Abstract
Cigarette smokers with past major depressive disorder (MDD) received 8 group sessions of standard, cognitive–behavioral smoking cessation treatment (ST; n = 93) or standard, cognitive–behavioral smoking cessation treatment plus cognitive–behavioral treatment for depression (CBT-D; n = 86). Although abstinence rates were high in both conditions (ST, 24.7%; CBT-D, 32.5%, at 1 year) for these nonpharmacological treatments, no main effect of treatment was found. However, secondary analyses revealed significant interactions between treatment condition and both recurrent depression history and heavy smoking (≥25 cigarettes a day) at baseline. Smokers with recurrent MDD and heavy smokers who received CBT-D were significantly more likely to be abstinent than those receiving ST (odds ratios = 2.3 and 2.6, respectively). Results suggest that CBT-D provides specific benefits for some, but not all, smokers with a history of MDD.
Cigarette smoking and depression frequently co-occur in both community and clinical samples. Smokers have generally been shown to exhibit higher rates of major depressive disorder (MDD; Breslau, Kilbey, & Andreski, 1991; Glassman et al., 1990; Kendler et al., 1993) and depressive symptoms (e.g., Anda et al., 1990) than nonsmokers, although exceptions have been noted (e.g., Covey, Hughes, Glassman, Blazer, & George, 1994). In one catchment area survey, smokers were more than twice as likely (6.6% vs. 2.9%) as nonsmokers to have a history of MDD (Glassman et al., 1990). Likewise, lifetime history of MDD has been associated with increased smoking prevalence in both men and women (Cohen, Schwartz, Bromet, & Parkinson, 1991) and decreased likelihood of quitting (Glassman et al., 1990). Lifetime prevalence rates of MDD are particularly high among smokers entering smoking cessation programs, with observed rates ranging from 22% to 61% (Glassman et al., 1988; Hall, Muñoz, & Reus, 1994; Hall et al., 1996, 1998). This is considerably higher than the lifetime prevalence of MDD in the general population, which is approximately 17% (Kessler, 1994).
Research suggests that smokers with a history of MDD have particular difficulty quitting. Although an association between lifetime MDD and smoking outcome has not been found in some smoking cessation treatment studies (Ginsberg, Hall, Reus, & Muñoz, 1995; Hall et al., 1994), a history of MDD has been associated with poorer smoking outcome at both 4 weeks (Glassman et al., 1988) and 10 weeks after the quit date (Glassman et al., 1993). Among smokers with past MDD, particularly poor cessation outcomes have been reported in women (Hall et al., 1998) and in smokers with recurrent episodes of MDD (Glassman et al., 1993).
If indeed smokers with lifetime MDD have difficulty in quitting, this may be attributable to elevations in negative mood and depressive symptoms. In retrospective studies, smokers consistently report that relapse to smoking often occurs in situations involving negative moods such as anxiety, anger, and depression (Bliss, Garvey, Heinold, & Hitchcock, 1989; Brandon, Tiffany, Obremski, & Baker, 1990; Marlatt & Gordon, 1980; Shiffman, 1982). A number of prospective studies have shown that affective distress at the beginning of treatment (Kinnunen, Doherty, Militello, & Garvey, 1996) and after quitting (Covey, Glassman, & Stetner, 1990; Ginsberg et al., 1995; West, Hajek, & Belcher, 1989) predicts poor outcome. Smokers with a positive history of MDD are at particular risk because they tend to begin treatment with higher levels of negative mood than smokers without past MDD (Ginsberg et al., 1995; Hall et al., 1994), report more depressed mood and higher overall withdrawal intensity during the week after quit date (Covey et al., 1990), and show greater increases in anger and depression during the 1st week of quitting (Ginsberg et al., 1995). Furthermore, pretreatment negative mood has been found to be a more powerful predictor of treatment failure for smokers with a positive history of MDD than for smokers without past MDD (Hall et al., 1994).
Cessation treatments that provide strategies for managing depressive symptoms and negative mood have become a recent focus of investigation. Three studies by Hall and colleagues have yielded mixed results on the efficacy of cessation programs incorporating mood management techniques. In an initial study in which participants received nicotine gum, smokers with a history of MDD in a 10-session cognitive–behavioral mood management treatment achieved higher rates of continuous abstinence than those in a standard 5-session treatment (Hall et al., 1994). The effect of treatment condition was nonsignificant for smokers without past MDD. However, the failure to equate for contact time between treatments in this study limits the interpretation of treatment effects. In a second study using nicotine gum and placebo, Hall and colleagues (Hall et al., 1996) equated for contact time (i.e., both interventions were 10 sessions) and used a health education control condition that expanded the 5-session treatment used in the Hall et al. (1994) study. Contrary to earlier findings, the mood management condition did not outperform the control condition among participants with past MDD, although the small number of past MDD participants provided limited statistical power for examination of treatment effects among this subpopulation of smokers. Recently, Hall and colleagues completed an outcome study incorporating nortriptyline and placebo (Hall et al., 1998). In this study, the mood management condition again had greater contact time than the control condition (10 vs. 5 sessions) and, as in the first study, mood management was more effective in smokers with a history of MDD but not for smokers without past MDD. Although the results of these studies suggest that smokers with past MDD benefit from more intensive behavioral treatment, they do not demonstrate the specific effects of mood management treatment for this subpopulation.
In the present study, we compared the efficacy of a standard, cognitive–behavioral smoking cessation treatment (ST) with that of this standard cessation treatment combined with cognitive–behavioral therapy for depression (CBT-D) in smokers with a positive history of MDD. Both treatment conditions provided eight 2-hr sessions and were equated for participant and therapist contact time. We used a sample consisting solely of smokers with a history of MDD because we were interested specifically in this subpopulation of smokers and wanted to have adequate power to test main effects of treatment, as well as mediators and moderators of treatment effects. We predicted that CBT-D would produce higher abstinence rates than ST in this population. We also predicted that CBT-D would help reduce negative mood and depressive symptoms leading up to quit date and would attenuate increases in these symptoms after quit date. Secondarily, we examined other factors that might increase risk for treatment failure and whose effects might be moderated by treatment condition, including demographic characteristics and measures of nicotine dependence and of depression severity and chronicity. Finally, we examined participants' subjective evaluations of treatment usefulness.
Method
Participants
Participants were 179 smokers recruited from the community through newspaper, radio, and television advertisements and flyers distributed to local health care professionals. Participants were included if they were between the ages of 18 and 70 years, had regularly smoked cigarettes for at least 1 year, were currently smoking at least 10 cigarettes per day, and had a history of MDD according to the Diagnostic and Statistical Manual of Mental Disorders (3rd ed. rev.; DSM–III–R; American Psychiatric Association, 1987), as determined by the Structured Clinical Interview for DSM–III–R—Non-Patient Edition (SCID-NP, Version 1.0; Spitzer, Williams, Gibbon, & First, 1990). Exclusion criteria were (a) DSM–III–R diagnosis of current MDD, dysthymia, or other Axis I disorder; (b) DSM–III–R diagnosis of psychoactive substance abuse or dependence within the past 6 months (other than nicotine); (c) current use of psychotropic medication; (d) current weekly psychotherapy; (e) use of other tobacco products; and (f) intent to use pharmacological aid to cessation.
Potential participants were screened by telephone according to inclusion–exclusion criteria prior to an intake interview to confirm eligibility. At intake, participants signed a statement of informed consent approved by the Butler Hospital Institutional Review Board. Eligible participants were required to provide a $75 deposit, refunded incrementally on completion of follow-up procedures. Smoking status was not considered in return of deposits. Reduced deposit amounts were collected for those who could not afford to deposit $75.
Three hundred fifty-eight participants met preliminary phone screen criteria and were invited to the study center for a diagnostic interview to confirm eligibility. One hundred of these participants did not show up for the diagnostic interview, leaving 258 participants who completed informed consent and participated in the interview. Of these 258 individuals, 5 withdrew prior to being assigned to a treatment condition and 74 did not meet inclusion–exclusion criteria (44 did not have a history of MDD, 14 met criteria for current MDD, 4 met criteria for current dysthymia, 5 had a history of bipolar disorder, 2 met criteria for current substance dependence, 4 had current psychotic symptoms, and 1 met criteria for current mania).
Of the 179 participants randomized to treatment, 107 (59.8%) were women, and 94 (52.5%) were married or living with a partner as if married. The mean age of the sample was 45.1 years (SD = 9.27), and the mean number of years of education completed was 14.5 (SD = 2.48). Almost all participants (n = 174, 97.2%) identified themselves as White. One participant was African American, and 4 were of other ethnic origins. Prior to treatment, participants reported smoking an average of 27.3 (SD = 11.27) cigarettes per day and had been smoking for an average of 27.1 years (SD = 9.47). The sample mean on the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) was 6.80 (SD = 1.93), and saliva cotinine levels averaged 383.7 ng/ml (SD = 170.59) at baseline. All but 7 participants (3.9%) had made a prior quit attempt. The sample mean on the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) administered immediately prior to Session 1 was 7.8 (SD = 6.31). Rates of history of substance use disorders were high, with 43.6% of participants (n = 78) having a history of alcohol abuse (n = 18) or dependence (n = 60), and 35.8% (n = 64) having a history of drug abuse (n = 22) or dependence (n = 42).1 Other disorders were more rare: Eighteen had a history of panic disorder, 1 had a history of agoraphobia without panic disorder, 9 had a history of social phobia, 2 had a history of simple phobia, and 1 had a history of bulimia nervosa.
Participants were randomized to treatment condition according to gender, current depressive symptoms (BDI cutoff = 9), and level of nicotine dependence (Fagerstrom Tolerance Questionnaire; FTQ; Fagerstrom, 1978; cutoff = 7) using the urn randomization technique (Wei, 1978). Ninety-three participants were randomized to the ST condition; 86 to CBT-D.
Measures
An assessment battery was administered at pretreatment. Questionnaires were also administered at each session during treatment. Follow-up phone interviews occurred at 1, 6, and 12 months posttreatment, and self-reported abstinence was verified biochemically. Five types of measures were included in the study: (a) descriptive and diagnostic measures, (b) measures of depressive symptoms and negative mood, (c) measures of smoking status, (d) measures of treatment integrity and compliance, and (e) measures of perceived treatment utility.
Descriptive and diagnostic measures
Participants provided demographic and background information such as age, gender, years of education, marital status, number of years of regular smoking, and average number of cigarettes smoked per day. Lifetime and current DSM–III–R Axis I diagnoses were determined with the SCID-NP (Spitzer et al., 1990). Severity of nicotine dependence was assessed using the FTND (Heatherton et al., 1991), which is derived from the FTQ (Fagerstrom, 1978). The FTND is a brief, six-item measure with scores ranging from 0 to 10, with higher scores indicating higher levels of nicotine dependence.
Measures of depressive symptoms and negative mood
The BDI (Beck et al., 1961) was used to assess depressive symptoms prior to and during treatment. The Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971) was used to assess changes in negative mood during treatment. A total negative mood score was created from the POMS by averaging the Tension–Anxiety, Anger–Hostility, and Depression–Dejection subscales.
Measures of smoking status
Self-reports of smoking status were collected at each treatment session from quit date to the end of treatment, and by telephone at 1-, 6-, and 12-month follow-up. Outcome analyses were based on 7-day point prevalence abstinence (i.e., reported abstinence of at least 7 days prior to the assessment day). Participants' reports of abstinence were verified biochemically.
Biochemical verification was obtained during treatment and follow-ups with alveolar carbon monoxide (CO) using a CMD/CO Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc., Auburn, ME). A 2-ml saliva sample was collected during treatment and follow-up and assayed for cotinine by the American Health Foundation (Valhalla, NY). All partici-pants provided breath samples for CO analysis and saliva samples for cotinine analysis at posttreatment. During follow-up, these biochemical measures were obtained in person, only from participants reporting 7-day abstinence (during telephone follow-up). Abstinence was confirmed by a combination of CO ≤ 10 ppm and cotinine ≤ 46 ng/ml (Cummings & Richard, 1988). In those few cases where biochemical verification could not be obtained (6.5%), self-reported abstinence was verified through interviews with significant others.
Measures of treatment integrity and compliance
To assess treatment integrity across conditions, we audiotaped each group session. One session was randomly selected from each 8-session treatment group (across both conditions), and that audiotape was rated by an independent rater not associated with the treatment to determine therapist adherence to the protocol. Specific topics for each session were provided to the rater according to major headings in the therapist manual, and the rater indicated whether or not each topic was covered during the session.
Treatment compliance was assessed by tracking participants' attendance at treatment sessions and by monitoring homework assignment completion. Participants were asked to complete homework assignments as a component of treatment. Homework assignment worksheets were provided on two-part, no-carbon-required paper. At each session, therapists collected all homework worksheets that had been assigned, regardless of degree of work completed. The ST condition included 6 different types of worksheets covering standard smoking cessation content. Because some worksheets were repeated for several consecutive sessions (e.g., identifying smoking trigger situations took place over several sessions), a total of 11 worksheet assignments were provided throughout the 6-week treatment. The CBT-D condition included 14 different types of worksheets (the same 6 as in the ST condition, as well as 8 more related to depression coping skills). Again, because some worksheets were repeated for several sessions, a total of 33 worksheets were assigned throughout the CBT-D treatment. All homework worksheets were subsequently rated by independent raters as to the quantity of the assignment completed, according to specific criteria provided to them.
Measures of perceived treatment utility
At the last treatment session, participants rated the effectiveness of the skills taught during treatment on a 5-point Likert-type scale (1 = Not at all effective, 5 = Extremely effective). Participants in ST rated 10 skills, and those in CBT-D rated the same 10 skills plus 4 condition-specific skills. Participants also answered the more general question, “To what extent did the program help you make positive changes in your life not related to smoking?” by providing ratings on a 5-point Likert-type scale (1 = Not at all, 5 = A great deal).
A second, anonymous posttreatment questionnaire asked participants, using 5-point Likert scales, to provide ratings of the “overall benefit” they received from participating in the smoking cessation program (1 = None at all, 5 = A great deal) and to answer the question “How useful were the specific skills learned in the program in helping you quit smoking?” (1 = Not useful, 5 = Extremely useful). Although anonymity was provided to individual participants, responses to this questionnaire were identifiable by treatment condition.
Treatments
Elements common to both treatments
Both treatments consisted of eight 2-hr sessions over 6 weeks. Quit date began on awakening on the morning of the fifth session. Sessions occurred weekly (in the early evening), except for the sixth session, which took place 3 days after quit date to provide a second session during the quit week. Patient manuals were used in both conditions and consisted of graphics and limited text presentations to enhance readability. Therapist manuals were used to ensure standard delivery of the program.
Therapists
Each group was co-led by 2 therapists. A total of 12 therapists (9 men, 3 women) conducted treatment sessions and were counterbalanced across treatments. Therapists included clinical psychologists, postdoctoral fellows in clinical psychology, and interns in clinical psychology. Richard A. Brown provided therapist training and conducted weekly group supervision during the study to ensure standard protocol delivery. All group sessions were audiotaped for purposes of group super-vision and assessment of therapist adherence to protocol.
Standard smoking cessation treatment (ST)
The standard smoking cessation treatment is a comprehensive, cognitive–behavioral program. The treatment included daily homework assignments applying elements of the skills being taught and included the following components.
Treatment rationale: Therapists provided a model of smoking as a learned habit and physical addiction.
Self-monitoring: Participants learned about their smoking habit by writing down time of day, situation, and perceived need for every cigarette smoked before smoking cessation.
Self-management: Participants identified common cues or “triggers” for smoking and learned to alter or avoid cues, or use substitutes in cue situations.
Nicotine fading: Nicotine fading (Brown, Lichtenstein, McIntyre, & Harrington-Kostur, 1984; Foxx & Brown, 1979) was accomplished by having participants systematically change their brand of cigarette consumed to a progressively lower nicotine content cigarette. Following a week of baseline smoking, participants changed to brands rated at 30%, 60%, and 90% lower than baseline brand in successive weeks leading up to quit date.
Relapse prevention: Participants were taught to identify high-risk situations for relapse and to rehearse behavioral or cognitive means of coping with these high-risk situations. Participants were also taught to anticipate and cope with negative emotional reactions that might occur following a slip (i.e., abstinence violation effect; Marlatt & Gordon, 1985).
Social support: Participants identified key behaviors by other people in their lives related to their effort to quit smoking and requested more of the supportive behaviors and less of (or total elimination of) the nonsupportive behaviors.
Standard smoking cessation plus cognitive–behavioral treatment for depression (CBT-D)
The CBT-D condition consisted of an integration of the standard, cognitive–behavioral smoking cessation skills described previously and cognitive–behavioral coping skills for depression. The same standard smoking cessation skills (described above) were included, but the amount of time devoted to presentation and discussion of these skills was condensed, relative to the ST condition. The Coping With Depression course (Brown & Lewinsohn, 1984a, 1984b; Lewinsohn, Antonuccio, Breckenridge, & Teri, 1984) served as the basis for the cognitive–behavioral treatment for depression in the CBT-D condition.
The application of the depression coping skills training to smoking involved daily homework assignments applying elements of the skills being taught and included the following components, which were presented as alternatives to smoking that could combat feelings of depression and fill the perceived void following the loss of smoking as a reinforcing activity.
Treatment rationale: The reciprocal relationship among behavior, thoughts, and mood was explained as an organizing model for the proposed treatment, and examples of how changes in behavior and thoughts can impact mood were provided. The relationship between depressive symptoms and cigarette smoking was discussed, and the importance of learning coping skills to control depressive symptoms that might otherwise serve as triggers to smoke was stressed.
Daily mood rating: Participants monitored their mood daily on a 9-point Likert scale throughout treatment to gain skills in identifying daily mood states and the factors that influence them and to determine the effect of mood-specific coping skills during the intervention. Each day, participants rated the extent to which they felt depressed, angry–irritable, and anxious.
Increasing pleasant activities: The Pleasant Events Schedule-Mood Related Form (Lewinsohn & Graf, 1973) was used to help participants create a personalized list of their “Top 20” pleasant activities. Participants then contracted for achievable, systematic increases in pleasant activities to maintain or improve their mood and to prevent the onset of depressive symptoms following cessation.
Increasing positive–decreasing negative thoughts: Participants monitored both positive and negative thoughts daily. Cognitive self-management techniques for reducing negative thoughts and for increasing positive thoughts were taught (Lewinsohn, Muñoz, Youngren, & Zeiss, 1986).
ABC technique: This procedure for identifying and challenging distorted, depressive thoughts was based on the rational–emotive therapy of Albert Ellis (Ellis & Harper, 1961) and incorporated instruction in the identification of cognitive distortions (Beck, Rush, Shaw, & Emery, 1978; Burns, 1980).
Social skills–assertiveness: Modeling, role-playing, and homework exercises were used to teach participants to respond more assertively in various situations, including situations involving social pressure to smoke.
Maintaining gains: Participants were encouraged to monitor their mood periodically, to identify the skills they found to be most effective for them, and to actively use those skills to manage or prevent depressed mood without smoking.
Results
Treatment Integrity and Compliance
Therapist adherence to protocol was excellent across cohorts and did not differ by condition (p = .86). Therapists in the ST condition completed 96.1% of treatment protocol topics across sessions sampled, compared with 95.4% by therapists in the CBT-D condition. Attendance at treatments sessions also did not differ by condition (p = .70). Participants attended an average of 5.8 sessions (SD = 2.2) in the ST condition and 5.9 sessions (SD = 2.1) in the CBT-D condition. Homework completion in the ST condition decreased from 50% (SD = 20.5) at Session 2 to 18.5% (SD = 20.2%) at Session 8 and decreased in the CBT-D condition from 52% (SD = 17.1%) at Session 2 to 19.5% (SD = 17.5%) at Session 8. The conditions did not differ in the percentage of smoking cessation skills homework completed at any session (ps > .05).
Preliminary tests were conducted to determine whether there were significant therapist effects. Using effects coding, we found that outcomes for any one therapist did not differ significantly from the average of the remaining therapists. Also, there were no therapist by treatment interactions. Similarly, we found that, within each treatment condition, no individual group had significantly different outcomes than the average outcome for the remaining groups.
Treatment Outcome
Following the intention-to-treat principle, data from all randomized participants were analyzed. Participants were followed for 12 months after the end of treatment, and assessments of interest, including point prevalence abstinence, were obtained at the end of treatment and at 1-, 6-, and 12-month follow-ups regardless of compliance with treatment. Completion rates at the 1-, 6-, and 12-month follow-ups, respectively, were 95.0%, 91.1%, and 92.2%.
Seven-day point prevalence abstinence rates at end of treatment and at the 1-month, 6-month, and 12-month follow-ups for the ST condition were 33.3%, 30.1%, 24.7%, and 24.7%, respectively, and, for the CBT-D condition, were 37.6%, 39.5%, 24.4%, and 32.5%, respectively. Chi-square analyses indicated that there were no significant differences in abstinence rates between treatment conditions at any one assessment point (all ps > .15). Continuous abstinence rates (confirmed abstinence at posttreatment, 1 month, 6 months, and 12 months) also did not differ by condition (15.0% in ST and 10.5% in CBT), χ2 (1, N = 179) = 0.84, ns.
To examine more thoroughly the effect of treatment within the context of other covariates that impact outcome, we conducted repeated measures analyses for categorical outcomes using the generalized estimating equation (GEE; Liang & Zeger, 1986; Zeger & Liang, 1986) with point prevalence abstinence at the four time periods as the dependent variable. GEE allows for inclusion of both categorical and continuous independent variables and for appropriate modeling of covariance structures when observations are correlated across time. Analyses were conducted in SAS using PROC GENMOD (SAS Institute, 1997) with the Logit link function and an unstructured correlation matrix specified.
Covariates for the GEE analysis were selected from among variables assessed prior to treatment and represented three domains: demographic characteristics (age, gender, marital status, and years of education), nicotine dependence severity (FTND score, number of years smoking, average daily smoking rate, and saliva cotinine), and depression severity and chronicity (BDI, negative mood on the POMS, and number of lifetime episodes of MDD). A variable carrying the effect of time was also added. Saliva cotinine, POMS, and BDI scores were square-root transformed to correct for positive skewness. Because of significant truncation in its distribution, number of lifetime episodes of MDD was dichotomized as recurrent (n = 98) versus nonrecurrent depression (n = 79). Valid data on number of depressive episodes were not available for 2 participants. Baseline smoking rate had a moderately bimodal distribution and was dichotomized as light (<25 cigarettes per day; n = 87) versus heavy (>25 cigarettes per day; n = 92) smoking. Treatment condition was not significantly associated with any of the covariates selected (ps > .15), suggesting that randomization was successful.
To reduce the set of covariates to those most relevant to treatment outcome, we conducted a backwards selection procedure in GEE with variables significant at p < .15 being retained. The time variable, years of education, heavy smoking at baseline, BDI, and recurrent depression were retained in the final model. Correlations between the variables selected were generally weak. Only recurrent depression and BDI score (r = .20) were significantly correlated at p < .05. Recurrent depression was not significantly cor-related with smoking rate (r = −.12, p = .10) or level of dependence (FTND score; r = .12, p = .09). The GEE model with the selected covariates is presented in the top of Table 1. The effect of time approached significance (p = .08), reflecting the modest tendency for abstinence rates to decrease across the follow-ups. Higher levels of education were significantly associated with a greater likelihood of abstinence across the four time periods (p = .048). Heavy smoking, BDI score, and history of recurrent depression were all significantly associated with a lower likelihood of abstinence (ps < .05). In the second step of a hierarchical analysis, the treatment condition variable was added to the GEE model. The effect of treatment approached statistical significance(p = .095), indicating a possible advantage of CBT-D over ST in smoking cessation outcome.
Table 1.
Hierarchical Generalized Estimating Equation Models Predicting 7-Day Point Prevalence Abstinence at the Posttreatment, 1-Month, 6-Month, and 12-Month Assessments
| Step and variable | B | SEB | Odds ratio |
|---|---|---|---|
| Step 1 | |||
| Intercept | −2.45 | 1.86 | — |
| Time | −0.12 | 0.07 | 0.88 |
| Years of educationa | 0.23 | 0.11 | 1.26* |
| Heavy smokingb | −1.29 | 0.22 | 0.27* |
| Pretreatment BDIc | −0.27 | 0.11 | 0.76* |
| Recurrent depressiond | −1.44 | 0.44 | 0.24** |
| Step 2 | |||
| Intercept | −1.40 | 0.91 | — |
| Time | −0.12 | 0.07 | 0.89 |
| Years of education | 0.12 | 0.06 | 1.13* |
| Heavy smoking | −0.69 | 0.27 | 0.50* |
| Pretreatment BDI | −0.21 | 0.11 | 0.81* |
| Recurrent depression | −0.82 | 0.28 | 0.44** |
| CBT-D treatmente | 0.45 | 0.27 | 1.57 |
| Step 3 | |||
| Intercept | −2.50 | 1.53 | — |
| Time | −0.19 | 0.11 | 0.83 |
| Years of education | 0.23 | 0.10 | 1.26* |
| Heavy smoking | −1.32 | 0.43 | 0.27** |
| Pretreatment BDI | −0.17 | 0.18 | 0.84 |
| Recurrent depression | −1.48 | 0.43 | 0.23*** |
| CBT-D treatment | −0.43 | 0.60 | 0.65 |
| CBT-D × Time | 0.12 | 0.14 | 1.12 |
| CBT-D × Years of Education | −0.20 | 0.12 | 0.82 |
| CBT-D × Heavy Smoking | 1.32 | 0.56 | 3.73* |
| CBT-D × Pretreatment BDI | −0.17 | 0.23 | 0.84 |
| CBT-D × Recurrent Depression | 1.29 | 0.57 | 3.62* |
Note. BDI = Beck Depression Inventory; CBT-D = standard, cognitive–behavioral smoking cessation treatment plus cognitive–behavioral treatment for depression.
Coded 0 for 12 years of education.
Coded 0 for light smoking (<25 cigarettes per day at baseline), 1 for heavy smoking (≥25 cigarettes per day at baseline).
Square-root transformed BDI Total score.
Coded 0 for nonrecurrent depression, 1 for recurrent depression.
Coded 0 for standard, cognitive–behavioral smoking cessation treatment, 1 for CBT-D.
p < .05.
p < .01.
p < .001.
In the final step of the GEE analysis, a set of variables carrying the interactions between treatment condition and the five covariates was added to the equation to test whether treatment moderated the effect of the covariates on outcome. This model is presented in the bottom of Table 1. A significant interaction was found between treatment and heavy smoking (p = .02) and between treatment and recurrent depression(p = .02). Heavy smokers and smokers with recurrent depression were more likely to abstain if they received CBT-D. For participants in the ST condition, being a heavy smoker (p = .002) and having a history of recurrent depression (p = .001) were associated with a lower likelihood of abstinence, whereas these variables were not associated with outcome among CBT-D participants (ps > .50). Controlling for education, heavy smoking, and depressive symptoms at Session 1, participants with recurrent depression were 2.30 (95% confidence interval = 1.05–5.03) times more likely to abstain across follow-ups if they received CBT-D rather than ST. Controlling for education, recurrent depression, and depressive symptoms at Session 1, heavy smoking participants were 2.62 (95% confidence interval = 1.18–5.83) times more likely to abstain across follow-ups if they received CBT-D rather than ST. Interactions between treatment and the other covariates were nonsignificant.
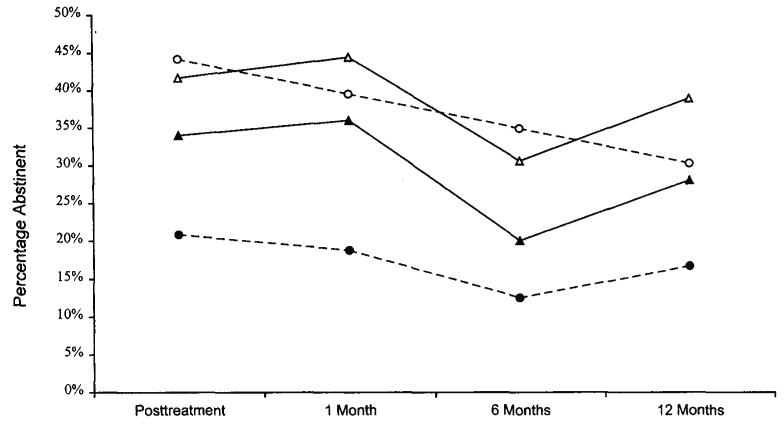
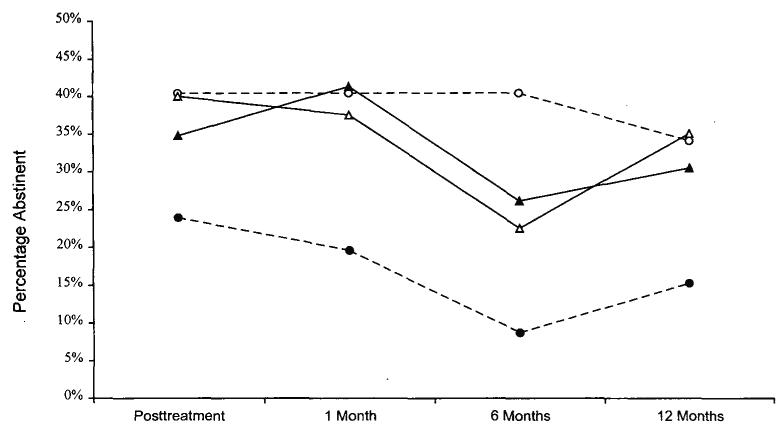
Figure 1 shows the abstinence rates across time for smokers with and without recurrent depression in both treatment conditions. Figure 2 illustrates abstinence rates across time for light (<25 cigarettes per day) and heavy (≥25 cigarettes per day) smokers by condition.
Figure 1.
Observed point prevalence abstinence rates by treatment condition for smokers with and without a history of recurrent major depressive disorder. Valid data on number of depressive episodes were not available for 2 participants. Open circles = standard, cognitive–behavioral smoking cessation treatment (ST-condition) with nonrecurrent depression (n = 43); filled circles = ST-condition with recurrent depression (n = 48); open triangles = ST-condition plus cognitive–behavioral treatment for depression (CBT-D condition) with nonre-current depression (n = 36); filled triangles = CBT-D condition with recurrent depression (n = 50).
Figure 2.
Observed point prevalence abstinence rates by treatment condition for light (<25 cigarettes per day) and heavy (≥25 cigarettes per day) smokers. Open circles = standard, cognitive–behavioral smoking cessation treatment (ST-condition) for light smokers (n = 47); filled circles = ST-condition for heavy smokers (n = 46); open triangles = ST-condition plus cognitive–behavioral treatment for depression (CBT-D condition) for light smokers (n = 40); filled triangles = CBT-D condition for heavy smokers (n = 46).
Differences In Depressive Symptoms and Mood Between Treatments
Prior to quit date
We hypothesized that the CBT-D condition would evidence a greater decrease (or less of an increase) in depressive symptoms and negative mood than the ST condition before quit date. Depressive symptoms were measured with the BDI, and negative mood was measured with the POMS. Before quit date, the BDI was administered at Sessions 1 and 3. The POMS was administered at Sessions 1, 2, 3, and 4. We used a repeated measures analysis of variance to analyze the BDI scores. The BDI scores were square-root transformed to account for positive skewness. Because POMS scores were assessed at each of the four sessions before quit date, a longitudinal random effects model for continuous responses was used (Laird & Ware, 1982). This model accounts for correlations between outcomes measured on the same person and also allows regression parameters to be estimated even though assessments may be missing for some participants. Valid inference depends on the assumption that data are missing for reasons unrelated to the unobserved outcome (the missing-at-random assumption; Little & Rubin, 1987). Because all participants were assessed regardless of compliance, we believe this assumption to be reasonable.
Prior to quit date, the model estimates on BDI scores showed no significant effect of treatment, time, or treatment by time interaction. The POMS scores showed strong evidence of a linear increase prior to quit date (B = 0.036, SE = 0.008, p = .0001). However, the main effect of treatment was nonsignificant as was the interaction between treatment and time. Because the analyses of smoking outcomes revealed significant interactions between treatment and both recurrent depression and heavy smoking, we also examined whether comparable interaction effects were present on measures of depressive symptoms and mood. Results indicated that these interactions were nonsignificant.
After quit date
We hypothesized that the CBT-D condition would show less of an increase in depressive symptoms and negative mood than the ST condition after quitting. The BDI was administered at quit date (Session 5), 1 week after quit date (Session 7), and 2 weeks after quit date (Session 8). The POMS was administered at quit date, 3 days after quit date (Session 6), 1 week after quit date, and 2 weeks after quit date. We analyzed BDI and POMS scores taken during Sessions 5 through 8 using the random effects model for continuous responses (Laird & Ware, 1982). In this analysis, we adjusted for baseline score (Session 1) and then made treatment condition comparisons stratified by smoking status. Abstinence was defined as no smoking in the 2-week period from quit date to the end of treatment. Smoking was defined as any smoking (even a puff of a cigarette) during this same 2-week period.
After quit date, we found a significant decrease in BDI scores among nonsmokers (B = −0.29, SE = 0.09, p = .0001). However, the magnitude of this change from quit date to 2 weeks after quit date was not significantly different between the treatment conditions, and there was no main effect of treatment. Among smokers, there was no evidence of either increases or decreases in BDI scores in either treatment condition, and no evidence of a between-groups difference. The POMS scores after quitting showed evidence of a linear decrease for nonsmokers (B = −0.07, SE = 0.017, p = .0002). However, the effect of treatment condition and the treatment by time interaction were nonsignificant. Among smokers, POMS scores also showed a significant decrease with time (B = −0.04, SE = 0.012, p = .0008) with no significant effects of treatment or treatment by time interaction. Follow-up analyses indicated that treatment did not interact significantly with recurrent depression or heavy smoking in predicting changes in mood and depressive symptoms after quit date. Also, depressed mood and depressive symptoms did not change (decrease) differentially between nonsmokers with single-episode versus recurrent past MDD.
Posttreatment Evaluation Measures
For participant ratings of the effectiveness of skills taught during treatment, we compared between-groups differences in the mean rating for the smoking cessation skills and within-group differences (in the CBT-D condition) for the mean rating for the smoking cessation skills versus the depression coping skills. There were no between-groups differences in the mean effectiveness rating of smoking cessation skills (p = .24). However, within the CBT-D condition, the mean effectiveness rating for the smoking cessation skills (M = 3.62, SD = 0.70) was greater than for the coping with depression skills (M = 3.02, SD = 0.99), t(66) = 6.12, p < .0001. Interestingly, however, participants in the CBT-D condition (M = 3.4, SD = 0.96) indicated that the overall program helped them make positive changes in their life not related to smoking more than did participants in the ST condition (M = 3.0, SD = 1.22), t(137) = 2.18, p = .03.
On the anonymous posttreatment questionnaire, t tests were used to determine whether treatment evaluations differed by treatment condition. On average, CBT-D participants rated the overall benefit received from the treatment program (M = 4.4, SD = 0.86) as greater than did the ST participants (M = 4.0, SD = 1.03), t(147) = 2.37, p = .02. CBT-D participants also rated the specific skills learned in the program as more useful in helping them quit smoking (M = 4.0, SD = 0.93) than did ST participants (M = 3.7, SD = 0.99), t(147) = 2.10, p = .04.
Discussion
Results of this study generally favored smoking cessation treatment that incorporated CBT-D over standard treatment alone, although the main effect of treatment did not attain traditional statistical significance (p = .095). Notably, both nonpharmacological treatments investigated in this study attained relatively high abstinence rates (ST, 24.7%; CBT-D, 32.5%, at 1 year) in smokers with past MDD, a population presumed to be at increased risk of failure. A unique characteristic of the present study was that the sample consisted exclusively of smokers with past MDD. This sample afforded sufficient statistical power to examine factors that impact outcome in this population and that might interact with treatment condition. In these secondary analyses, we found significant interactions between treatment condition and both recurrent MDD and heavy smoking at baseline. Smokers with recurrent MDD and heavy smokers who received CBT-D had significantly higher abstinence rates than those receiving ST. These findings suggest specific benefits of CBT-D independent of treatment contact intensity.
The hypothesis that CBT-D would produce superior outcomes to ST in a sample selected for positive history of MDD was predicated on the assumption that smokers with past MDD fare poorly in standard treatment. However, the ST condition in the present study yielded relatively high abstinence rates, resulting in a particularly stringent test of CBT-D. The lack of a significant main effect of CBT-D may also have been due to MDD history, in itself, being an insufficiently robust indicator of poor prognosis. Indeed, findings on the association between MDD history and poor smoking cessation outcome have been mixed: no association (Ginsberg et al., 1995; Hall et al., 1994), association only among women (Hall et al., 1998), and positive association (Glassman et al., 1988, 1993). MDD history as a dichotomous variable may have poor predictive power because it does not differentiate smokers with a single episode versus multiple episodes of past MDD. There may be important differences between these groups. Covey, Glassman, and colleagues (Covey, Glassman, & Stetner, 1997) found that the negative effect of MDD history on smoking outcome was primarily attributable to smokers with recurrent MDD rather than single-episode MDD. Extrapolating from the data presented in the text, the rates of abstinence in the Covey et al. (1997) study were 29.3%, 31.3%, and 13.4%, respectively, among smokers with no history of MDD, one prior episode of MDD, and multiple episodes of MDD. These data are consistent with our finding that recurrent MDD predicts poor cessation outcome in standard treatment, whereas single-episode MDD does not.
Samples of smokers selected for past MDD include many participants with only one past episode, who may not be at increased risk for poor outcome and are unlikely to benefit from the addition of CBT-D. Studies by Hall and colleagues (Hall et al., 1994, 1996, 1998), investigating a similar mood management treatment, have not differentiated between single-episode and recurrent MDD when evaluating the effects of treatment. This may account for their lack of a treatment effect with past MDD smokers in the one study that equated for contact time (Hall et al., 1996). In a similar vein, the exclusion of smokers with current MDD in the present study and in the Hall studies may have contributed to the lack of effects of CBT-D, because one might expect currently depressed smokers to receive particular benefit from CBT-D. Future studies on enhancing treatments for poor-prognosis smokers should be careful to use robust prognostic indicators when selecting target populations.
The suggestion that smoking treatment enhanced with CBT-D will only improve outcomes for smokers with an especially poor prognosis mirrors recent discussions on the treatment of major depression with “combined” therapies. Generally, studies whose participants have been outpatients with mild to moderate depression, who tend to have high rates of response in any treatment condition, have not demonstrated an advantage of combined pharmacotherapy and psychotherapy over monotherapy (Miller & Keitner, 1996). However, studies whose participants have been inpatients with more severe depression have demonstrated significant advantages of treatments that combine pharmacotherapy, individual psychotherapy, and/or family therapy (Miller & Keitner, 1996). The CBT-D condition in the present study represents a type of combined therapy that offers both behavioral smoking cessation and cognitive–behavioral mood management interventions. As in the treatment of major depression, a combined smoking cessation treatment may offer advantages primarily to those smokers who are high on characteristics that predict poor treatment outcome.
Although recurrent depression history predicted poor outcome in the ST condition, the mechanisms underlying this effect are unclear. It also is unclear how CBT-D benefited smokers with recurrent depression. The poor outcomes of smokers with recurrent depression in ST became evident early in the treatment process, as only 21% of these participants were abstinent during the 2nd week after quitting. We expected that CBT-D would reduce negative mood increases during these first weeks of quitting and thereby reduce relapse. However, changes in depressive symptoms and negative mood states after quitting did not differ by treatment condition. Also, contrary to the findings of Hall and colleagues (Ginsberg et al., 1995; Hall et al., 1994), for most participants, current depressive symptoms and negative mood either remained the same or decreased in the 2 weeks after quit date. These changes in depressive symptoms and negative mood did not differ between participants with single versus recurrent episodes of past MDD. Thus, it appears that the affective processes that were measured did not account for differences in outcome by treatment condition and recurrent depression. Future research should examine other possible mechanisms whereby smokers with past recurrent MDD might benefit from CBT-D.
Although CBT-D significantly reduced the risk of poor outcome associated with recurrent depression, it did not reduce the risk associated with depressive symptoms prior to treatment. Elevated pretreatment depressive symptoms, in general, may lead to poor engagement in any type of behavioral program. Indeed, in an earlier analysis of treatment attrition in the present sample, we found that pretreatment depressive symptoms were especially high in participants who dropped out of treatment prior to quit date (Curtin, Brown, & Sales, 2000). Although reducing elevated depressive symptoms might result in improved treatment retention and outcome, CBT-D was ineffective in this regard. Contrary to expectation, in the 4 weeks prior to quit date, depressive symptoms did not change significantly in the CBT-D condition. Similarly, in the 2 weeks after quit date, CBT-D did not result in improved depressive symptoms relative to ST. Interestingly, no relative advantage in regard to depressive symptoms (or negative mood) was found, even in the two subgroups that showed improved long-term abstinence, smokers with recurrent depression and heavy smokers. Treating depressive symptoms while concurrently initiating nicotine fading in anticipation of a quit attempt may not be optimal or even feasible. In the CBT-D treatment, only half of the eight 2-hr sessions were devoted to teaching depression coping skills. Apparently this was not sufficient to overcome the pharmacological (e.g., nicotine withdrawal due to fading) and psychological factors that contributed to increases in negative mood prior to quit date. In light of the fact that the “typical” course of cognitive–behavioral treatment for major depression is 16–20 sessions (Sacco & Beck, 1985), a more intensive depression intervention may be needed in its application for smoking cessation treatment.
An unexpected finding was that CBT-D produced better outcomes than ST in heavy smokers. A few explanations of this effect seem possible. First, heavier smokers may be more likely to smoke for self-medication purposes. A recent study found that smoking rate is positively associated with smoking for stimulation and smoking for negative affect reduction (Lerman et al., 1998). CBT-D teaches a broader variety of coping skills than ST and may have been more effective in providing heavier smokers with alternatives to self-medication smoking. Overall, participants did rate the skills taught in CBT-D as more useful in helping them quit. Heavier smokers also may be more physically dependent on nicotine and therefore may depend more heavily on cigarettes for positive reinforcement and be more prone to reward deprivation when quitting. CBT-D, with its emphasis on increasing pleasant events and activities, may help heavier smokers make use of other sources of positive reinforcement. Consistent with this hypothesis, participants in CBT-D, compared with those in ST, reported that they received more overall benefit from treatment and that treatment was more helpful in fostering positive changes in life areas not related to smoking.
Methodological limitations of the present study must be considered when interpreting results. First, the treatment sample was restricted to smokers with a history of MDD. This restricted sample allowed us to conduct a detailed exploration of the factors affecting outcome in this population, but it also prevented us from making comparisons to smokers without a history of MDD. Large-scale treatment outcome studies that include substantial numbers of smokers with no history, one prior episode, and more than one episode of MDD will be able to document more fully the effects of lifetime MDD, both single episode and recurrent, on smoking cessation. Studies targeting smoker subgroups at greatest risk, such as smokers with current MDD, may also be informative. Second, it should be noted that the hypothesized main effect in this study approached, but did not attain, traditional statistical significance. This lack of main effect may have been due, in part, to the high rates of abstinence achieved in the ST condition in this study. Significant effects of treatment emerged only during secondary analyses of treatment response in patient subgroups. Therefore, results should be interpreted with appropriate caution pending replication.
Nonetheless, this study has potentially important implications. Results suggest that CBT-D provides specific benefits for some, but not all, smokers with a history of MDD. Further research is needed to determine which smokers benefit most from CBT-D and what elements of the treatment account for its efficacy. Different elements of CBT-D (e.g., pleasant event scheduling, cognitive restructuring, assertiveness) may be useful for different risk factors. CBT-D includes interventions designed both to decrease negative mood and to increase positive mood. Research that examines the role of both positive and negative mood in CBT-D may further elucidate its mechanisms of action. Future studies that evaluate the efficacy of CBT-D with specific subgroups of smokers and that more clearly specify the risk profiles that predict poor response to standard cessation programs should enhance our ability to select the interventions most likely to benefit individual smokers who enter treatment.
Footnotes
This study was partially supported by Grant DA08511 from the National Institute on Drug Abuse and Grant PBR-74 from the American Cancer Society. We wish to thank Peter M. Lewinsohn for his helpful comments on an earlier version of this article. We gratefully acknowledge the research assistance of Michelle Ricci, Jessica Whiteley, Virginia Smith, and Amber Novak. Thanks also to Matt Evans, Kevin Everett, Wilson McDermut, Thane Dykstra, Sheri Johnson, Mark Myers, Eric Wagner, Jon Kassel, Janet Levenson, and Arthur Kaye, who served as therapists in the study.
Although we did not hypothesize effects of substance use disorder history on outcome, we did examine these variables because of their high prevalence. Results indicated that neither alcohol nor drug use disorder predicted outcome, nor did either variable interact significantly with treatment (ps > .40). Therefore, these variables were not included in the main analyses.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed. Author; Washington, DC: 1987. [Google Scholar]
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking: A national perspective. Journal of the American Medical Association. 1990;264:1541–1545. [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. Guilford Press; New York: 1978. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bliss RE, Garvey AJ, Heinold JW, Hitchcock JL. The influence of situation and coping on relapse crisis outcomes after smoking cessation. Journal of Consulting and Clinical Psychology. 1989;57:443–449. doi: 10.1037//0022-006x.57.3.443. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Archives of General Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM. Coping with depression: Course workbook. Castalia Press; Eugene, OR: 1984a. [Google Scholar]
- Brown RA, Lewinsohn PM. A psychoeducational approach to the treatment of depression: Comparison of group, individual, and minimal contact procedures. Journal of Consulting and Clinical Psychology. 1984b;52:774–783. doi: 10.1037//0022-006x.52.5.774. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lichtenstein E, McIntyre KO, Harrington-Kostur J. Effects of nicotine fading and relapse prevention on smoking cessation. Journal of Consulting & Clinical Psychology. 1984;52:307–308. doi: 10.1037//0022-006x.52.2.307. [DOI] [PubMed] [Google Scholar]
- Burns DD. Feeling good. Morrow; New York: 1980. [Google Scholar]
- Cohen S, Schwartz JE, Bromet EJ, Parkinson DK. Mental health, stress, and poor health behaviors in two community samples. Preventive Medicine. 1991;20:306–315. doi: 10.1016/0091-7435(91)90029-4. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Comprehensive Psychiatry. 1990;31:350–354. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. American Journal of Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- Covey LS, Hughes DC, Glassman AH, Blazer DG, George LK. Ever-smoking, quitting, and psychiatric disorders: Evidence from the Durham, North Carolina, epidemiologic catchment area. Tobacco Control. 1994;3:222–227. [Google Scholar]
- Cummings SR, Richard RJ. Optimum cutoff points for biochemical validation of smoking status. American Journal of Public Health. 1988;78:574–575. doi: 10.2105/ajph.78.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin L, Brown RA, Sales SD. Determinants of attrition from cessation treatment in smokers with a history of major depressive disorder. Psychology of Addictive Behaviors. 2000;14:134–142. doi: 10.1037//0893-164x.14.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A, Harper RA. A guide to rational living. Wilshire; Hollywood, CA: 1961. [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Foxx RM, Brown RA. Nicotine fading and self-monitoring for cigarette abstinence or controlled smoking. Journal of Applied Behavior Analysis. 1979;12:111–125. doi: 10.1901/jaba.1979.12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D, Hall SM, Reus VI, Muñoz RF. Mood and depression diagnosis in smoking cessation. Experimental and Clinical Psychopharmacology. 1995;3:389–395. [Google Scholar]
- Glassman AH, Covey LS, Dalack GW, Stetner F, Rivelli SK, Fleiss JF, Cooper TB. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clinical Pharmacology and Therapeutics. 1993;54:670–679. doi: 10.1038/clpt.1993.205. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottier LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. Journal of the American Medical Association. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Glassman AH, Stetner F, Walsh BT, Raizman PS, Fleiss JL, Cooper TB, Covey LS. Heavy smokers, smoking cessation, and clonidine. Journal of the American Medical Association. 1988;259:2863–2866. [PubMed] [Google Scholar]
- Hall SM, Muñnoz RF, Reus VI. Cognitive–behavioral intervention increases abstinence rates for depressive-history smokers. Journal of Consulting and Clinical Psychology. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, Hartz DT. Mood management and nicotine gum in smoking treatment: A therapeutic contact and placebo-controlled study. Journal of Consulting and Clinical Psychology. 1996;64:1003–1009. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Muñoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E. Nortriptyline and cognitive–behavioral therapy in the treatment of cigarette smoking. Archives of General Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Health AC, Eaves LJ, Kessler RC. Smoking and major depression: A causal analysis. Archives of General Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The National Comorbidity Survey of the United States. International Review of Psychiatry. 1994;6:365–376. [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: Characteristics of depressed smokers and effects of nicotine dependence. Journal of Consulting and Clinical Psychology. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lerman C, Caporaso N, Main D, Audrain J, Boyd NR, Bowman ED, Shields PG. Depression and self-medication with nicotine: The modifying influence of the dopamine D4 receptor gene. Health Psychology. 1998;17:56–62. doi: 10.1037//0278-6133.17.1.56. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Antonuccio DO, Breckenridge JS, Teri L. The Coping With Depression Course: A psychoeducational intervention for unipolar depression. Castalia Press; Eugene, OR: 1984. [Google Scholar]
- Lewinsohn PM, Graf M. Pleasant activities and depression. Journal of Consulting and Clinical Psychology. 1973;41:261–268. doi: 10.1037/h0035142. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Muñoz RF, Youngren MA, Zeiss AM. Control your depression. Prentice-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrica. 1986;73:13–22. [Google Scholar]
- Little RJ, Rubin DB. Statistical analysis with missing data. Wiley; New York: 1987. [Google Scholar]
- Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral medicine: Changing health lifestyles. Brunner/Mazel; New York: 1980. pp. 410–452. [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention. Guilford Press; New York: 1985. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EITS manual for the Profile of Mood States. Educational and Industrial Testing Service; Sao Diego, CA: 1971. [Google Scholar]
- Miller IW, Keitner GI. Combined medication and psychotherapy in the treatment of chronic mood disorders. Psychiatric Clinics of North America. 1996;19:151–171. doi: 10.1016/s0193-953x(05)70279-3. [DOI] [PubMed] [Google Scholar]
- Sacco WP, Beck AT. Cognitive therapy of depression. In: Beckham EE, Leber WR, editors. Handbook of depression: Treatment, assessment and research. Dorsey Press; Homewood, IL: 1985. pp. 3–38. [Google Scholar]
- SAS Institute . SAS/STAT software: Changes and enhancements through Release 6.12. Author; Gary, NC: 1997. [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured clinical interview for DSM-III-R—Non-patient edition (SCID-NP, Version 1.0) American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- Wei I. Application of an urn model to the design of sequential controlled clinical trials. Journal of the American Statistical Association. 1978;73:559–563. [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychological Medicine. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]