Microsc Soc. 1973 Jul;92(3):416–421. [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Hirano S. S., Upper C. D. Diel Variation in Population Size and Ice Nucleation Activity of Pseudomonas syringae on Snap Bean Leaflets. Appl Environ Microbiol. 1989 Mar;55(3):623–630. doi: 10.1128/aem.55.3.623-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff K. A. Rapid and simple method for double staining of bacteria with 4',6-diamidino-2-phenylindole and fluorescein isothiocyanate-labeled antibodies. Appl Environ Microbiol. 1988 Dec;54(12):2949–2952. doi: 10.1128/aem.54.12.2949-2952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger J. Near-UV radiation effects on microorganisms. Photochem Photobiol. 1981 Dec;34(6):761–768. [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Linder K., Oliver J. D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989 Nov;55(11):2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel D. A., Van Netten P. Harmful effects of selective media on stressed micro-organisms: nature and remedies. Soc Appl Bacteriol Symp Ser. 1984;(12):329–369. [PubMed] [Google Scholar]
- Oliver J. D., Nilsson L., Kjelleberg S. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol. 1991 Sep;57(9):2640–2644. doi: 10.1128/aem.57.9.2640-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Singh A., Pyle B. H., McFeters G. A. Rapid enumeration of viable bacteria by image analysis. J Microbiol Methods. 1989;10:91–101. doi: 10.1016/0167-7012(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Singh A., Yu F. P., McFeters G. A. Rapid detection of chlorine-induced bacterial injury by the direct viable count method using image analysis. Appl Environ Microbiol. 1990 Feb;56(2):389–394. doi: 10.1128/aem.56.2.389-394.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



