Abstract
Previously we discovered that holocarboxylase synthetase (HCS) is a chromatin-associated protein in Drosophila melanogaster, and that HCS deficiency alters chromatin structure and gene expression patterns, leading to decreased heat tolerance. The effects of HCS deficiency were attributed to decreased biotinylation of histones. However, HCS is known to mediate biotinylation of carboxylases in cytoplasm and mitochondria in addition to mediating biotinylation of histones. A challenge posed by the genetic analysis of HCS is to distinguish between the effects of decreased biotinylation of carboxylases from the effects of decreased histone biotinylation in the gene expression patterns and phenotypes observed in HCS-deficient flies. Here, we tested whether 3-methylcrotonyl-CoA carboxylase (MCC) mutant flies exhibit gene expression patterns and heat susceptibility similar to that in HCS-deficient Drosophila. Biotin transporter (SMVT) mutants were used to investigate effects of cellular biotin depletion on gene expression and heat susceptibility. Deficiencies of MCC and SMVT in mutant flies were confirmed by real-time PCR, streptavidin blotting of holocarboxylases, and analysis of MCC activities; expression of HCS and biotinylation of histones were not altered in MCC and SMVT mutants. Gene expression patterns in MCC and SMVT mutants were different from that seen with HCS-deficient flies, as judged by the abundance of mRNA coding for defective chorion 1, chitin binding peritrophin-A, dopamine receptor 2, and yolk protein 2. MCC mutants exhibited increased resistance to heat stress compared with wild-type flies. We conclude that gene expression patterns and phenotypes in HCS-deficient flies in previous studies are caused by decreased biotinylation of histones rather than MCC.
Keywords: biotin, carboxylases, Drosophila melanogaster, heat stress, sodium-dependent multivitamin transporter
Biotin uptake into eukaryotic cells is mediated by the sodium-dependent multivitamin transporter (SMVT)3 (1). Intracellular biotin is then conjugated to carboxylases and histones by holocarboxylase synthetase (HCS) in an ATP-requiring reaction (2,3). Five biotin-dependent carboxylases have been identified: acetyl-CoA carboxylase α, acetyl-CoA carboxylase β, pyruvate carboxylase (PC), propionyl-CoA carboxylase, and 3-methylcrotonyl-CoA carboxylase (MCC). These carboxylases play essential roles in intermediary metabolism. For example, PC and MCC catalyze steps in gluconeogenesis and leucine metabolism, respectively (2). The biological functions of carboxylases are distinct from the second family of biotinylated proteins, histones. Histones comprise five major classes (H1, H2A, H2B, H3, and H4) of DNA-binding proteins that play essential roles in chromatin structure, DNA repair, and gene regulation (4). Ten distinct lysine residues in histones H2A, H3, and H4 (5-7) are known to be targets for biotinylation in both humans and Drosophila melanogaster (8,9). Biotinylation of histones has been implicated in the cellular response to DNA double-strand breaks (10), cell proliferation and mitotic chromatin condensation (8,11), and heterochromatin structures and gene silencing in humans (12).
As of today, no HCS null individuals have been identified in the general population, consistent with essentiality of HCS-dependent biotinylation reactions and fetal lethality of the HCS null genotype. The prevalence of hcs gene mutations that cause decreased (as opposed to absent) HCS activity is also low in the general population, e.g., less than 1 in one hundred thousand live births in Japan (13). Symptoms of HCS deficiency include excretion of abnormal organic acids in the urine, skin rashes, and developmental delays (13,14). Afflicted individuals typically respond well to administration of pharmacological doses of biotin, in particular if the mutation of the gene resides in the sequences encoding biotin-binding region of the protein (13).
Recently, we showed that HCS is a chromatin-associated protein in Drosophila. We used RNAi to generate HCS-deficient Drosophila for the investigation of biological functions of HCS and biotinylated histones (15). We showed that HCS deficiency affects the extent of histone biotinylation and gene expression patterns, and results in flies that have decreased heat tolerance. Notwithstanding the importance of these previous studies, the possibility was not formally excluded that decreased biotinylation of carboxylases, rather than decreased histone biotinylation, caused the gene expression and phenotypic changes observed in HCS-deficient flies. This uncertainty has been addressed in the studies presented here. Specifically, we tested whether mutations in the gene coding for MCC causes gene expression patterns and heat susceptibility similar to those observed in HCS-deficient Drosophila; in addition, SMVT mutants were used to investigate effects of cellular biotin depletion on gene expression and heat susceptibility.
The selection of mutants was based on the following lines of reasoning. First, MCC mutants were selected because only a very small number of human cases of deficiency have ever been reported for this carboxylase (16-19). This observation suggests that null mutations of MCC are lethal and thus that MCC activity is essential for normal biotin metabolism. Second, an SMVT mutant was selected because deficiency of this transporter decreases uptake of biotin into eukaryotic cells, leading to moderate biotin depletion (20). Previous studies in our laboratory showed that moderate biotin deficiency is associated with selective depletion of biotinylated carboxylases, whereas biotinylation of histones is maintained at normal levels in human cells and Drosophila (9,21-23) under such conditions. Hence, SMVT deficiency is a model for moderate biotin deficiency that does not detectably impair chromatin structure.
MATERIALS AND METHODS
Drosophila stocks
MCC (PBac{GAL4D,EYFP}CG2118PL00054) and SMVT (PBac{RB}CG2191e01332) mutant Drosophila melanogaster were obtained from the Bloomington stock center (Bloomington, IN). In addition, a PC (P{w[+mGT]=GT1}CG1516BG01252) mutant stock was tested, but the decrease of PC expression in these flies was insufficient to permit meaningful analysis (see below). A laboratory strain of wild-type flies was used as control; in addition, HCS-deficient flies (denoted “HCS-RNAi”) were generated by RNA interference (15) and were used as controls in some experiments.
Drosophila husbandry
Flies were housed at 25°C on standard cornmeal diet (9,24) and were transferred to fresh tubes every 72 h. This diet contains approximately 60 pmoles of biotin per gram (9). Flies were kept on a 12 h light/dark cycle.
Expression of HCS, MCC, PC, and SMVT
Total RNA was isolated from 100 flies with TriZol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Genomic DNA was removed enzymatically using TURBO DNA-free™ (Ambion, Austin, TX). Briefly, cDNA was synthesized using the ImProm-II reverse transcriptase system from Promega (Madison, USA). PCR primers for HCS, MCC, PC, and SMVT (online supplemental Table S1) were designed using the Beacon designer 4.0 software (Premier Biosoft International, Palo Alto, CA). Real-time PCR was performed using the iCycler IQ™ multicolor real-time detection system (Bio-Rad, Palo Alto, CA), using histone H4 as reference gene.
Biotinylated carboxylases
The abundance of holocarboxylases and the activities of PC and MCC were quantified to estimate levels of gene knockdown in mutant flies. Briefly, 50 flies were homogenized as described (9) and proteins (∼ 100 μg) were resolved using 4-8% Tris-acetate gels (Invitrogen). Transblots were probed with streptavidin peroxidase (21). Band intensities were quantified by gel densitometry (25). Note that MCC and propionyl-CoA carboxylase contain biotinylated and non-biotinylated subunits, and that only the biotin-containing α subunits are detectable in streptavidin blots. Alpha subunits of MCC and propionyl-CoA carboxylase co-migrate under the conditions used here. Activities of PC and MCC in fly homogenates were quantified as described (26) with minor modifications (9).
HCS abundance
Abundance of HCS in whole fly homogenates was quantified by Western blot analysis using rabbit anti-human HCS (5); this antibody cross-reacts with HCS from Drosophila. Proteins were resolved using 4-8% Bis-Tris gels (Invitrogen), and transblots were probed with anti-HCS serum (20,000-fold dilution) and goat anti-rabbit IgG peroxidase conjugate (Sigma, St. Louis, MO; 10 μg/L). Histone H3 was used as loading control as described (15).
Biotinylated histones
Drosophila histones were extracted as described (15). Histones were resolved by gel electrophoresis and probed using either streptavidin-peroxidase or antiserum to K18-biotinylated histone H3 (15). Equal loading of lanes was confirmed by using bicinchoninic assay (Pierce; Rockford, IL), densitometric quantitation of gels stained with Commassie blue, and Western blot analysis using an antibody to the C-terminus in histone H3.
Gene expression patterns
HCS deficiency in flies is associated with altered expression of about 200 genes (15); here we selected the following HCS-dependent genes for comparing gene expression patterns of carboxylase mutants and SMVT mutants to that of HCS-deficient flies: (i) defective chorion 1 (GenBank NP_511072) and chitin binding peritrophin-A (CG9307), the expression of which is known to increase by 100 to 200% in HCS-deficient flies compared with wild-type controls (15); and (ii) yolk protein 2 (CG2979) and dopamine receptor 2 (CG18741), the expression of which is known to decrease by 50 to 75% in HCS-deficient flies compared with wild-type controls (15). Here, we quantified the relative abundance of these HCS-dependent genes in MCC and SMVT mutants, HCS-RNAi transgenic flies (15), and wild-type controls. The goal was to determine whether gene expression patterns are similar in MCC and SMVT mutants compared with HCS-deficient flies. mRNA was quantified by real-time PCR as described above by using gene-specific primers (online supplemental Table S1).
Heat tolerance
Previous studies suggested that HCS-deficient flies have an increased susceptibility to heat stress (15). Here we determined whether carboxylase and SMVT mutants have a phenotype similar to HCS-deficient flies. In heat stress experiments, four tubes (25 flies each) were kept at 34°C up to 9 h; dead flies were counted every 0.5 h. Data are presented in units of “% survival time.” For example, the “75% survival time” indicates the time of heat exposure that was survived by 75% of flies.
Statistics
Homogeneity of variances among groups was tested using Bartlett's test (27). Significance of differences among groups was tested by one-way ANOVA. Fisher's Protected Least Significant Difference procedure was used for posthoc testing (27). Effects of treatment over time (e.g., resistance to heat stress) were analyzed by repeated measures ANOVA. If effects of treatment over time were statistically significant, treatment effects at individual time points were assessed by one-way ANOVA and Fisher's Protected Least Significant Difference procedure for individual time points. StatView 5.0.1 (SAS Institute; Cary, NC) was used to perform all calculations. Differences were considered significant if P < 0.05. Data are expressed as means ± SD.
RESULTS
Expression and activities of carboxylases in MCC, and SMVT mutant flies
Expression of MCC and SMVT is substantially decreased in mutant flies compared with wild-type controls. This conclusion is based on the following observations. First, the abundance of MCC mRNA was decreased by 67 ± 0.8 % in MCC mutants compared with wild-type flies (P < 0.02; n = 3). Likewise, the abundance of SMVT mRNA was decreased by 68 ± 6.0 % in SMVT mutants compared with wild-type flies (P < 0.05; n = 3). In contrast, the abundance of PC mRNA was decreased by only 28 ± 2.1 % in PC mutants compared with wild-type flies (P < 0.05; n = 3). This relatively moderate decrease of PC expression was further confirmed by analysis of PC activity (see below), and was the reason for excluding PC mutant flies from the majority of further analyses.
Second, the abundance of holocarboxylases was reduced in mutant flies compared to wild-type controls. Specifically, holo-MCC was barely detectable in MCC mutants compared with wild-type flies; for unknown reasons, the abundance of holo-acetyl-CoA carboxylase was moderately decreased in MCC mutants, whereas the abundance of holo-PC was not affected (Fig. 1 and Table 1). Mutation of the smvt gene caused decreased abundance of holo-acetyl-CoA carboxylase, holo-MCC, and holo-PC. This observation is consistent with cellular biotin depletion by transporter deficiency.
FIGURE 1.
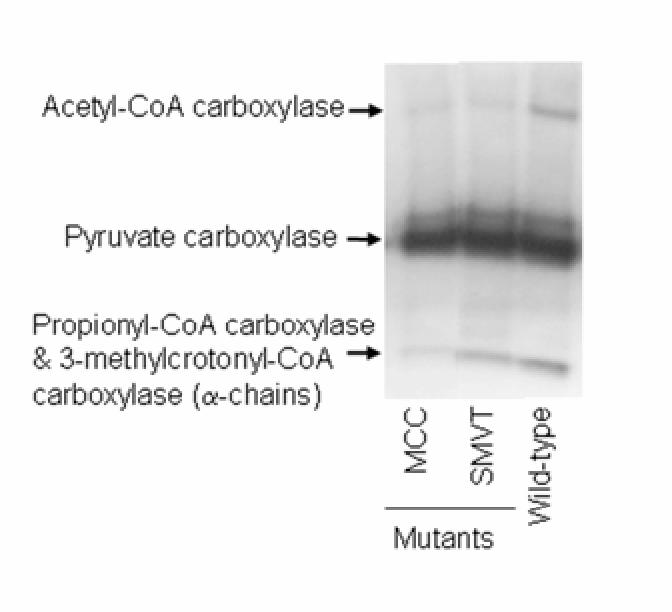
Mutations of mcc and smvt genes decrease the abundance of holocarboxylases in Drosophila melanogaster. Holocarboxylases in fly extracts were probed by using streptavidin peroxidase. PCC = Propionyl-CoA carboxylase.
TABLE 1.
Mutations of mcc, pc, and smvt genes decrease the abundance of holocarboxylases in Drosophila melanogaster1
| Genotype | ACC | MCC & PCC | PC |
|---|---|---|---|
| Aritrary units | |||
| MCC mutant | 9.0 ± 0.5a | 13 ± 2.1a | 95 ± 5.0 |
| SMVT mutant | 15 ± 5.5b | 43 ± 6.4b | 84 ± 2.9 |
| Wild type | 36 ± 2.1c | 62 ± 3.8c | 91 ± 2.9 |
Values are means ± SD, n = 3. Means in a column with superscripts without a common letter differ, P < 0.05.
Third, the activity of MCC was significantly lower in both MCC and SMVT mutants compared with wild-type controls (Fig. 2). These findings suggest that this mutant allele of mcc decreases the expression of MCC protein, and that the mutant allele of smvt impairs cellular biotin uptake, leading to decreased carboxylase activities. In contrast, PC activity in PC mutants was decreased only moderately, by 30 ± 1.2 % compared with wild-type flies (P < 0.05; n = 3). Note that all three of these mutant lines were identified based on P-element transposon insertions at the respective loci. These are the first functional assays for each of these mutant alleles.
FIGURE 2.
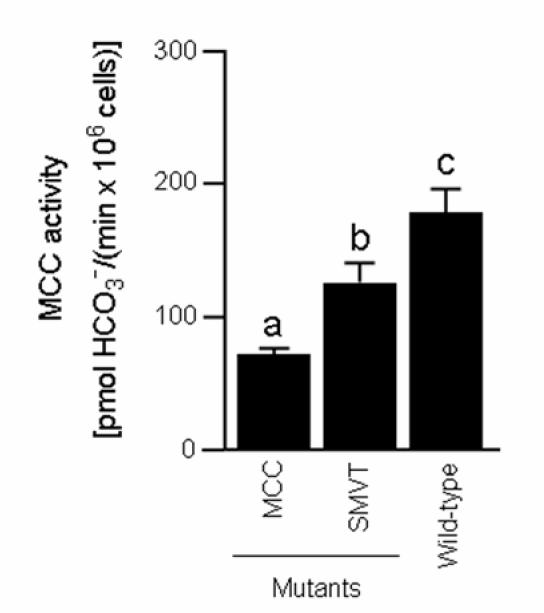
Mutations in mcc and smvt genes decrease activities of MCC in Drosophila melanogaster. a,b,cColumns not sharing the same letter are significantly different (P < 0.05). Values are means ± SD (n = 3).
Expression of HCS and biotinylation of histones
MCC and SMVT mutants expressed normal levels of HCS and exhibited a normal abundance of biotinylated histones. This conclusion is based on the following observations. First, the abundance of HCS mRNA in MCC and SMVT mutants was within 93-104 % of the abundance in wild-type flies. Second, the abundance of HCS protein and histone H3 (control) was similar in mutants and wild-type flies (Fig. 3A). Third, the abundance of biotinylated histones was similar in carboxylase and SMVT mutant males and females compared with wild-type controls (Fig. 3B). Our observation that histone biotinylation remains unaltered despite SMVT deficiency is consistent with previous studies (9,21-23) showing that moderately biotin-deficient mammalian cultured cells maintain normal histone biotinylation (see Discussion).
FIGURE 3.
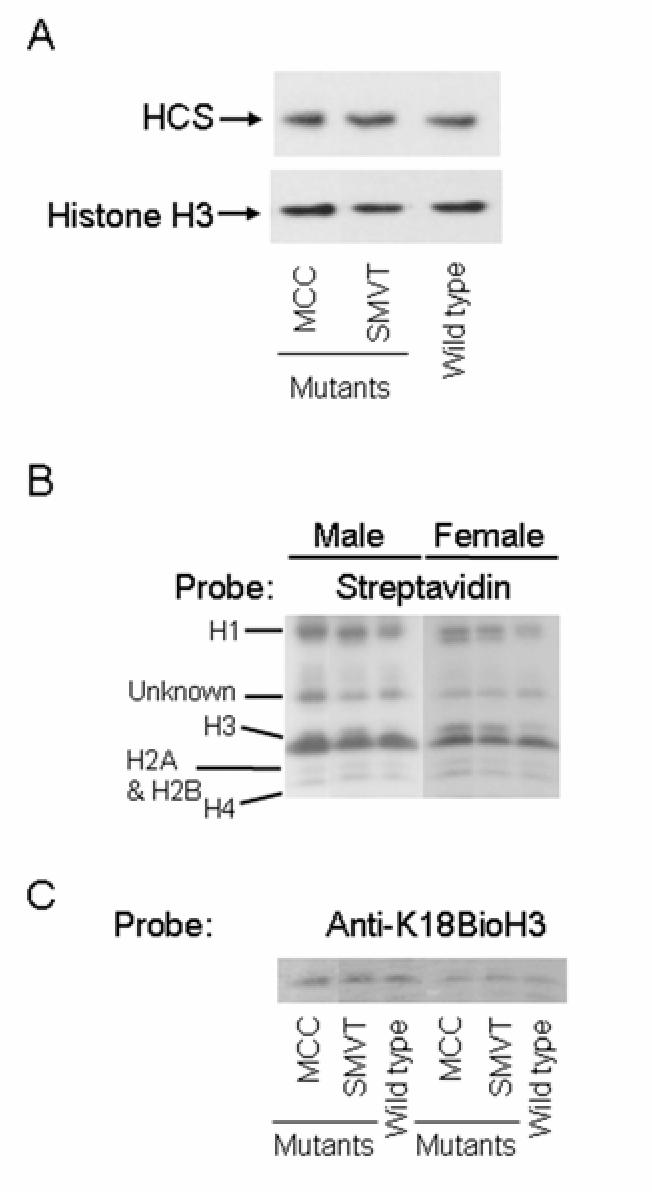
Mutations in mcc and smvt genes do not affect the abundance of HCS (panel A) and biotinylated histones (panel B) in Drosophila melanogaster. Histone H3 was used as a loading control.
Gene expression patterns
MCC and SMVT mutants exhibited gene expression patterns that are different from the patterns known from HCS-deficient flies in previous studies (15). The expression of HCS-dependent genes (see Materials and Methods) was not affected by deficiencies of MCC or SMVT. For example, the abundance of mRNA coding for defective chorion 1 and chitin binding peritrophin-A increased significantly in HCS-deficient flies compared with wild-type controls, but was not altered in MCC and SMVT mutants (Fig. 4A). Likewise, the abundance of mRNA coding for yolk protein 2 and dopamine receptor 2 decreased significantly in HCS-deficient flies compared with wild-type controls, but was not altered in MCC and SMVT mutants (Fig. 4B).
FIGURE 4.
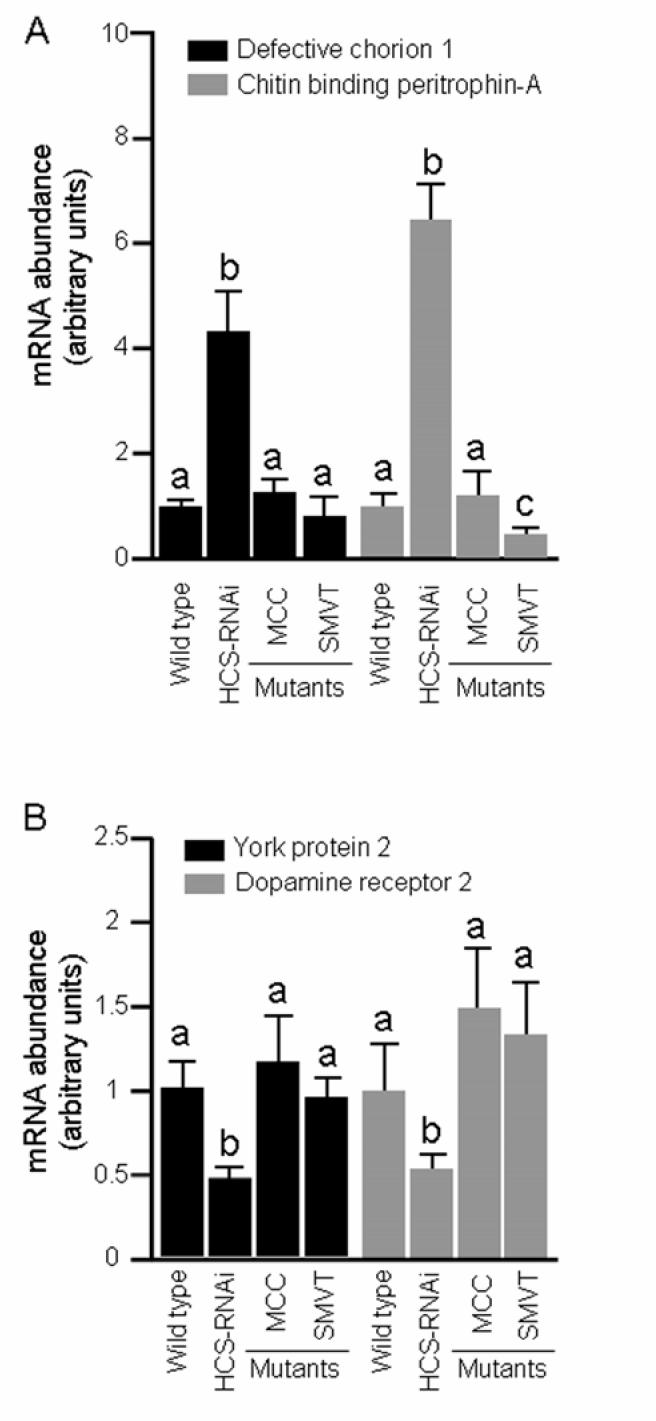
Mutations in mcc and smvt genes do not affect the relative abundance of mRNA coding for defective chorion 1 and chitin-binding peritrophin 1 (panel A) and Yolk protein 2 and dopamine receptor 2 (panel B) in Drosophila melanogaster; Wild-type flies and HCS-deficient flies (“HCS-RNAi”) were used as controls. mRNA abundance in wild-type flies was set at “1.” a,bColumns not sharing the same letter are significantly different (P < 0.05). Values are means ± SD (n = 3).
Heat tolerance
Mutation of mcc was associated with increased tolerance to heat stress in both male and female flies compared with wild-type controls (Table 2). Mutations of the smvt gene did not affect heat tolerance. These phenotypes contrast with the decreased thermotolerance observed for HCS-deficient flies in previous studies (see Discussion).
TABLE 2.
Mutation of mcc gene but not the smvt gene increases heat tolerance in Drosophila melanogaster1
| Genotype | ||||||
|---|---|---|---|---|---|---|
| Male |
Female |
|||||
| % survival | MCC mutant | SMVT mutant | Wild type | MCC mutant | SMVT mutant | Wild type |
| Survival at 34°C, h | ||||||
| 75 | 3.3 ± 0.2a | 1.9 ± 0.3b | 2.4 ± 0.3b | 3.7 ± 0.4a | 2.4 ± 0.1b | 2.8 ± 0.2b |
| 50 | 4.3 ± 0.0a | 2.7 ± 0.1b | 3.2 ± 0.1c | 4.9 ± 0.1a | 3.3 ± 0.4b | 3.5 ± 0.3b |
| 25 | 5.4 ± 0.1a | 6.3 ± 0.0b | 4.0 ± 0.2c | 6.3 ± 0.2a | 4.3 ± 0.1b | 4.3 ± 0.1b |
| 5 | 6.4 ± 0.1a | 4.6 ± 0.3b | 5.0 ± 0.2b | 7.2 ± 0.1a | 5.3 ± 0.1b | 5.6 ± 0.4b |
Values are means ± SD, n = 100. Within gender, means in a row without a common letter differ, P < 0.05.
DISCUSSION
Here we demonstrate that gene expression patterns and phenotypes of MCC-deficient and SMVT-deficient Drosophila are distinct from those observed with HCS-deficient flies. This is a critical observation in the context of our previous work, in which we characterized patterns of gene expression and heat susceptibility in HCS-deficient flies (15). In these previous studies, we proposed that gene expression patterns and phenotypes of HCS deficiency were caused by altered histone biotinylation and chromatin structure. Note that HCS deficiency decreases the abundance both of biotinylated histones and of biotinylated carboxylases. In the studies presented here, we formally excluded the possibility that decreased biotinylation of MCC explains gene expression patterns and phenotypes observed in HCS-deficient flies. This conclusion is based on the following observations: (i) patterns of gene expression are different in MCC mutants compared with HCS-deficient flies; and (ii) heat tolerance increases in MCC mutants but decreased in HCS-deficient flies. Taken together, these findings support recent proposals that a major function of HCS is to mediate chromatin remodeling events.
Theoretically, both SMVT mutants and HCS-deficient flies could have an impaired ability to biotinylate carboxylases and histones. In SMVT mutants, impaired biotinylation of proteins is due to low cellular levels of biotin as previously described for humans (28); in HCS-deficient flies, impaired biotinylation of proteins is due to low biotinyl protein ligase activity. One might speculate that SMVT mutants and HCS-deficient flies would exhibit similar gene expression patterns and phenotypes, if these variables were regulated by protein biotinylation. However, the present study provides evidence that gene expression and phenotypes are different in SMVT mutants and HCS knockdowns. We offer the following explanation for this observation. SMVT deficiency is associated with impaired cellular uptake of biotin (20), causing moderate rather than severe biotin depletion in SMVT mutant flies. For example, abundance and activities of holo-carboxylases decreased by only 20-30 % in SMVT mutants compared with controls. Previous studies suggested that moderate biotin deficiency is associated with depletion of holocarboxylases while biotinylation of histones is maintained at normal levels in human cells and flies (9,21-23). This selective preservation of histone biotinylation over carboxylase biotinylation was confirmed in this study, where histone biotinylation was not altered in SMVT mutants. Collectively, the protein biotinylation patterns in SMVT mutants are similar to carboxylase mutants but distinct from HCS-deficient flies. Based on these patterns, it is not surprising that gene expression patterns and phenotypes in SMVT mutants resemble those in carboxylase mutants but are distinct from those in HCS-deficient flies.
Drosophila melanogaster has proven a useful model to study biological functions of HCS in chromatin remodeling, mediated by biotinylation of histones. We have generated novel tools for the investigation of biotin-dependent chromatin remodeling, including transgenic flies, human cells, and histone biotinylation site-specific antibodies. Now that these tools are in hand, and gene expression patterns and phenotypes of HCS deficiency have been characterized, an important next step is to identify the mechanisms involved in chromatin remodeling by biotin. Currently, we are pursuing this goal by employing a variety of techniques, including studies of protein-protein interactions, chromatin immunoprecipitation assays, Drosophila genetics, and biotin feeding studies.
Supplementary Material
Footnotes
A contribution of the University of Nebraska Agricultural Research Division, supported in part by funds provided through the Hatch Act. Additional support was provided by NIH grants DK 063945 and ES 015206-01, USDA grant 2006-35200-01540, and by NSF grants EPSCoR EPS-0346476, MCB 0131414, and MCB 6552870.
Supplemental Table S1 is available with the online posting of this paper at jn.nutrition.org.
The abbreviations used are: HCS, holocarboxylase synthetase; MCC, 3-methylcrotonyl-CoA carboxylase; PC, pyruvate carboxylase; SMVT, sodium-dependent multivitamin transporter.
Publisher's Disclaimer: This is an un-copyedited author manuscript that has been accepted for publication in The Journal of Nutrition, copyright © American Society for Nutrition (ASN). This manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, the ASN. The final copyedited article, which is the version of record, can be found at http://pubs.nutrition.org/. The ASN disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
LITERATURE CITED
- 1.Said HM. Recent advances in carrier-mediated intestinal absorption of water-soluble vitamins. Annu. Rev. Physiol. 2004;66:419–446. doi: 10.1146/annurev.physiol.66.032102.144611. [DOI] [PubMed] [Google Scholar]
- 2.Camporeale G, Zempleni J. In: Biotin. In: Present Knowledge in Nutrition. 9th ed. Bowman BA, Russell RM, editors. International Life Sciences Institute; Washington, D.C: 2006. pp. 314–326. [Google Scholar]
- 3.Narang MA, Dumas R, Ayer LM, Gravel RA. Reduced histone biotinylation in multiple carboxylase deficiency patients: a nuclear role for holocarboxylase synthetase. Hum. Mol. Genet. 2004;13:15–23. doi: 10.1093/hmg/ddh006. [DOI] [PubMed] [Google Scholar]
- 4.Wolffe A. Chromatin. 3th ed. Academic Press; San Diego, CA: 1998. [Google Scholar]
- 5.Chew YC, Camporeale G, Kothapalli N, Sarath G, Zempleni J. Lysine residues in N- and C-terminal regions of human histone H2A are targets for biotinylation by biotinidase. J. Nutr. Biochem. 2006;17:225–233. doi: 10.1016/j.jnutbio.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobza K, Camporeale G, Rueckert B, Kueh A, Griffin JB, Sarath G, Zempleni J. K4, K9, and K18 in human histone H3 are targets for biotinylation by biotinidase. FEBS J. 2005;272:4249–4259. doi: 10.1111/j.1742-4658.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camporeale G, Shubert EE, Sarath G, Cerny R, Zempleni J. K8 and K12 are biotinylated in human histone H4. Eur. J. Biochem. 2004;271:2257–2263. doi: 10.1111/j.1432-1033.2004.04167.x. [DOI] [PubMed] [Google Scholar]
- 8.Stanley JS, Griffin JB, Zempleni J. Biotinylation of histones in human cells: effects of cell proliferation. Eur. J. Biochem. 2001;268:5424–5429. doi: 10.1046/j.0014-2956.2001.02481.x. [DOI] [PubMed] [Google Scholar]
- 9.Landenberger A, Kabil H, Harshman LG, Zempleni J. Biotin deficiency decreases life span and fertility but increases stress resistance in Drosophila melanogaster. J. Nutr. Biochem. 2004;15:591–600. doi: 10.1016/j.jnutbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Kothapalli N, Sarath G, Zempleni J. Biotinylation of K12 in histone H4 decreases in response to DNA double strand breaks in human JAr choriocarcinoma cells. J. Nutr. 2005;135:2337–2342. doi: 10.1093/jn/135.10.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kothapalli N, Zempleni J. Biotinylation of histones depends on the cell cycle in NCI-H69 small cell lung cancer cells. FASEB J. 2005;19:A55. [abstract] [Google Scholar]
- 12.Camporeale G, Oommen AM, Griffin JB, Sarath G, Zempleni J. K12-biotinylated histone H4 marks heterochromatin in human lymphoblastoma cells. J. Nutr. Biochem. 2006 doi: 10.1016/j.jnutbio.2006.12.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki Y, Yang X, Aoki Y, Kure S, Matsubara Y. Mutations in the holocarboxylase synthetase gene HLCS. Human Mutation. 2005;26:285–290. doi: 10.1002/humu.20204. [DOI] [PubMed] [Google Scholar]
- 14.Wolf B, Grier RE, Allen RJ, Goodman SI, Kien CL. Biotinidase deficiency: An enzymatic defect in late-onset multiple carboxylase deficiency. Clin. Chim. Acta. 1983;131:273–281. doi: 10.1016/0009-8981(83)90096-7. [DOI] [PubMed] [Google Scholar]
- 15.Camporeale G, Giordano E, Rendina R, Zempleni J, Eissenberg JC. Drosophila holocarboxylase synthetase is a chromosomal protein required for normal histone biotinylation, gene transcription patterns, lifespan and heat tolerance. J. Nutr. 2006;136:2735–2742. doi: 10.1093/jn/136.11.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf B, Feldman GL. The biotin-dependent carboxylase deficiencies. Am. J. Hum. Genet. 1982;34:699–716. [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson BH, Oei J, Saudubray JM, Marsac C, Bartlett K, Quan F, Gravel R. The French and North American phenotypes of pyruvate carboxylase deficiency, correlation with biotin containing protein by 3H-biotin incorporation, 35S-streptavidin labeling, and northern blotting with a cloned cDNA probe. Am. J. Hum. Genet. 1987;40:50–59. [PMC free article] [PubMed] [Google Scholar]
- 18.Desviat LR, Perez-Cerda C, Perez B, Esparza-Gordillo J, Rodriguez-Pombo P, Penalva MA, Rodriguez De Cordoba S, Ugarte M. Functional analysis of MCCA and MCCB mutations causing methylcrotonylglycinuria. Mol. Genet. Metab. 2003;80:315–320. doi: 10.1016/S1096-7192(03)00130-6. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner MR, Dantas MF, Suormala T, Almashanu S, Giunta C, Friebel D, Gebhardt B, Fowler B, Hoffmann GF, et al. Isolated 3-methylcrotonyl-CoA carboxylase deficiency: evidence for an allele-specific dominant negative effect and responsiveness to biotin therapy. Am. J. Hum. Genet. 2004;75:790–800. doi: 10.1086/425181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balamurugan K, Vaziri ND, Said HM. Biotin uptake by human proximal tubular epithelial cells: cellular and molecular aspects. Am. J. Physiol. Renal Physiol. 2005;288:F823–831. doi: 10.1152/ajprenal.00375.2004. [DOI] [PubMed] [Google Scholar]
- 21.Manthey KC, Griffin JB, Zempleni J. Biotin supply affects expression of biotin transporters, biotinylation of carboxylases, and metabolism of interleukin-2 in Jurkat cells. J. Nutr. 2002;132:887–892. doi: 10.1093/jn/132.5.887. [DOI] [PubMed] [Google Scholar]
- 22.Scheerger SB, Zempleni J. Expression of oncogenes depends on biotin in human small cell lung cancer cells NCI-H69. Int. J. Vitam. Nutr. Res. 2003;73:461–467. doi: 10.1024/0300-9831.73.6.461. [DOI] [PubMed] [Google Scholar]
- 23.Crisp SERH, Camporeale G, White BR, Toombs CF, Griffin JB, Said HM, Zempleni J. Biotin supply affects rates of cell proliferation, biotinylation of carboxylases and histones, and expression of the gene encoding the sodium-dependent multivitamin transporter in JAr choriocarcinoma cells. Eur. J. Nutr. 2004;43:23–31. doi: 10.1007/s00394-004-0435-9. [DOI] [PubMed] [Google Scholar]
- 24.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc. R. Soc. Lond. B Biol. Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 25.Griffin JB, Rodriguez-Melendez R, Zempleni J. The nuclear abundance of transcription factors Sp1 and Sp3 depends on biotin in Jurkat cells. J. Nutr. 133:3409–3415. doi: 10.1093/jn/133.11.3409. [DOI] [PubMed] [Google Scholar]
- 26.Zempleni J, Trusty TA, Mock DM. Lipoic acid reduces the activities of biotin-dependent carboxylases in rat liver. J. Nutr. 1997;127:1776–1781. doi: 10.1093/jn/127.9.1776. [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute . StatView Reference. 3rd ed. SAS Publishing; Cary, NC: 1999. [Google Scholar]
- 28.Mardach R, Zempleni J, Wolf B, Cannon MJ, Jennings ML, Cress S, Boylan J, Roth S, Cederbaum S, et al. Biotin dependency due to a defect in biotin transport. J. Clin. Invest. 2002;109:1617–1623. doi: 10.1172/JCI13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


