Abstract
Oxidative stress has been implicated in the pathogenesis of several neurodegenerative disorders including primary open angle glaucoma (POAG) an optic neuropathy characterized by loss of retinal ganglion cell (RGC) axons and remodeling of the optic nerve head (ONH). Previous findings in glaucomatous astrocytes suggested increased oxidative stress and lipid peroxidation in human optic nerves. We studied the dose and time dependent effects of 4-hydroxynonenal (HNE), a by-product of lipid peroxidation, on the viability of primary cultures of human ONH astrocyte. A significant depletion of glutathione (GSH) level was observed in normal astrocytes after exposure to HNE for 1h and 3h. Untreated glaucomatous astrocytes exhibited depleted levels of GSH which increased slightly after exposure to HNE. Both normal and glaucomatous astrocytes recovered GSH levels after 24h of removal of HNE. HNE caused significant increases in expression of antioxidant enzymes, glutamate cysteine ligase catalytic subunit (GCLC), aldoketo reductase 1C family member 1 (AKR1C1) and glutathione-S-transferase-α4 (GSTA4). HNE induced expression of the transcription factor Nrf2, which coordinates the upregulation of detoxification enzymes. In addition, ONH astrocytes responded to HNE by activation and transcription of cFOS and NFkB, which regulate physiological protective responses against oxidative stress. Our results indicate that ONH astrocytes exhibit a strong antioxidant response to HNE treatment by inducing the transcription factors cFOS, NFkB, and Nrf2, which upregulate the expression of GCLC, to produce more GSH in the cell. AKR1C1 was also upregulated after HNE treatment to inactivate HNE, independent of GSH availability in the cells. Collectively these data indicate that ONH astrocytes can efficiently counteract the neurotoxic effects of HNE offering protection in the optic nerve by releasing GSH and antioxidant enzymes to eliminate the products of chronic oxidative stress.
Keywords: optic nerve, astrocytes, HNE, lipid peroxidation, oxidative stress
Introduction
Oxidative stress is believed to play an important role in neuronal cell death associated with many neurodegenerative diseases such as Alzheimer’s Disease (AD), amyotrophic lateral sclerosis (ALS), and Parkinson’s Disease (PD) (Keller and Mattson, 1998). Oxidative DNA damage has been found in the trabecular meshwork of the eye in patients with primary open angle glaucoma (POAG) (Izzotti et al., 2003) and may contribute to the pathogenesis of POAG (Sacca et al., 2005). POAG is an optic neuropathy that can lead to blindness and is characterized by loss of retinal ganglion cell (RGC) axons and cupping of the optic disk. This disease affects an estimated 67 million people worldwide (Quigley and Broman, 2006; Friedman et al., 2004). In human glaucoma and experimental models of glaucoma, irreversible damage of the axons occurs at the level of the lamina cribrosa in the optic nerve head (ONH), anterior to the RGCs of the optic nerve (Hernandez and Pena, 1997; Quigley, 1999).
Astrocytes are the most abundant glial cell type in the adult CNS. Normally, astrocytes provide metabolic and structural support to neurons and participate in the maintenance and detoxification of the extracellular space of the CNS. In neurodegenerative diseases or following CNS injury, quiescent astrocytes acquire a reactive phenotype and produce many enzymes, proteins, cytokines, and free radicals that are not produced under normal conditions (Hernandez et al., 2000; Ridet et al., 1997; Theodosis and Poulain, 1999). Previous studies from our laboratory reported that several genes related to lipid synthesis and metabolism, steroid metabolism and glutathione (GSH) metabolism were upregulated in ONH astrocytes cultured from patients with POAG (Hernandez et al., 2002). Glaucomatous ONH astrocytes express high levels of 20α (3α)-dihydrodiol dehydrogenase mRNA (AKR1C1 or DDH), a steroid metabolizing enzyme that can inactivate products of lipid peroxidation (Hernandez et al., 2002; Agapova et al., 2003). In addition, expression of several members of the alcohol and aldehyde dehydrogenase families were also upregulated in glaucomatous ONH astrocytes suggesting that these cells have multiple enzymatic pathways to inactivate products of lipid peroxidation. These findings led to the present study of oxidative stress in ONH astrocytes.
During oxidative stress, reactive oxygen species (ROS) are generated which can induce peroxidation of lipids (either cellular membrane lipids or circulating lipoprotein molecules) and generate highly reactive aldehydes (Esterbauer et al., 1991). The brain exhibits high oxygen consumption and is enriched with polyunsaturated fatty acids, so it is quite vulnerable to lipid peroxidation (Neely et al., 1999). Aldehydes are more stable than ROS, so they can diffuse within or escape from the cell and attack targets far from the site of origin (Uchida, 2003). One of the most important and toxic products of lipid peroxidation is the highly reactive aldehyde 4-hydroxy-2E-nonenal (HNE) (Uchida and Stadtman, 1992).
HNE is neurotoxic and present at increased concentrations in brain tissue and cerebrospinal fluid of AD patients, in spinal cord of ALS patients, and is found in Lewy bodies in brain stem neurons in PD patients (Zarkovic, 2003). There are significantly higher plasma levels of another aldehyde by-product of lipid peroxidation, malondialdehyde, in POAG patients compared to controls (Yildirim et al., 2005). HNE detoxification primarily occurs via conjugation to GSH, which can be catalyzed by glutathione S-transferases (GSTs). Izzotti et al (2003) discovered that lack of the GSTM1 gene predisposes patients to have increased oxidative DNA damage and to have enhanced prevalence of POAG. Alternative ways of inactivating HNE also includes reduction by aldoketoreductases such as AKR1C1 and alcohol dehydrogenases, or oxidation by aldehyde dehydrogenases and carbonyl reductases (Esterbauer et al., 1991).
The purpose of this study is to determine the effects of oxidative stress generated by HNE in human ONH astrocytes. We have found that ONH astrocytes are able to efficiently protect themselves, and possibly neurons, from oxidative stress by way of several mechanisms following treatment with HNE. First, normal ONH astrocytes conjugate HNE to GSH, which depletes the cell of GSH while simultaneously inactivating HNE. After allowing a recovery, astrocytes are able to rebound and produce great amounts of GSH above basal levels. Also, interestingly, our data indicates that glaucomatous ONH astrocytes are able to handle HNE treatment according to GSH levels after recovery. Second, HNE induces the upregulation of alternative antioxidant enzymes including AKR1C1, which can reduce HNE to the inactive 1, 4-dihydroxy-2-nonene (DHN) (Burczynski et al., 2001), and glutamate cysteine ligase catalytic subunit (GCLC), which catalyzes the first step in de novo synthesis of GSH (Forman et al., 2003). Finally, HNE upregulates the transcription factors cFOS, nuclear transcription factor kappaB (NFkB), and nuclear factor erythroid 2-related factor 2 (Nrf2), which are involved in the transcriptional activation of detoxification enzymes and antioxidant proteins.
Materials and Methods
Materials
Cell proliferation/viability assay kit was purchased from Chemicon International (Temecula, CA). HNE and GSH Assay Kit were purchased from Cayman Chemical (Ann Arbor, MI). Rabbit polyclonal anti-HNE-Michael adduct antibody was purchased from Calbiochem (San Diego, CA). Rabbit polyclonal cFOS and rabbit polyclonal NFkB antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal Nrf2 antibody was purchased from Stressgen Bioreagents (Victoria, BC Canada).
Astrocyte cell cultures
Normal human eyes from three male donors (ages: 19, 29, and 65) and glaucomatous human eyes from three male donors (ages: 61, 70, and 83) were obtained from the National Disease Research Interchange (NDRI) and from Mid-America Eye Bank (St. Louis, MO) within 24 h of death. ONHs were dissected and processed to generate ONH (type 1B) astrocyte cultures. Primary cultures of human ONH astrocytes were purified, characterized, and maintained as previously described (Kobayashi et al., 1997; Yang and Hernandez, 2003). ONH astrocytes were cultured at 37°C in Dulbecco’s Modified Eagle’s Medium (DMEM)/F-12 containing 5-10% fetal bovine serum (FBS) under a humidified atmosphere of 95% air and 5% CO2. All experiments used astrocytes at the fourth or fifth passages with 5% FBS DMEM/F-12.
Cell viability assay
Cells were grown to confluence in a 24-well plate. Cells were treated with varying concentrations of HNE (1-250 μM) for 1–24 h, and then each well was incubated with 50 μl of the WST-1/ECS solution for 3 h under a humidified atmosphere of 95% air and 5% CO2. The plate was put on a shaker for one minute and absorbance was measured at 430 nm using a spectrophotometer. Cell viability was calculated as a percentage of treated absorbance divided by control absorbance. This assay is based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases. Therefore, as cells die there is a decrease in overall activity of the mitochondrial dehydrogenases in the sample, which leads to a decrease in the amount of the formazan dye formed. Changes in the amount of formazan dye produced by viable cells are measured by the absorbance of the dye solution at 430 nm. All cultures and treatments were carried out in triplicate and student’s t-test was used to calculate statistical significance.
RNA Isolation
ONH astrocytes were treated for 1 h and 3 h with 25 μM HNE in 5% FBS DMEM/F-12. After HNE treatment, HNE-containing medium was removed and replaced with fresh 5% FBS DMEM/F-12 for 6 h, then cytoplasmic RNA was isolated from ONH astrocytes as previously described (Agapova et al., 2001). Briefly, following HNE treatment, total RNA from ONH astrocytes was isolated in TRIzol (Invitrogen Life Technologies; Carlsbad, CA). After isolation, RNA was treated with DNase I, precipitated, and resuspended in 10 μl nuclease-free water. RNA absorbance at 260 nm and the absorbance ratio at 260/280 nm were measured.
Real-time quantitative RT-PCR
First-strand cDNA was prepared from 1 μg of total RNA and RT-PCR was performed as described by Agapova et al. ( 2003). Primers were designed for real-time PCR against AKR1C1, alpha-B-crystallin, alcohol dehydrogenase class I alpha polypeptide, alcohol dehydrogenase class I beta polypeptide, aldehyde dehydrogenase 1 family member 3, cFOS, cJUN, GCLC, glutathione-S-transferase-alpha4 (GSTA4), glutathione S-transferase-theta2 (GSTT2), heat shock 27 kDa protein (HSP27), NFkB, Nrf2, and super oxide dismutase 2 (SOD2) using the Primer Express program (PE Applied Biosystems; Forster City, CA) (Table 1). Quantitative RT-PCR was preformed by monitoring in real time the increase in fluorescence of SYBR-Green using the MyiQ Single Color Real-Time PCR Detection System (Bio-Rad; Hercules, CA). Relative amounts of RNA for target genes were determined and normalized to relative amounts of reference gene RNA (18S). The mean and standard deviation of relative expression level of triplicate determinations were calculated for each target gene. Experiments were carried out in three cultures of normal ONH astrocytes. Data were analyzed using Student’s t-test and ANOVA, and significance was determined as P < 0.05.
Table 1.
| Gene Symbol | Accession Number | Primer | Sequences (from 5′ to 3′) | PCR products (bp) |
|---|---|---|---|---|
| AKR1C1 | NM_001353 | Forward
Reverse |
ACCAAATTGGCAATTGAAGCTGGC
TTCCATTTTCATCTTTTGGGATCAC |
295 |
| CRYAB | NM_001885 | Forward
Reverse |
GTCAACCTGGATGTGAAGCACTT
TTCATCCTGGCGCTCTTCAT |
102 |
| ADH1A | M12963 | Forward
Reverse |
ACAAGAAACCCATCCAGGAGGT
CAGGGAAGCCATCATGGTGT |
101 |
| ADH1B | X03350 | Forward | CGGCTTGACACCATGATGG | 91 |
| ALDH1A3 | U07919 | Forward
Reverse |
GGGCTCAGCCATGGAAGAC
CTGGCCCGAAAATCTCCTCT |
91 |
| cFOS | NM_005252 | Forward
Reverse |
CGGAGGAGGGAGCTGACTG
TTCTCCTTCAGCAGGTTGGC |
101 |
| cJUN | J04111 | Forward
Reverse |
CCCCAGCGTATCTATATGGAATTG
GCTGTCCCTCTCCACTGCAA |
91 |
| GCLC | M90656 | Forward
Reverse |
TTGAGGCCAACATGCGAAA
AGGACAGCCTAATCTGGGAAATG |
101 |
| GSTA4 | NM_001512 | Forward
Reverse |
GGATGGTAACCACCTGCTGTTC
GCTTGTCTGCTATGTAGTGGAGAATG |
101 |
| GSTT2 | NM_000854 | Forward
Reverse |
GGCAGCACAAGAGCAAGGAG
AGTCCTACCGGCTCGAAAG |
102 |
| HSP27 | NM_001540 | Forward
Reverse |
ACCGCTGGCGCGTGT
CGTGCTTGCCGGTGATCT |
96 |
| NFkB | L19067 | Forward
Reverse |
GCTCAGTGAGCCCATGGAAT
TGATGCTCTTGAAGGTCTCATATGTC |
101 |
| Nrf2 | NM_006164 | Forward
Reverse |
GCCCACATTCCCAAATCAGAT
CGTAGCCGAAGAAACCTCATTG |
102 |
| SOD2 | Y00985 | Forward
Reverse |
GGGCAGTGTGCGGCA
TCGTAGGGCAGGTCGGG |
91 |
| 18S | X03205 | Forward
Reverse |
TCTAGATAACCTCGGGCCGA
ACGGCGACTACCATCGAAAG |
91 |
Immunofluorescence staining
ONH astrocytes were treated with 25 μM HNE for 1 h and 3 h, and were immediately prepared for immunofluorescence staining. Coverslips were transferred to phosphate-buffered saline (PBS), and then fixed in 4% paraformaldehyde in PBS and processed for standard indirect immunofluorescence. Fixed coverslips were washed in PBS and then permeabilized with 0.1% Triton X-100 in distilled water. Coverslips were blocked with 10% donkey serum (Sigma) in 0.5% bovine serum albumin (BSA)/PBS for 30 min. Primary antibodies (cFOS 1:400 and NFkB 1:100) were diluted in the blocking mixture and incubated with the coverslips for 1 h. Then coverslips were washed in PBS and incubated with the appropriate secondary antibody [Alexa Fluor 488 goat anti-mouse IgG or Alexa Fluor 568 goat anti-rabbit IgG (Molecular Probes)] for 45 min. Finally, coverslips were washed with PBS and mounted on slides using Vectashield mounting medium with DAPI (Vector Laboratories; Burlingame, CA).
Quantitation of cFOS- and NFkB-labeled ONH astrocyte nuclei
Following HNE treatment, astrocytes were stained with a cFOS or NFkB antibody as described above and mounted with DAPI for nuclear staining. Quantitation of cFOS- and NFkB-labeled nuclei was carried out as previously described by Hashimoto et al. (Hashimoto et al., 2005). Briefly, images were photographed at 20x magnification for 15–20 random μm2 areas per coverslip and saved as .tif files. Total number of nuclei (DAPI) and cFOS or NFkB nuclei were counted using Optimas 6.2 image analysis software (Bothel, WA). Experiments were carried out in all three ONH astrocyte cultures and run in triplicate coverslips per condition. Data were expressed as percentage of the cFOS- or NFkB-positive nuclei divided by the total number of nuclei. Data were analyzed using Student’s t-test and significance was determined as P < 0.05.
Western blots
ONH astrocytes were treated for 1 h and 3 h with 25 μM HNE in 5% FBS DMEM/F-12. After HNE treatment, HNE-containing medium was removed and replaced with fresh 5% FBS DMEM/F-12 for 6 h prior to collecting protein. Western blot analysis was carried out as previously described by Salvador-Silva et al. ( 2001). Briefly, for protein extraction, ONH astrocytes were grown on 35 or 100 mm plates to ~95% confluence, washed twice in cold PBS and incubated for 15 min in 150 or 300 μl of ice-cold IP buffer [50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, .05% sodium azide, Roche protease inhibitors (Roche Molecular Biochemicals; Indianapolis, IN)]. Cells were then scraped with disposable cell lifters and centrifuged for 15 min at 4°C and 14,000 rpm. The supernatant was recovered and protein concentrations in cell lysates were determined by the Bio-Rad Protein Assay Kit (Bradford method). Cell lysates were stored at −80°C until further use. For nuclear proteins, cell pellets were washed twice with lysis buffer (20 mM HEPES, pH 7.0, 10 mM KCl, 2 mM MgCl2, 0.3% IGEPAL CA-630), and nuclear proteins were extracted at 4°C overnight in 50 μl lysis buffer with 0.5 M NaCl. Samples were run on 4–15% Tris-HCl SDS-PAGE and transferred to Bio-Rad nitrocellulose membranes. Membranes were blocked for 30 min in blocking solution [Tris buffered saline solution containing 0.2% Tween-20 (TBS-T) with 5% Amersham blocking agent (Amersham Pharmacia Biotech; Piscataway, NJ)] and incubated overnight with primary antibody diluted in TBS-T/2.5% blocking agent. The following primary antibodies were used: polyclonal anti-cFOS (1:500), polyclonal NFkB (1:500), polyclonal Nrf2 (1:1000). Between 5–20 μg protein were used per lane. Membranes were washed in TBS-T and then incubated with the appropriate secondary antibody conjugated to horseradish peroxidase for 1.5 h. For detection of membrane-bound primary antibodies, we used the enhanced chemiluminescence plus Western blotting detection system (Amersham Pharmacia). All cytoplasmic protein was normalized to actin and all nuclear protein was normalized to Histone H3 as loading controls and measured for significance (data not shown). Western blots were run in triplicate.
Glutathione (GSH) assay
Total GSH content was quantified in astrocyte cell lysates based on the method of Tietze (1969) using the Glutathione Assay Kit from Cayman Chemical as previously described by Hu et al. (2003). Normal or glaucomatous ONH astrocytes were grown to ~95% confluence in 100 mm dishes, and then treated for 1 h and 3 h with 25 μM HNE in 5% FBS DMEM/F-12. Immediately after HNE treatment, or after a 24 h recovery in 5% FBS DMEM/F-12, cells were collected in 1.0 mL sterile PBS using disposable cell lifters. Cells were centrifuged, supernatant was removed, and 100 μl of 50 mM phosphate buffer were added to the cell pellet and sonicated for 10 min. Samples were then centrifuged for 15 min at 4°C, the supernatant was collected, and 5 μl of the supernatant were used to measure the protein concentration using the Bio-Rad Protein Assay Kit (Bradford method). The remaining supernatant was deproteinated as described in the assay protocol. Standards and samples were diluted and aliquoted in a 96-well plate. Freshly prepared assay cocktail was added to each well, then the plate was incubated in the dark on an orbital shaker for 25 min. Absorbance was measured at 405 nm using a platereader. Total GSH content for each sample was calculated based on the standard curve, and results were expressed as total GSH content (μM) per μg protein. Statistical significance was calculated on three sets of determinations using Student’-t-test
Results
Effect of HNE on cell viability
To determine the appropriate concentration of HNE for use in further experiments, astrocytes were cultured and treated with increasing concentrations of HNE (1 μM –250 μM) for 1, 3, 6, and 24 h. Cell viability was measured using a cell proliferation assay kit as described in the methods. The optimal concentration of HNE was determined to be 25 μM for 1 h and 3 h. Higher concentrations and longer treatments with HNE resulted in drastic reductions in cell viability; 50 μM HNE resulted in 50% reduction of cell viability and 100–250 μM HNE was lethal to the cells within 6 h of treatment (Figure 1 A). Treatment with 25 μM HNE for 3 h only lead to a 10% reduction in cell viability, however, after 6 h 25 μM HNE treatment there was a 70% reduction in cell viability (Figure 1 B). The percentage of viable cells for 1 h, 3 h, 6 h, and 24 h treatment with 25 μM HNE were 100%, 90%, 32%, and 7%, respectively.
Figure 1. A. Cell viability assay.
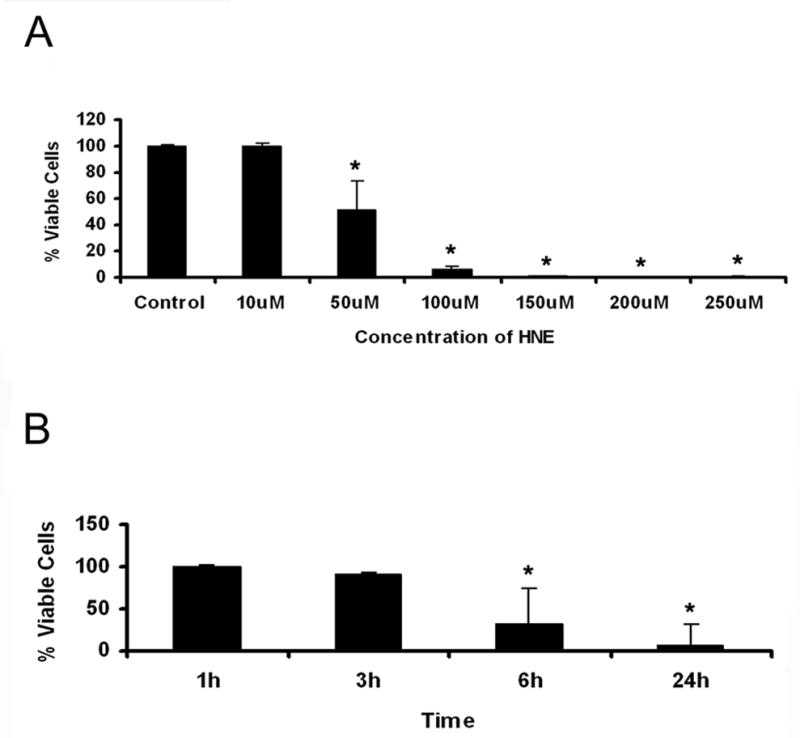
Normal ONH astrocytes were treated with varying concentrations of HNE for 6 h. Treatment with HNE at concentrations of 50 μM and higher led to a greater than 50% reduction in cell viability over 6 h. The data is expressed as % of viable cells of the untreated control. Asterisks indicate significant difference (*P<0.05).
B. Time course of HNE (25 μM) treatment. Normal ONH astrocytes were treated with 25 μM HNE for different exposure times. Treatment with 25 μM HNE for 3 h led to a 10% reduction in cell viability and was used in future experiments. Asterisks indicate significant difference (*P<0.05).
Effect of HNE on Glutathione (GSH) levels
To determine the effect of HNE treatment on cellular GSH content, ONH astrocytes were grown to ~95% confluence, treated for 1 h and 3 h with 25 μM HNE and cell lysates were collected. Normal ONH astrocytes have high levels of GSH that become depleted after 25 μM HNE treatment for 1h. After 3h the levels of GSH are undetectable (Fig. 2A).
Figure 2. A. Effect of HNE on Glutathione (GSH) content of normal astrocytes.
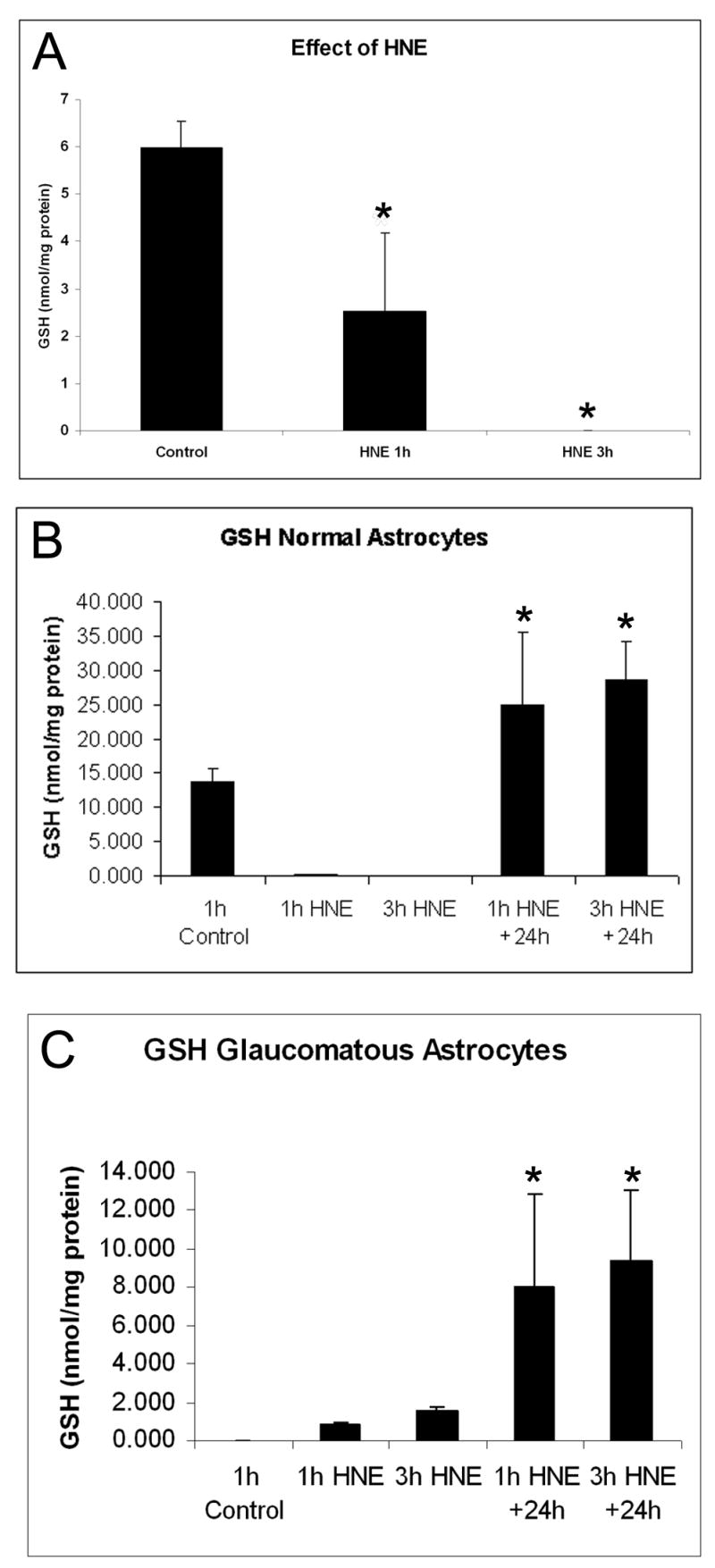
Normal ONH astrocytes from a 29 year old donor were treated with 25 μM HNE for 1 h and 3 H. In normal ONH astrocytes levels of GSH become depleted after 25 μM HNE treatment. Asterisks indicate significant difference (*P<0.05).
B. Glutathione (GSH) content of normal astrocytes. Normal ONH astrocytes from a 65 year old donor were treated with 25 μM HNE for 1 h and 3 h, and GSH content was measured immediately after treatment, as well as after a 24 h recovery without HNE-containing medium following each treatment duration. Levels of GSH become depleted after 25 μM HNE treatment for 1h and 3h in normal astrocytes. After a 24 h recovery from HNE treatment, the levels of GSH are over 2-fold higher than basal levels. Asterisks indicate significant difference (*P<0.05) from control and from treatment with HNE.
C. Glutathione (GSH) content of glaucomatous astrocytes. Glaucomatous ONH astrocytes from a 70 year old donor were treated with 25 μM HNE for 1 h and 3 h, and GSH content was measured immediately after treatment, as well as after a 24 h recovery without HNE-containing medium following each treatment duration. Glaucomatous astrocytes exhibit basal levels of GSH below the level of detection compared to normal astrocytes, which increase slightly after 25 μM HNE treatment for 1h and 3h. After a 24 h recovery from HNE treatment, the levels of GSH are higher. Asterisks indicate significant difference (*P<0.05) from control and from treatment with HNE.
To determine whether the effects of HNE were reversible, age-matched normal and glaucomatous ONH astrocytes were treated with 25 μM HNE for 1 h and 3 h and had the HNE-containing media removed and replaced with fresh 5% FBS DMEM/F-12, allowed to recover for 24h. In normal OHN astrocytes, the levels of GSH are over 2-fold higher than basal level after a 24 h recovery from the initial HNE treatment for 1h or 3h (Fig. 2 B). These results indicate that ONH astrocytes fully recover from oxidative stress induced by HNE.
The GSH concentration in untreated glaucomatous ONH astrocytes was undetectable, after exposure to HNE for 1h and 3 h GSH levels increased by 40% and 78% respectively (P<0.05). After 24h recovery levels of GSH were markedly increased by ~ 400% in glaucomatous ONH astrocytes (Fig 2 C). Similar results were obtained from three astrocyte cultures from glaucomatous donors. These results demonstrate the ability of glaucomatous ONH astrocytes to recover from oxidative stress as well as normal astrocytes.
Effect of HNE on expression of antioxidant enzymes
To determine the effect of HNE on mRNA, astrocytes were grown to ~95% confluence, treated for 1 h and 3 h with 25 μM HNE, and then the HNE-containing media was removed and replaced with fresh media. RNA was isolated 6 h later and prepared for RT-PCR as described above. According to recent microarray data, AKR1C1 upregulation in glaucomatous astrocytes (Hernandez et al., 2002) suggested that these cells reduce HNE into the non-toxic 1, 4-dihydroxy-2-nonene (DHN). Here we determined whether HNE induced upregulation of AKR1C1 mRNA by quantitative RT-PCR. The relative expression of AKR1C1 mRNA is normalized to 1.00 in controls, and is 5.56 times higher after 1 h HNE treatment, and 2.77 times higher after 3 h HNE treatment, when compared to controls. The relative expression of AKR1C1 mRNA increased by a large amount after 1 h HNE treatment (~5.6 fold change) and increased to a lesser degree versus controls after 3 h HNE treatment (~2.8 fold change) (Figure 3 A).
Figure 3. Effects of exposure to HNE in gene expression of antioxidant enzymes.
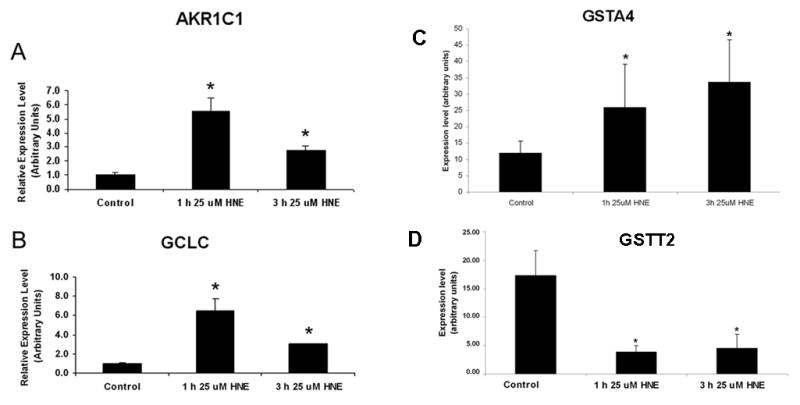
Normal astrocytes were treated for 1 h and 3 h with 25 μM HNE, then the HNE-containing media was removed and RNA was collected 6 h later. Relative amount of target mRNA in ONH astrocytes measured by quantitative RT-PCR as described in the Methods. Values represent mean ±SD of target mRNA level normalized to 18S RNA (internal control). All experiments were done in triplicate using three normal astrocytes cultures. Asterisk indicates significance of P <0.05
A. Expression of AKR1C1. The relative expression of AKR1C1 mRNA is 5.56 times higher after 1 h HNE treatment, and 2.77 times higher after 3 h HNE treatment, when compared to controls.
B. Expression of GCLC. The relative expression of GCLC mRNA increased after 1 h HNE treatment to 6.49 times higher than control levels, and to a lesser extent after 3 h HNE treatment to 3.02 times higher than control levels.
C. Expression of GSTA4. The relative expression of GSTA4 mRNA increased after 1 h HNE treatment to 2.2 times higher than control levels, and 2.8 times higher than control levels after 3 h HNE treatment.
D. Expression of GSTT2. The relative expression of GSTT2 mRNA was 4.60 times lower than control levels after 1 h HNE treatment to, and 3.89 times lower than control levels after 3 h HNE treatment.
Glutamate cysteine ligase, catalytic subunit (GCLC) mRNA, the first rate limiting enzyme in the synthesis of GSH was upregulated in glaucomatous astrocytes in microarray experiments (Hernandez et al., 2002). The relative expression of GCLC mRNA increased 6.5 fold and 3 fold after HNE treatment for 1h and 3 h respectively compared to control levels. HNE-induced upregulation of GCLC suggests that these cells are actively synthesizing GSH. Here we demonstrate that exposure to HNE induces upregulation of GCLC.
The alcohol dehydrogenases class I α polypeptide, ADH1A, and ADH1 β polypeptide, ADH1B, exhibited a decrease in expression levels after 1h and 3 h of exposure to HNE which was not significant. Aldehyde dehydrogenase 1 family member 3, ALDH1A3, did not exhibit any change in expression level (data not shown).
Expression of glutathione S-transferase A4 (GSTA4) increased after 1h and 3h exposure to HNE (P<0.005) (Fig 3C) whereas glutathione S-transferase theta 2 (GSTT2) exhibited a marked decrease in expression in the same experimental conditions.
We also tested other genes involved in responses to oxidative stress that were upregulated in glaucomatous ONH astrocytes (Table 1) however, changes in mRNA levels induced by exposure to HNE were not consistent amongst three normal ONH astrocyte cultures used in the present study thus significance was not achieved.
Regulation of transcription factors by HNE
Effect of HNE on expression of transcription factors
To determine the effect of HNE on mRNA, total RNA was isolated and prepared for real time RT-PCR as described above. The relative expression of Nrf2 mRNA increased in response to HNE treatment to 1.80 and 1.75 times higher than control levels after 1h and 3h, respectively (Figure 4 A).The relative expression of cFOS mRNA increased after 1 h and 3 h HNE treatment. A smaller increase of cFOS mRNA occurred after 1 h HNE treatment (~1.9 fold change) and a great increase of cFOS mRNA occurred after 3 h HNE treatment (~35 fold change) (Figure 4 B). No other significant changes in mRNA expression were detected in the expression of other transcription factors listed in Table 1, including cJUN and NFkB, either because the level of the target gene was undetectable, or there was no effect of the HNE treatment on expression of the mRNA.
Figure 4. Effects of HNE on transcription factor gene expression.
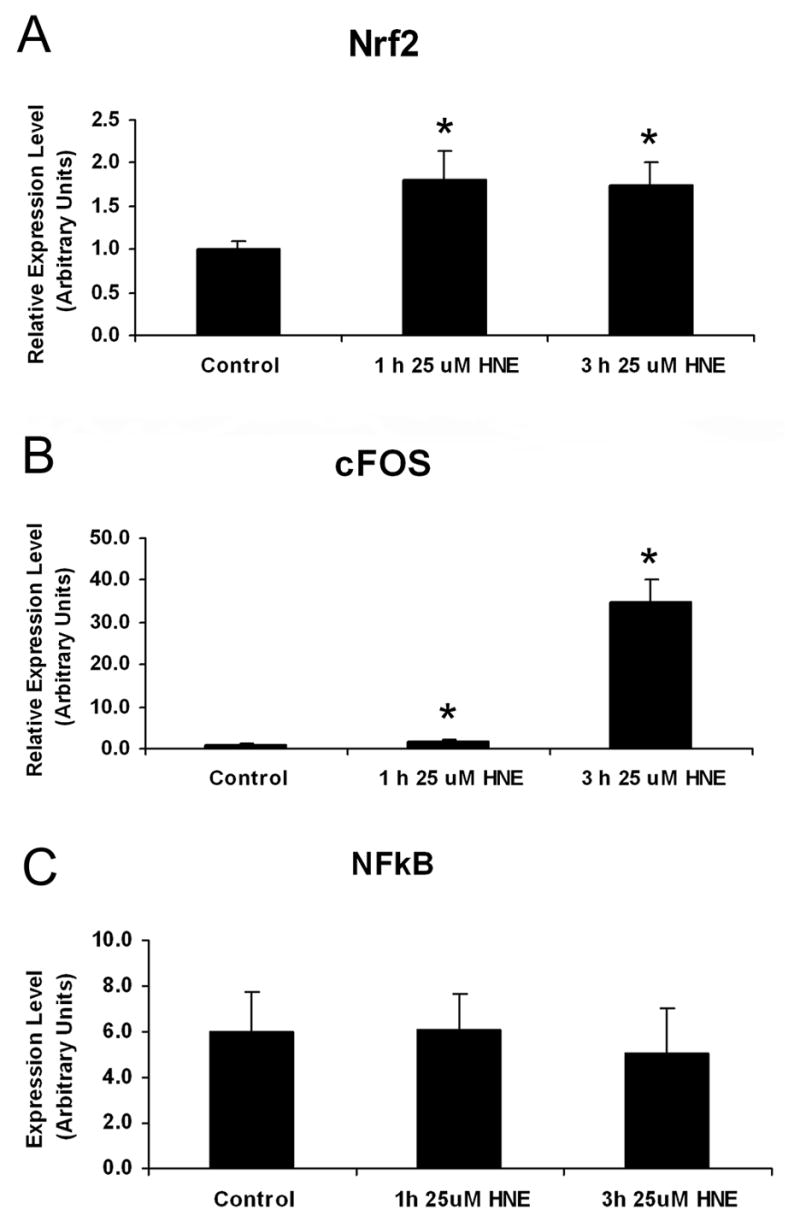
Normal astrocytes were treated for 1 h and 3 h with 25 μM HNE, then the HNE-containing media was removed and RNA was collected 6 h later. Relative amount of target mRNA in ONH astrocytes measured by quantitative RT-PCR as described in the Methods. Values represent mean ±SD of target mRNA level normalized to 18S RNA (internal control). All experiments were done in triplicate using three normal astrocytes cultures. Asterisk indicates significance of P <0.05.
A. Increased expression of Nrf2 using qRT-PCR. The relative expression of Nrf2 mRNA increased in response to both a 1 h and 3 h HNE treatment to 1.80 and 1.75 times higher than control levels, respectively.
B. Induction of cFOS expression. The relative expression of cFOS mRNA increased after 1 h and 3 h HNE. A smaller increase of cFOS mRNA occurred after 1 h HNE treatment to 1.86 times higher than control levels, and a great increase of cFOS mRNA to 34.96 times higher than control levels occurred after 3 h HNE treatment.
C. Expression of NFkB. The relative expression of NFkB did not change after treatment with HNE. All experiments were done in triplicate using three normal astrocytes cultures.
Effect of HNE on nuclear translocation of transcription factors
To determine if HNE treatment led to nuclear translocation of cFOS or NFkB, astrocytes were grown to ~95% confluence, treated for 1 h and 3 h with 25 μM HNE, and then stained with a cFOS or NFkB antibody and DAPI for nuclear staining (Figure 5 A). HNE treatment consistently increased the number of nuclei labeled with cFOS and NFkB all cultures of primary ONH astrocytes. Percentage of cFOS-positive nuclei in control, 1 h HNE, and 3 h HNE-treated astrocytes were 28.82%, 53.67%, and 75.07%, respectively. The increase in cFOS nuclear localization with HNE treatment was ~45% and ~60% at 1h and 3h respectively. Percentage of NFkB-positive nuclei in control, 1 h HNE, and 3 h HNE-treated astrocytes were 54.15%, 63.46%, and 73.29%, respectively. (Figure 5 B). A ~20% increase in NFkB nuclear localization with 3h HNE treatment was significant at P<0.05.
Figure 5. HNE affects the nuclear localization of c-Fos in normal human ONH astrocytes.
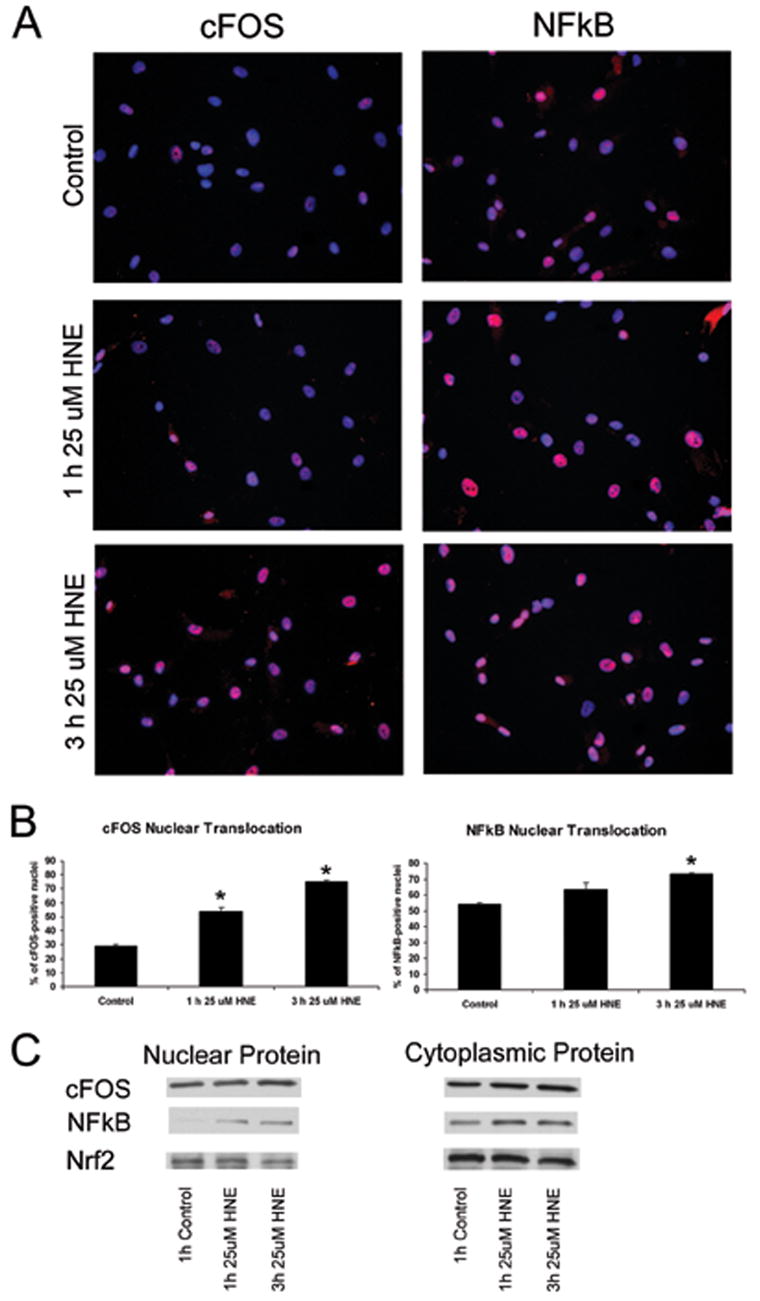
A. cFOS and NFkB nuclear localization. Treatment of normal ONH astrocytes with 25 μM HNE for 1 h and 3 h led to nuclear localization of both cFOS and NFkB, identified by immunofluorescence staining. cFOS and NFkB are stained in red; nuclei are stained in blue with DAPI, and localization of cFOS or NFkB to the nucleus results in a pink staining of nuclei.
B. cFOS and NFkB nuclear translocation measurements. Treatment of normal ONH astrocytes with 25 μM HNE led to nuclear translocation of cFOS and NFkB. Percentage of nuclear localization of cFOS in control astrocytes was 28.82%. Upon exposure to HNE for 1h and 3 h, c-Fos nuclear localization increased to 53.67%, and 75.07%, respectively. Percent of NFkB-positive nuclei in control, 1 h HNE, and 3 h HNE-treated astrocytes were 54.15%, 63.46%, and 73.29%, respectively. Asterisk indicates significance of P <0.05. All experiments were done in triplicate I ONH astrocytes fro three normal donors.
C. Western blots of nuclear and cytoplasmic protein. Normal astrocytes were treated for 1 h and 3 h with 25 μM HNE, then the HNE-containing media was removed and protein was collected 6 h later. In controls we detected abundant cFOS protein in the nucleus and cytoplasm. No discernable changes in cFOS cytoplasmic or nuclear protein were detected, nor any changes in NFkB cytoplasmic protein levels. In controls there were very low levels of NFkB protein detected in the nucleus which increased after treatment for 1 h and 3 h with 25 μM HNE. Nrf2 protein was detected in the nucleus and cytoplasm but there were no changes after exposure to HNE.
To further determine the effect of HNE on cytoplasmic and nuclear translocation of protein, ONH astrocytes were grown to ~95% confluence and treated for 1 h and 3 h with 25 μM HNE, then the media containing HNE was removed and replaced with fresh media. Protein was collected 6 h following HNE treatment as described above. Immunoblots of cytoplasmic protein showed no change in levels of NFkB in the cytoplasm. Immunoblots of nuclear protein showed that there is no NFkB protein in the nucleus in untreated astrocytes. After treatment for 1 h and 3 h with 25 μM HNE, NFkB protein was detected in the nucleus. Nrf2 and cFOS were detected in the nucleus and cytoplasm without discernable changes after HNE treatment (Figure 5 C). It is possible that no changes in cFOS and Nrf2 nuclear protein were detected because translocation of protein occurred earlier than when protein was collected. Alternatively, cFOS and Nrf2 protein levels were abundant in the nucleus and cytoplasm under control conditions, obscuring small changes in protein translocation from the cytoplasm to the nucleus that could not be detected by Western blot. Immunocytochemistry of Nrf2 nuclear translocation was not performed because no differences were seen in the protein by Western blot and no change in mRNA level was detected by qRT-PCR after exposure to HNE.
Discussion
Recent microarray analysis demonstrated that glaucomatous ONH astrocytes express high levels of lipid metabolizing enzymes which, as a byproduct, produce HNE a neurodestructive product of lipid peroxidation involved in the pathogenesis of several neurodegenerative diseases.(Hernandez et al., 2002).
HNE covalently binds to cellular proteins and DNA, causing extensive and permanent damage to the cell. As a defense mechanism, GSH binds covalently to HNE in order to inactivate HNE. GSH is the most abundant nonprotein thiol in the cell and a key cellular antioxidant important in the inactivation of HNE (Dickinson et al., 2004; Iles and Liu, 2005) . Human OHN astrocytes treated with HNE showed immediate decreased cellular levels of GSH, which in turn induced expression of GCLC, the rate-limiting enzyme in the synthesis of GSH. The strong induction of GCLC and new synthesis of GSH after a 24 h recovery from the HNE treatment indicates that ONH astrocytes can manage oxidative stress. These results are consistent with similar experiments performed with Madin-Darby canine kidney II cells (MDCK II) (Ji et al., 2004), transformed human bronchial epithelial (HBE1) cells (Dickinson et al., 2002), and rat astrocytes (Ahmed et al., 2002).
Glaucomatous ONH astrocytes compared to normal ONH astrocytes have lower basal levels of GSH, which may imply a compromised oxidation-reduction system or a depleted antioxidant response in the glaucomatous optic nerve. This observation is consistent with a study that assessed the levels of plasma GSH in patients with untreated POAG and found lower levels of circulating GSH in glaucoma patients when compared to control subjects (Gherghel et al., 2005). However, unlike the normal ONH astrocytes, glaucomatous ONH astrocytes exhibited increased levels of GSH immediately following treatment with HNE. This result may be consistent with findings by Ferreira et al (Ferreira et al., 2004) that found 3-fold higher GSH activity in the aqueous humor of patients with glaucoma compared to patients with cataracts. If the same phenomenon occurs in the glaucomatous ONH our data suggests faster mobilization of GSH in glaucomatous astrocytes immediately following HNE exposure compared normal astrocytes. Interestingly, glaucomatous astrocytes also exhibit a strong recovery following HNE treatment. Our data shows that basal GSH levels are lower in glaucomatous astrocytes suggesting active detoxification activity. Moreover GSH can recover to levels similar or higher than normal astrocytes suggesting that in vivo a primed antioxidant system exists in the glaucomatous ONH as a consequence of chronic exposure to oxidative stress.
Normal ONH astrocytes treated with HNE led to the increased production of GCLC mRNA. The strong induction of GCLC as a result of decreased GSH levels led to high levels of de novo synthesis of GSH, as seen after a 24 h recovery following HNE treatment. The GCLC promoter contains many cis-acting elements, including NFkB and AP-1 (Dickinson et al., 2004; Iles and Liu, 2005).
ONH astrocytes upregulate expression of the alternative antioxidant enzyme AKR1C1 following treatment with HNE, which can inactivate HNE. AKR1C1 acts as a reductase converting HNE to DHN (an inactive metabolite), thereby eliminating the ability of HNE to bind to protein or DNA (Burczynski et al., 2001) . AKR1C1 has been shown to be over-expressed in glaucomatous optic nerves, suggesting a constitutive response to chronic oxidative stress (Agapova et al., 2003). AKR1C1 may play a more important role in the inactivation of HNE when the primary stores of GSH are depleted in the cell, as exhibited immediately following treatment with HNE in normal ONH astrocytes, because AKR1C1 can reduce HNE independent of GSH.
Glutathione-S-transferases (GST) catalyze the conjugation of HNE to GSH thus contributing to the detoxification of HNE. GTSA4 encodes an enzyme with glutathione peroxidase activity that functions in the detoxification of lipid peroxidation products such as HNE (Raza et al., 2002;Cheng et al., 2001); GTSA4 has been localized to astrocytes in the rat cerebellum (Martinez-Lara et al., 2003). In contrast, expression of GSTT2, another member of the GST family exhibited a decrease in ONH astrocytes exposed to HNE suggesting that GST isoforms may have differential responses to HNE as demonstrated in transfected neuroblastoma cells (Xie et al., 2001).
HNE induces expression of the transcription factor Nrf2, which coordinates the upregulation of detoxification enzymes and antioxidant proteins. Nrf2 is a transcription factor that binds to the promoter sequence antioxidant responsive element (ARE) leading to the coordinate upregulation of ARE-driven detoxification and antioxidant genes (Lee et al., 2005). Nrf2 can upregulate GCLC (Sun et al., 2005), which was also found after HNE treatment.
ONH astrocytes respond to HNE by the activation and transcription of cFOS and NFkB, which in turn regulate physiological responses related to cellular protection against oxidative stress. cFOS and NFkB are also able to act as cis-regulatory elements in the promoter of GCLC (Dickinson et al., 2004); (Iles and Liu, 2005), helping to promote the synthesis of GSH, which can conjugate to HNE and is the primary method of inactivating HNE.
In this study, we found that normal ONH astrocytes exhibit a strong antioxidant response to HNE treatment by inducing the transcription factors cFOS, NFkB, and Nrf2, which can all upregulate the expression of GCLC, to produce more GSH in the cell. Oxidative stress has been identified as a component of the pathogenesis of glaucoma (Izzotti et al., 2003; Sacca et al., 2005). Moreno et al ( 2004) analyzed retinal oxidative damage in rats with induced glaucoma and found decreased SOD and catalase activities, as well as decreased GSH levels, suggesting that retinal oxidative stress may be involved in the glaucomatous cell death of RGCs.
Glial cells are well know protectors of neurons and are involved in the antioxidant defense in the brain by synthesizing GSH, mostly located in astrocytes in the brain (Pearce et al., 1997) and by providing precursors that neurons require to make their own GSH (Sagara et al., 1993; Dringen et al., 1999). Since astrocytes have high levels of antioxidants (Makar et al., 1994; Dringen et al., 1993), they can sustain the presence of ROS and therefore prevent their neurotoxicity (Wilson, 1997), which we have demonstrated after HNE treatment.
Neurons when cultured alone have low levels of cellular GSH whereas neurons co-cultured with astrocytes exhibit a significant increase in neuronal GSH content (Bolanos et al., 1996); (Dringen et al., 1999). Astrocytes can transport GSH conjugated to HNE out of the cell via the multidrug-resistance protein (MRP1) (Hirrlinger et al., 2002) or through the RLIP76 (Ral binding protein) (Yang et al., 2003). Once outside of the cell, the released GSH cannot be taken up by neurons, so gamma-glutamyl transpeptidase (GGT) present on the plasma membrane of astrocytes cleaves GSH into components that can be further cleaved and taken up by neurons. (Heales et al., 2004). Therefore, ONH astrocytes may offer neuroprotection in the optic nerve by releasing GSH and antioxidant enzymes to eliminate the products of chronic oxidative stress that may be contributing to the progression of neurodegeneration in POAG.
Footnotes
This work was supported by National Institutes of Health Grants EY-06416 and by National Research to Prevent Blindness (RPB).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Agapova OA, Ricard CS, Salvador-Silva M, Hernandez MR. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia. 2001;33:205–216. doi: 10.1002/1098-1136(200103)33:3<205::aid-glia1019>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Agapova OA, Yang P, Wang WH, Lane DA, Clark AF, Weinstein BI, Hernandez MR. Altered expression of 3 alpha-hydroxysteroid dehydrogenases in human glaucomatous optic nerve head astrocytes. Neurobiol Dis. 2003;14:63–73. doi: 10.1016/s0969-9961(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Ahmed I, John A, Vijayasarathy C, Robin MA, Raza H. Differential modulation of growth and glutathione metabolism in cultured rat astrocytes by 4-hydroxynonenal and green tea polyphenol, epigallocatechin-3-gallate. Neurotoxicology. 2002;23:289–300. doi: 10.1016/s0161-813x(02)00042-6. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Heales SJ, Peuchen S, Barker JE, Land JM, Clark JB. Nitric oxide-mediated mitochondrial damage: a potential neuroprotective role for glutathione. Free Radic Biol Med. 1996;21:995–1001. doi: 10.1016/s0891-5849(96)00240-7. [DOI] [PubMed] [Google Scholar]
- Burczynski ME, Sridhar GR, Palackal NT, Penning TM. The reactive oxygen species--and Michael acceptor-inducible human aldoketo reductase AKR1C1 reduces the alpha,beta-unsaturated aldehyde 4-hydroxy-2-nonenal to 1,4-dihydroxy-2-nonene. J Biol Chem. 2001;276:2890–2897. doi: 10.1074/jbc.M006655200. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic Biol Med. 2002;33:974. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Levonen AL, Moellering DR, Arnold EK, Zhang H, rley-Usmar VM, Forman HJ. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic Biol Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–69. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Dickinson DA, Iles KE. HNE--signaling pathways leading to its elimination. Mol Aspects Med. 2003;24:189–194. doi: 10.1016/s0098-2997(03)00013-x. [DOI] [PubMed] [Google Scholar]
- Friedman DS, Wolfs RC, O'Colmain BJ, Klein BE, Taylor HR, West S, Leske MC, Mitchell P, Congdon N, Kempen J. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–883. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Parker A, Malone P, Gabelt BT, Rasmussen C, Kaufman PS, Hernandez MR. Long-term activation of c-Fos and c-Jun in optic nerve head astrocytes in experimental ocular hypertension in monkeys and after exposure to elevated pressure in vitro. Brain Res. 2005;1054:103–115. doi: 10.1016/j.brainres.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Heales SJ, Lam AA, Duncan AJ, Land JM. Neurodegeneration or neuroprotection: the pivotal role of astrocytes. Neurochem Res. 2004;29:513–519. doi: 10.1023/b:nere.0000014822.69384.0f. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Agapova OA, Yang P, Salvador-Silva M, Ricard CS, Aoi S. Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia. 2002;38:45–64. doi: 10.1002/glia.10051. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Pena JD. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 1997;115:389–395. doi: 10.1001/archopht.1997.01100150391013. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Pena JD, Selvidge JA, Salvador-Silva M, Yang P. Hydrostatic pressure stimulates synthesis of elastin in cultured optic nerve head astrocytes. Glia. 2000;32:122–136. doi: 10.1002/1098-1136(200011)32:2<122::aid-glia20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Schulz JB, Dringen R. Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res. 2002;69:318–326. doi: 10.1002/jnr.10308. [DOI] [PubMed] [Google Scholar]
- Hu XM, Hirano T, Oka K. Arsenic trioxide induces apoptosis in cells of MOLT-4 and its daunorubicin-resistant cell line via depletion of intracellular glutathione, disruption of mitochondrial membrane potential and activation of caspase-3. Cancer Chemother Pharmacol. 2003;52:47–58. doi: 10.1007/s00280-003-0629-5. [DOI] [PubMed] [Google Scholar]
- Iles KE, Liu RM. Mechanisms of glutamate cysteine ligase (GCL) induction by 4-hydroxynonenal. Free Radic Biol Med. 2005;38:547–556. doi: 10.1016/j.freeradbiomed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Sacca SC, Cartiglia C, De FS. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–646. doi: 10.1016/s0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- Ji B, Ito K, Horie T. Multidrug resistance-associated protein 2 (MRP2) enhances 4-hydroxynonenal-induced toxicity in Madin-Darby canine kidney II cells. Chem Res Toxicol. 2004;17:158–164. doi: 10.1021/tx034067m. [DOI] [PubMed] [Google Scholar]
- Keller JN, Mattson MP. Roles of lipid peroxidation in modulation of cellular signaling pathways, cell dysfunction, and death in the nervous system. Rev Neurosci. 1998;9:105–116. doi: 10.1515/revneuro.1998.9.2.105. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Vidal I, Pena JD, Hernandez MR. Expression of neural cell adhesion molecule (NCAM) characterizes a subpopulation of type 1 astrocytes in human optic nerve head. Glia. 1997;20:262–273. doi: 10.1002/(sici)1098-1136(199707)20:3<262::aid-glia10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem. 1994;62:45–53. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Lara E, Siles E, Hernandez R, Canuelo AR, Luisa del MM, Jimenez A, Blanco S, Lopez-Ramos JC, Esteban FJ, Pedrosa JA, Peinado MA. Glutathione S-transferase isoenzymatic response to aging in rat cerebral cortex and cerebellum. Neurobiol Aging. 2003;24:501–509. doi: 10.1016/s0197-4580(02)00139-2. [DOI] [PubMed] [Google Scholar]
- Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Neely MD, Sidell KR, Graham DG, Montine TJ. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J Neurochem. 1999;72:2323–2333. doi: 10.1046/j.1471-4159.1999.0722323.x. [DOI] [PubMed] [Google Scholar]
- Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J Neural Transm. 1997;104:661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza H, Robin MA, Fang JK, Avadhani NG. Multiple isoforms of mitochondrial glutathione S-transferases and their differential induction under oxidative stress. Biochem J. 2002;366:45–55. doi: 10.1042/BJ20020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- Sagara JI, Miura K, Bannai S. Maintenance of neuronal glutathione by glial cells. J Neurochem. 1993;61:1672–1676. doi: 10.1111/j.1471-4159.1993.tb09802.x. [DOI] [PubMed] [Google Scholar]
- Salvador-Silva M, Ricard CS, Agapova OA, Yang P, Hernandez MR. Expression of small heat shock proteins and intermediate filaments in the human optic nerve head astrocytes exposed to elevated hydrostatic pressure in vitro. J Neurosci Res. 2001;66:59–73. doi: 10.1002/jnr.1197. [DOI] [PubMed] [Google Scholar]
- Sun X, Erb H, Murphy TH. Coordinate regulation of glutathione metabolism in astrocytes by Nrf2. Biochem Biophys Res Commun. 2005;326:371–377. doi: 10.1016/j.bbrc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol. 1999;468:175–182. doi: 10.1007/978-1-4615-4685-6_14. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Uchida K. Histidine and lysine as targets of oxidative modification. Amino Acids. 2003;25:249–257. doi: 10.1007/s00726-003-0015-y. [DOI] [PubMed] [Google Scholar]
- Uchida K, Stadtman ER. Selective cleavage of thioether linkage in proteins modified with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992;89:5611–5615. doi: 10.1073/pnas.89.12.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75:1149–1163. [PubMed] [Google Scholar]
- Xie C, Lovell MA, Xiong S, Kindy MS, Guo J, Xie J, Amaranth V, Montine TJ, Markesbery WR. Expression of glutathione-S-transferase isozyme in the SY5Y neuroblastoma cell line increases resistance to oxidative stress. Free Radic Biol Med. 2001;31:73–81. doi: 10.1016/s0891-5849(01)00553-6. [DOI] [PubMed] [Google Scholar]
- Yang P, Hernandez MR. Purification of astrocytes from adult human optic nerve heads by immunopanning. Brain Res Brain Res Protoc. 2003;12:67–76. doi: 10.1016/s1385-299x(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma A, Sharma R, Patrick B, Singhal SS, Zimniak P, Awasthi S, Awasthi YC. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J Biol Chem. 2003;278:41380–41388. doi: 10.1074/jbc.M305766200. [DOI] [PubMed] [Google Scholar]
- Yildirim O, Ates NA, Ercan B, Muslu N, Unlu A, Tamer L, Atik U, Kanik A. Role of oxidative stress enzymes in open-angle glaucoma. Eye. 2005;19:580–583. doi: 10.1038/sj.eye.6701565. [DOI] [PubMed] [Google Scholar]
- Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003;24:293–303. doi: 10.1016/s0098-2997(03)00024-4. [DOI] [PubMed] [Google Scholar]


