Abstract
Objective
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is frequently complicated by severe infections and graft versus host disease (GVHD). Saliva contains many components of adaptive and innate immune response crucial for local host defenses. Changes in salivary constituents could reflect systemic processes such as immune reconstitution and development of GVHD that occur post-transplant. This study was an initial evaluation of salivary protein changes that occur after allo-HCT.
Patients and Methods
Serially collected saliva samples from 41 patients undergoing allo-HCT were evaluated. Changes in salivary proteome were initially examined by SELDI-TOF mass spectrometry. Individual protein changes were identified by 2-dimensional differential in-gel electrophoresis (2D-DIGE) with subsequent MS/MS sequencing and ELISA.
Results
Significant increases and decreases in multiple salivary proteins that lasted at least 2 months post-transplant were detected by SELDI-TOF mass spectrometry. Lactoferrin and secretory leukocyte protease inhibitor demonstrated elevations 1 month post-HCT that persisted at least 6 months. Secretory IgA (sIgA) levels were decreased 1 month post transplant, with recovery at approximately 6 months. Levels of salivary β2-microglobulin were elevated at 6 months and correlated with sIgA levels.
Conclusion
Allo-HCT is associated with long-term changes in several salivary proteins important for innate immune responses. These results support further studies on the association of salivary proteins with post-transplant complications including infections and GVHD.
Keywords: Hematopoietic stem cell transplantation, saliva, biomarkers, proteomics, SELDI-TOF-MS, innate immunity
Introduction
Despite tremendous advances, significant challenges remain that limit more widespread application of allogeneic hematopoietic stem cell transplantation (HCT). Non-myeloablative conditioning regimens have decreased treatment related mortality, but major complications such as graft versus host disease (GVHD) and severe infections still occur 1.
Many complications related to HCT directly or indirectly involve the oral cavity. Mucositis is the most common early oral complication of pre-transplant conditioning 2. Studies suggest that changes in oral microbiota and salivary function may influence the severity of oral mucositis 3. Microbial translocation through oral mucosa damaged by chemotherapeutic agents used for conditioning is one of the major causes of septic complications in this patient population 4. Mucosal sites, including the oropharynx, were the most commonly identified areas of colonization by Staphylococcus causing bacteremia in one analysis 5. Chronic GVHD affects oral mucosa with a frequency second only to skin, causing significant morbidity 6.
Homeostasis in the oral cavity is maintained by a complex network of innate and adaptive immune proteins and normal oral flora. Saliva contributes many important components to this system 7. Whole saliva, a mixture major and minor salivary gland secretions, gingival crevicular fluid, transudating plasma proteins and products of oral keratinocyte and microbial flora, contains components of both innate and adaptive immunity. Several families of proteins with antimicrobial, immunomodulatory and anti-inflammatory activity are present in saliva, including histatins, alpha and beta defensins, cystatins, cathelicidin, lactoferrin, lysozyme, and mucins 8. The fact that many of these proteins are highly conserved between various distant species provides evidence of their evolutionary importance 9. In addition to direct antimicrobial effects, some innate immune proteins can attract immature dendritic cells acting through the chemokine receptors 10 and activate antigen presenting cells by serving as endogenous ligands for toll like receptors 11.
Successful treatment and prevention of infections and GVHD requires a more complete understanding of oral cavity changes following HCT. Although the influence of HCT on salivary IgA has been reported 12, no studies have evaluated global changes in salivary proteome following HCT. Given the significance of salivary constituents in oral mucosal immunity, we examined changes in salivary proteome after HCT using several approaches, including SELDI-TOF (surface enhanced laser desorption ionization – time of flight) mass spectrometry in conjunction with 2-dimensional differential in-gel electrophoresis (2D-DIGE). We found numerous changes in the salivary proteome, some of which persisted for at least 6 months post-HCT.
Methods
Patient population
Saliva samples from forty one patients undergoing matched sibling peripheral blood mobilized HCT for a variety of indications at the NIH Clinical Center were evaluated in this study. All patients were consented to a protocol previously approved by the NIDCR Institutional Review Board. Patient and transplant characteristics are summarized in Table 1.
Table 1.
Patient population
| Sex and Age (years) | Male | N = 24, 42 ± 14 |
| Female | N = 17, 40 ± 13 | |
| Race | Caucasian | 23 |
| Hispanic | 11 | |
| Asian | 4 | |
| Black | 3 | |
| Primary Diagnosis | Hematologic Malignancy | 25 |
| Acute Leukemia | 7 | |
| Chronic Leukemia | 5 | |
| Lymphomas | 7 | |
| Multiple Myeloma | 4 | |
| Other | 2 | |
| Solid Malignancy | 13 | |
| Sarcomas | 5 | |
| Renal Cell Carcinoma | 3 | |
| Other | 5 | |
| Non-malignant Conditions | 3 | |
| Conditioning Regimen* | Non-Myeloablative | 29 |
| Cy + Flu | 22 | |
| Cy + Flu + Mel | 4 | |
| Other | 3 | |
| Myeloablative | 12 | |
| Cy + Flu + TBI | 12 | |
| GVHD Prophylaxis† | CSA | 19 |
| CSA + MTX | 11 | |
| CSA + Sirolimus | 5 | |
| Other | 6 | |
| Acute GVHD | Grade I | 4 |
| Grade II | 9 | |
| Grade III | 1 | |
| Grade IV | 0 |
Cy – Cyclophosphamide, Flu – Fludarabine, Mel – Melphalan, TBI – Total Body Irradiation
CSA – Cyclosporine, MTX - Methotrexate
Saliva collection and storage
To isolate the impact of transplant on salivary secretions, saliva from major salivary glands rather than whole saliva was collected. Stimulated submandibular/sublingual gland saliva was collected with a custom-made device from the oral orifice of Wharton’s duct after blocking the parotid ducts and applying 2% citric acid to the dorsal surface of the tongue every 30 seconds as described previously 13. Collection continued until about 1.5 to 2cc of saliva was collected. Samples were kept on ice at all times, aliquoted and frozen at −80° C until studied. Saliva was collected at 4 different time points: pre-HCT and approximately 1 month, 2 months and 6 months post-HCT.
Reagents
Cy dyes, immobilines IPG strips, α-cyano-4-hydroxycinnamic acid, and chemicals for 2D-gel electrophoresis were purchased from Amersham Pharmacia Biotech (Piscataway, NJ, USA). Trypsin and calibrant mixture for the 4700 Proteomics Analyzer were from Promega (Madison, WI, USA) and Applied Biosystems (Foster City, CA, USA), respectively. ProteinChips and sinapinic acid were purchased from Ciphergen (Fremont, CA, USA) and Sigma. ELISA kits were purchased to quantify lactoferrin (Calbiochem, La Jolla, CA, USA), secretory IgA (ALPCO Diagnostics, Windham, NH, USA), beta-2 microglobulin and SLPI (R & D systems, Minneapolis, MN, USA).
SELDI-TOF MS: Sample Preparation and Spectra Acquisition
Saliva was thawed, centrifuged, and applied to Q10 (strong anion exchange) and CM10 (weak cation exchange) ProteinChip surfaces (Ciphergen Biosystems, Inc., Fremont, CA) were that were first equilibrated with 150 ul of binding buffer (100 mM Tris-HCl, pH 9.0). Samples were kept at 4° C. Q10 and CM10 chips were selected because of the hydrophilic charged nature of the many salivary secretory proteins. Pilot experiments demonstrated that these affinity surfaces yielded optimal spectra quality. Individual saliva samples were mixed, applied and processed in duplicate as described previously 14. Samples were analyzed using SELDI-TOF-MS (Protein Biology System II, Ciphergen Biosystems). Spectra consisted of 130 averaged laser shots and were externally calibrated using All-in-One Protein Standard II (Ciphergen Biosystems, Inc). Data were processed using CiphergenExpress 3.1 Data Management Software. Spectra were mass aligned, baseline-subtracted using a smoothing feature and a fitting width of 4.5 times expected peak width, and then normalized by total ion current. Spectra were visually examined and poor quality spectra were excluded from further analysis. Peaks with signal to noise ratios > 3 and valley depths > 3 were automatically detected using the Biomarker Wizard Peak Picking Algorithm.
Two-dimensional - differential in-gel electrophoresis (2D-DIGE): Sample Preparation, Dye-labeling, Imaging, and Data Analysis
Given the large sample requirement for this assay (about 1mg of protein per gel) and the limited sample availability, saliva from 24 patients with the greatest volume of saliva available was pooled into 2 groups for analyses by 2D gel electrophoresis. These consisted of pre-transplant samples (pool one) and their corresponding 1 month samples (pool two). Salivary proteins were precipitated with absolute ethanol and resuspended in lysis buffer (7M urea, 2M thiourea, 4% CHAPS, and 15 mM Tris, pH 8.5) with 1/10 of original salivary volume. Protein concentration was determined by the Bradford method (BioRad, Carlsbad, CA). Equal amounts of pooled protein samples were minimally labeled with Cy3 and Cy5 fluorescent dyes on ice and in the dark and quenched with 10 mM lysine. For isoelectrofocusing (IEF), Cy dye labeled samples were mixed together, reconstituted with rehydration buffer, loaded on an immobilized pH 3–10 nonlinear gradient (IPG) strip of 7 cm, focused, equilibrated and separated as previously described 14. Cy dye images were collected using a 9400 Typhoon scanner (Amersham) and analyzed using DeCyder V 5.0 (Amersham) software for quantitative spot analysis14. Differentially expressed protein spots (with >1.5 fold difference in fluorescent intensity) were visualized using 0.1% Coumassie blue (SimpleBlue SafeStain kit, Invitrogen, Carlsbad, CA), with a modified Neuhoff solution 15, removed and analyzed with MALDI-TOF-MS.
Protein identification with MALDI-Time of Flight - Mass Spectrometry (MALDI-TOF-MS)
Spots identified by 2D-DIGE with differing intensities were manually excised from gels, prepared for MALDI-TOF-MS and analyzed as described previously 14. Database search was based on Mascot 2.0 (Matrix Science) individual MS/MS ion score and searched against SwissProt. Ion score >95% CI was considered significant.
ELISA
Lactoferrin, secretory IgA, β2-microglobulin, and secretory leukocyte protease inhibitor (SLPI) concentrations were determined from aliquots of individual patient samples used in the experiments above by ELISA following the manufacturers’ protocols.
Statistical Analysis
CiphergenExpress 3.1 Data Management Software was employed for SELDI-TOF-MS data peak detection and cluster generation. Raw peak intensity data were analyzed using the SAS 9.1 statistical package. Standardized mean-intensity values (three replicates) of each recognized peak at the molecular mass range of 1–200 kDa were analyzed by principle components to determine patterns among the M/Z intensity scores at baseline (pre-HCT treatment) and 1 and 2 months post HCT treatment, separately. The differences between the standardized intensity values for pre-HCT and the one and two month post-HCT treatment, separately, were used to investigate the effect of HCT using paired t-tests. In these analyses the p = 0.001 value was used for significance to adjust for multiple comparisons.
In multivariable analyses the following covariates were tested and found not to be significant for acute GVHD prediction at p=0.05: age, sex, race, diagnosis (solid tumor, hematologic malignancy, non-malignant), conditioning (myeloablative vs non-myeloablative), GVHD prophylaxis (cyclosporine only, cyclosporine + methotrexate, sirolimus containing regimens). Logistic regression models were used to investigate associations between acute GVHD and standardized mean intensity values for each identified peak for the one and two month post HCT treatment time-points, separately. No adjustment for multiple comparisons were made (p = 0.05 was used to define significance) due to the high correlations between intensities for pairs of M/Z values.
Linear regression models were employed to fit the post HCT standardized peak intensities to serum IgA and β2-microglobulin levels and acute GVHD status.
Results
Differentially expressed proteins detected by SELDI-TOF MS
To assess the general pattern of differential salivary protein expression after HCT, SELDI-TOF-MS was used to compare pre-transplant, 1 and 2 months post-transplant salivary samples. A total of 462 peaks and 278 peaks were detected using Q10 and CM10 chips respectively. Comparisons of peak intensities of the pre-transplant and 1 month post-transplant saliva samples found 42 from the CM10 chip and 36 from the Q10 chip differed at p<0.001 (Table 2). Next, pre-transplant and 2 month post-transplant peaks were compared. Notably, the same pattern was detected. Fifty-one of the 78 significantly different salivary peaks detected at one month were still significantly different two months post transplant. The directions of the peak changes (either increased or decreased) were always the same in the one and two-month post transplant samples (Table 2). Only 8 additional significantly different peaks (4 from CM10 and 4 from Q10) were found in the 2 months post-transplant samples (Table 2). When the peaks from the 1 and 2 months post HCT samples were compared, only one peak (Q10 chip, M/Z = 18847, p=0.00079) differed, indicating that the majority of differences persisted 2 months post-HCT.
Table 2.
SELDI-TOF MS peaks differing in 1 or 2 month post HCT salivary samples as compared to pre-transplant samples. Saliva was collected pre-HCT (mean ± SD 52 ± 40 days to HCT) and at 1 month (mean ± SD − 27 ± 6 days) and 2 months (mean ± SD − 59 ± 6) post HCT.
| CM10 Chip | Q10 Chip | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Changes at 1 and 2 months | Changes at 1 month only | Changes at 2 months only | Changes at 1 and 2 months | Changes at 1 month only | Changes at 2 months only | ||||||||||||
| M/Z* | P-value† | Change‡ | M/Z | P-value | Change | M/Z | P-value | Change | M/Z | P-value† | Change | M/Z | P-value | Change | M/Z | P-value | Change |
| 14513 | 1.10E-08 | ↓ | 94737 | 1.30E-05 | ↑ | 11481 | 2.30E-04 | ↑ | 14483 | 9.30E-08 | ↓ | 11773 | 1.30E-05 | ↑ | 7191 | 3.60E-04 | ↓ |
| 14509 | 1.30E-08 | ↓ | 30033 | 1.90E-05 | ↓ | 14735 | 5.50E-04 | ↑ | 14530 | 1.90E-07 | ↓ | 14445 | 1.60E-05 | ↓ | 104856 | 4.30E-04 | ↑ |
| 11760 | 2.00E-08 | ↑ | 11524 | 4.50E-05 | ↑ | 14930 | 7.10E-04 | ↑ | 11980 | 4.80E-07 | ↑ | 14058 | 2.20E-05 | ↓ | 5462 | 4.60E-04 | ↑ |
| 14526 | 2.20E-08 | ↓ | 43850 | 6.90E-05 | ↓ | 18252 | 8.70E-04 | ↑ | 15586 | 6.30E-07 | ↑ | 20521 | 2.70E-05 | ↑ | 20843 | 5.90E-04 | ↑ |
| 14497 | 3.00E-08 | ↓ | 13386 | 9.10E-05 | ↑ | 15791 | 7.30E-07 | ↑ | 14321 | 3.00E-05 | ↓ | ||||||
| 14572 | 3.10E-08 | ↓ | 38788 | 1.00E-04 | ↑ | 14405 | 4.70E-06 | ↓ | 28528 | 1.40E-04 | ↓ | ||||||
| 29740 | 2.50E-07 | ↓ | 80197 | 1.10E-04 | ↑ | 20714 | 2.00E-05 | ↑ | 7146 | 1.50E-04 | ↓ | ||||||
| 14463 | 3.10E-07 | ↓ | 2592 | 5.90E-04 | ↑ | 15683 | 3.10E-05 | ↑ | 28651 | 1.60E-04 | ↓ | ||||||
| 28985 | 8.90E-07 | ↓ | 28395 | 6.40E-04 | ↓ | 11666 | 4.20E-05 | ↑ | 28429 | 2.00E-04 | ↓ | ||||||
| 28649 | 1.80E-06 | ↓ | 27885 | 7.10E-04 | ↑ | 19717 | 4.50E-05 | ↓ | 21613 | 2.40E-04 | ↓ | ||||||
| 14285 | 2.40E-06 | ↓ | 19261 | 7.30E-04 | ↓ | 25041 | 4.60E-05 | ↓ | 10722 | 5.60E-04 | ↓ | ||||||
| 7264 | 3.00E-06 | ↓ | 79385 | 6.60E-05 | ↑ | 17872 | 5.80E-04 | ↑ | |||||||||
| 14278 | 3.10E-06 | ↓ | 39675 | 8.50E-05 | ↑ | 14236 | 5.90E-04 | ↓ | |||||||||
| 11691 | 4.60E-06 | ↑ | 78014 | 1.00E-04 | ↑ | 17702 | 6.90E-04 | ↑ | |||||||||
| 14397 | 5.00E-06 | ↓ | 19814 | 1.00E-04 | ↓ | 7778 | 7.10E-04 | ↑ | |||||||||
| 11946 | 8.20E-06 | ↑ | 25438 | 2.30E-04 | ↓ | 1136 | 7.10E-04 | ↑ | |||||||||
| 14308 | 1.30E-05 | ↓ | 43012 | 2.50E-04 | ↓ | 10752 | 8.00E-04 | ↓ | |||||||||
| 7165 | 4.20E-05 | ↓ | 14619 | 2.60E-04 | ↓ | 33308 | 8.90E-04 | ↑ | |||||||||
| 5864 | 4.30E-05 | ↑ | 77452 | 2.90E-04 | ↑ | ||||||||||||
| 14220 | 5.90E-05 | ↓ | 38758 | 5.20E-04 | ↑ | ||||||||||||
| 15149 | 9.50E-05 | ↑ | |||||||||||||||
| 42900 | 1.10E-04 | ↓ | |||||||||||||||
| 14350 | 1.30E-04 | ↓ | |||||||||||||||
| 7136 | 2.10E-04 | ↓ | |||||||||||||||
| 7194 | 3.00E-04 | ↓ | |||||||||||||||
| 18711 | 3.60E-04 | ↑ | |||||||||||||||
| 7146 | 4.00E-04 | ↓ | |||||||||||||||
| 17556 | 4.30E-04 | ↑ | |||||||||||||||
| 11041 | 4.80E-04 | ↑ | |||||||||||||||
| 15350 | 5.70E-04 | ↓ | |||||||||||||||
| 10007 | 5.70E-04 | ↑ | |||||||||||||||
M/Z – Mass/charge ratio;
p-values for pre-HCT vs. 1 month comparison;
↓- decreased post-HCT, ↑ - increased post-HCT
We examined the possibility that identified peaks could be influenced by conditioning regimen (non-myeloblative vs myeloablative). In this analysis, differences of means of individual peak intensities pre- and 1 month after transplant were compared between the two groups. There were no peaks different at the p<0.001. Only 6 peaks were identified in both analyses. We therefore conclude that the type of conditioning regimen does not contribute greatly to the differential expression of salivary proteins after allo-HCT but some contribution is certainly possible.
Intensities of thirty peaks (9 from CM10 Chips and 21 from Q10 Chips) were associated with subsequent diagnosis of acute GVHD at the level of significance p<0.05 (Table 3). In contrast, only one peak was associated with subsequent diagnosis of acute GVHD at p<0.05 when the same analysis was applied to pre-HCT time-point peak intensities (data not shown). If the associations with acute GVHD found in the 1 month post-transplant samples were simply secondary to random changes in salivary protein expression, we would expect to see roughly the same number of correlated peaks at all time points. Instead, 30 times more peaks were present in the 1 month samples that correlated with acute GVHD. Principal components analysis of the pre-HCT peak intensities produced 5 clusters (components) that accounted for 46%, 42% and 43% of the variation in the pre-HCT, 1 and 2 month post-HCT treatment data, respectively. Thus large correlations are inherent in the peak intensity values, effectively producing a small number of independent peak intensity comparisons.
Table 3.
Peaks associated with the development of the acute GVHD at 1 month post HCT.
| M/Z | Odds Ratio | Lower CL* | Upper CL | P-value |
|---|---|---|---|---|
| CM10 Chip – positively correlated peaks | ||||
| 5071 | 2.6950 | 1.1289 | 6.4335 | 0.026 |
| 29393 | 5.1159 | 1.2177 | 21.4927 | 0.026 |
| 4973 | 3.5664 | 1.1004 | 11.5584 | 0.034 |
| 3237 | 2.7720 | 1.0702 | 7.1802 | 0.036 |
| 27473 | 3.6862 | 1.0183 | 13.3438 | 0.047 |
| Negatively correlated peaks | ||||
| 5110 | 0.1639 | 0.0406 | 0.6613 | 0.011 |
| 9420 | 0.0047 | 0.0000 | 0.5978 | 0.030 |
| 3375 | 0.0689 | 0.0056 | 0.8489 | 0.037 |
| 5796 | 0.1797 | 0.0339 | 0.9521 | 0.044 |
| Q10 Chip – positively correlated peaks | ||||
| 5944 | 3.5407 | 1.2211 | 10.2669 | 0.020 |
| 1031 | 2.8833 | 1.1479 | 7.2422 | 0.024 |
| 1046 | 2.7268 | 1.1278 | 6.5928 | 0.026 |
| 16714 | 3.1474 | 1.1176 | 8.8635 | 0.030 |
| 1536 | 2.4182 | 1.0706 | 5.4619 | 0.034 |
| 4964 | 3.5226 | 1.0958 | 11.3237 | 0.035 |
| 1369 | 2.3934 | 1.0648 | 5.3797 | 0.035 |
| 70487 | 3.9769 | 1.1013 | 14.3608 | 0.035 |
| 1116 | 2.9271 | 1.0401 | 8.2376 | 0.042 |
| 1146 | 2.1521 | 1.0076 | 4.5967 | 0.048 |
| 3396 | 15.4892 | 1.0211 | 234.9536 | 0.048 |
| Negatively correlated peaks | ||||
| 1905 | 0.0002 | 0.0000 | 0.3639 | 0.026 |
| 9442 | 0.0018 | 0.0000 | 0.2398 | 0.011 |
| 35146 | 0.3671 | 0.1492 | 0.9034 | 0.029 |
| 19537 | 0.3256 | 0.1138 | 0.9309 | 0.036 |
| 19620 | 0.2915 | 0.0904 | 0.9392 | 0.039 |
| 49490 | 0.3576 | 0.1346 | 0.9500 | 0.039 |
| 1577 | 0.3914 | 0.1603 | 0.9560 | 0.040 |
| 18847 | 0.2102 | 0.0470 | 0.9393 | 0.041 |
| 5370 | 0.2472 | 0.0641 | 0.9536 | 0.042 |
| 3494 | 0.1373 | 0.0200 | 0.9441 | 0.044 |
CL – confidence limit
Differentially expressed proteins detected by 2D-DIGE
2D-DIGE was used to further analyze salivary proteins differentially expressed between 1 month post-transplant and pre-transplant since these time-points had the greatest differences by SELDI-TOF-MS. Fluorescence intensity differed by at least 1.5 fold (either increased or decreased) between the two groups (Figure 1) for 13 protein spots. All showed increased fluorescence at 1 month, with the exception of one at ~ 12 kDa. After Coomassie staining, eight protein spots that differed in intensity by at least 1.5 fold were visualized. Four were subsequently identified with high degree of confidence (Figure 1, Table 4). These were lactoferrin (increased 1.5 fold), cystatin SN (increased 1.8 to 2.5 fold), albumin (increased 1.5–1.7 fold) and salivary amylase (increased 1.7–2.1 fold). The unidentified spots could have either contained insufficient amounts of protein or belonged to proteins with fragmentation patterns unfavorable for reliable MS/MS sequencing (e.g. proline-rich proteins).
Figure 1.
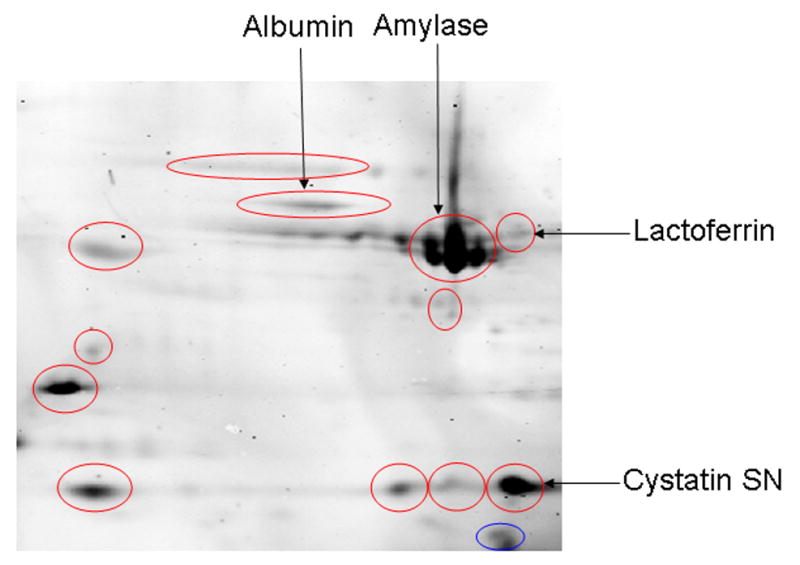
2D-Differential in-Gel Electrophoresis (2D-DIGE) of salivary proteins pre-transplant and at 1 month post-transplant. Spots that were increased ≥ 1.5 fold in the 1 month samples are indicated by the red circles, those decreased ≥ 1.5 fold by blue circles. After Coomassie staining, ten of these thirteen spots that differed were visualized and removed for MALDI-TOF MS/MS. The four that were identified are labeled on the figure. The other spots were not identified positively.
Table 4.
Differentially expressed proteins identified by 2D-DIGE, MALDI-TOF MS/MS, and Mascot database search.
| Protein | Nominal Mass (Mr) | Calculated pI* | Number of Tryptic Peptides | % Sequence Coverage | Mowse Score |
|---|---|---|---|---|---|
| Lactoferrin | 80242 | 8.50 | 3 | 5 | 86 |
| Cystatin SN (SA-1) | 16579 | 6.82 | 3 | 32 | 240 |
| Albumin | 46442 | 5.77 | 2 | 7 | 70 |
| Salivary Amylase | 56859 | 8.82 | 8 | 25 | 593 |
pI – isoelectric point
Measurement of salivary proteins by ELISA
Lactoferrin
One protein with differential expression by 2D-DIGE at 1 month post-transplant was subsequently identified as lactoferrin. To confirm these changes, lactoferrin levels were determined by ELISA in the individual samples used for the 2D-DIGE gels. There was a trend to increased lactoferrin concentration in the 1 month samples when compared with the pre-transplant values (5.63 ± 0.72 vs. 4.26 ± 0.54 mcg/ml, p=0.076, N=21). To determine whether lactoferrin elevations persisted, levels were determined in samples collected from the same patients 6 months post-transplant. The concentration of this protein was even higher at six months (6.50 ± 0.70 mcg/ml, p=0.006, Fig. 2A).
Figure 2.

Salivary proteins as measured by ELISA that differed significantly between pre-transplant and 1 month-post samples or pre-transplant and 6 months (mean ± SD − 191 ± 25 days) post-transplant: These were lactoferrin (A), secretory leukocyte protease inhibitor (SLPI, Panel B), secretory IgA (C) and β2-microglobulin (D). Individual p values are indicated on the panels.
Secretory Leukocyte Protease Inhibitor (SLPI)
Many differentially expressed peaks were detected using anionic surface SELDI chip (CM10), which preferentially binds cationic domains. Several salivary innate defense proteins are cationic species. The M/Z of one peak with highly increased expression from the CM10 Chip at both 1 month and 2 month post transplant (11.7 kDa, p<2E-07, Table 2) corresponds to the molecular weight of SLPI, a salivary cationic host defense protein 16. Therefore, concentrations of salivary SLPI were measured by ELISA. Compared to pre-transplant levels, salivary SLPI levels were elevated at 1 month post HCT (3.48 ± 0.36 vs 2.26 ± 0.23 ng/ml, p=0.016, N=27, Fig. 5D) and remained elevated 6 months post HCT (3.30 ± 0.50 ng/ml, p=0.048, Fig. 2B).
Salivary secretory IgA
Prior studies reporting decreased of salivary secretory IgA post HCT were done in the setting of fully myeloablative HCT 12. Since the majority of patients in this cohort (70%) received non-myeloablative transplants, concentrations of salivary secretory IgA by ELISA were determined. Secretory IgA concentrations were decreased 1 month post HCT compared to pre-transplant values (68.8 ± 10.1 vs 101.3 ± 12.7 mcg/ml, p=0.019, N=27), but returned to pre-transplant levels 6 months post transplant (117.7 ± 16.7 mcg/ml p=0.49, Fig. 2C). No significant differences in salivary IgA levels were observed between myeloablative and non-myeloablative conditioning regimens (data not shown).
Since acute GVHD is associated with impaired immune reconstitution following allogeneic HCT, the association of salivary IgA levels to acute GVHD was determined. In a multivariable analysis, prior diagnosis of acute GVHD was the only covariate associated with significantly lower salivary IgA values at 6 months post transplant (F value = 4.10, p=0.05).
β2-microglobulin
Serum β2-microglobulin level is a marker of lymphocyte turnover 17. Increased levels of salivary β2-microglobulin have been associated with lymphocytic infiltration of the salivary glands in Sjögren’s syndrome 18. Since similar salivary infiltrates have been associated with the development of chronic GVHD 19, levels of salivary β2-microglobulin were determined. While β2-microglobulin concentration did not increase significantly 1 month after HCT (0.821 ± 0.131 vs. 0.701 ± 0.104 mcg/ml, p=0.43, N=24), the concentration was significantly higher 6 months post-HCT (1.239 ± 0.181mcg/ml, p=0.008, Fig. 2D). β2-microglobulin levels correlated with sIgA 6 months post-transplant (Pearson’s r=0.47, p=0.0163).
Discussion
Recently, saliva-based biomarker assays have received significant attention given the ease and non-invasiveness of saliva collection. The recent consensus statement supported development of salivary biomarkers predictive of the onset or progression of chronic GVHD 6. The goal of this study was to investigate the impact of HCT on salivary proteins, which constitute a large portion of the mucosal immune system in the oral cavity. Submandibular/sublingual (SM/SL) saliva, rather than whole saliva that contains many non-salivary proteins, was evaluated in this study so that the effects of transplant on salivary secretions could be ascertained. Specimens were collected serially from the same patients pre-and post-transplant to control for individual variation. Complementary proteomic techniques of SELDI-TOF MS, 2D-DIGE and selective ELISA assays were employed for evaluation. Using this approach, multiple alterations in the proteome of SM/SL saliva were identified that persisted at least two months after transplant. Three of the identified differentially expressed proteins –lactoferrin, cystatin-SN and SLPI - are known innate immune proteins 8.
SELDI-TOF-MS is applied commonly in the discovery phase of proteomics to assess global changes in proteome under various conditions. The advantages of this technique include high sensitivity and throughput 20. Serum and urine proteomic patterns have been previously reported to discriminate between acute GVHD and other complications of HCT 21,22. We evaluated the possibility that changes in salivary proteins as detected by SELDI-TOF-MS might be associated with the development of acute GVHD, a condition that involves multiple mucosal tissues, and found intensity changes of several salivary proteins one month post-HCT were associated with the subsequent development of acute GVHD. While this finding should be interpreted with caution due to the preliminary nature of the current study, relatively small sample size and the liberal level of significance (0.05), it provides initial evidence that assays of salivary proteins could be useful for prediction of GVHD.
Using MALDI-MS/MS sequencing, four proteins of the thirteen differently expressed protein spots detected by 2D-DIGE in 1 month post-transplant samples were identified. These were lactoferrin, cystatin SN, albumin and salivary amylase. We were not able to identify others. While 2D-DIGE allows comparison of samples from two or more different populations on a single 2-D gel23, a relatively large amount of protein is required per spot for accurate MS identification. Also, salivary proteins of low molecular weight or those having highly acidic or basic pI – properties are not easily identified by MALDI-MS/MS.
Lactoferrin is a highly conserved protein present in multiple mucosal glandular secretions, keratinocytes and neutrophil secondary granules. Lactoferrin has broad spectrum antimicrobial activity related to its iron-binding capacity and membrane disrupting cationic domains. Additionally, lactoferrin has immunomodulatory properties, such as inhibition of Langerhans cell migration and suppression of allergic inflammation in the skin 24,25. As host dendritic cells play a key role in initiation of allogeneic immune responses 26, the role of this innate immune defense protein in the development of GVHD should be investigated.
We hypothesized that salivary changes similar to those found in Sjögren’s syndrome may occur post HCT, as salivary glands are frequently targeted by chronic GHVD 19. The continued increase of salivary lactoferrin at patients 6 months post transplant (Figure 2A) reflect salivary infiltrates in a portion of these patients. Elevated salivary lactoferrin is well described in Sjögren’s syndrome, as is salivary β2-microglobulin, a part of the MHC-I complex highly expressed on lymphocytes 14,27. Increased levels of salivary β2-microglobulin were positively associated with the presence of salivary gland lymphocytic infiltrate in Sjogren’s in earlier studies 18. While β2-microglobulin levels were significantly increased 6 months following HCT in our cohort (Figure 2C), the elevations were modest and correlated with sIgA levels. Since secretory IgA levels generally recover around this time 12, increased salivary β2-microglobulin levels may relate to the repopulation of the salivary glands with B-cells and plasma cells rather than chronic GVHD.
In addition to lactoferrin, two other elevated proteins identified in this study have antimicrobial and immunomodulatory properties. Salivary cystatin SN (SA-1) belongs to a family of widely expressed cysteine protease inhibitors with activity against bacterial, viral and protozoal organisms 28. Another protein elevated post-HCT in our study, secretory leukocyte protease inhibitor (SLPI), was also originally noted for its ability to inhibit proteolytic enzymes. SLPI has direct antimicrobial properties and is active against several bacterial and fungal pathogens including E. coli and Aspergillus 16. Additionally, SLPI has other functions 29,30, including modulation of macrophage responses to LPS 31. SLPI knock-out animals demonstrate increased septic shock mortality in response to intra-peritoneal LPS injection 32. Since LPS mediated inflammatory cytokine release plays a major role in the initiation phase of the acute GVHD 33, SLPI and other endogenous modulators of response to LPS could prove important for acute GVHD pathogenesis.
Increased LPS access to lamina propria macrophages and systemic circulation in the setting of acute GVHD is attributable in large degree to increased GI mucosal permeability 34. In our study, elevated salivary albumin in the 1 month post transplant group may have been related to leakage of plasma secondary to increased oral mucosa permeability. Increased mucosal permeability could also be compounded by low secretory IgA levels, as mice lacking secretory component demonstrated increased mucosal permeability and secondarily decreased secretory IgA 35. The significance of increased amylase expression following HCT is unclear. Amylase has some antibacterial properties in vitro thought to be related to production of starch products that are toxic to some species 36. It does bind certain streptococcal species in the oral cavity37.
In summary, this study is the first to demonstrate global changes in the salivary proteome after allogeneic HCT. Many salivary proteins have multiple functions in innate host defense and immunoregulation, and changes in their profiles could impact clinical outcomes after transplant. Polymorphisms in genes associated with changes of several innate host defense proteins have been linked to increased incidence of inflammatory conditions including GVHD 38–41. Innate host defense proteins are produced by glandular secretions and cells lining the GI and respiratory tract, and are independent of the peripheral blood cell counts. Therefore, their roles in transplant related complications such as post-transplant infections and GVHD warrants further investigation.
Acknowledgments
We would like to thank Drs. Ram Srinivasan, Gauri Alvarez and Bipin Savani, Rebecca Babb, Jeanne Odom, Michael Krumlauf, Kate Castro, Lisa Cook, Catalina Ramos, Cindy Love, Paula Layton, Laura Wisch, Laura Musse, and Beth Link for the help with clinical data and patient care coordination, Dr. Peter Munson for help with statistical analysis, Drs. Bruce Baum, Gabor Illei and Frances Hakim for critical reading of the manuscript and helpful discussions, and Sherry Gollins and Sharon Mitchell for excellent data management and patient scheduling. Finally, we would like to sincerely thank all the patients who participated in this study. We declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Eisen D, Essell J, Broun ER. Oral cavity complications of bone marrow transplantation. Semin Cutan Med Surg. 1997;16:265–272. doi: 10.1016/s1085-5629(97)80015-6. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JB, Tsang AH, Warkentin D, Ship JA. The role of salivary function in modulating chemotherapy-induced oropharyngeal mucositis: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:39–44. doi: 10.1067/moe.2002.126018. [DOI] [PubMed] [Google Scholar]
- 4.Ruescher TJ, Sodeifi A, Scrivani SJ, Kaban LB, Sonis ST. The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer. 1998;82:2275–2281. [PubMed] [Google Scholar]
- 5.Costa SF, Miceli MH, Anaissie EJ. Mucosa or skin as source of coagulase-negative staphylococcal bacteraemia? Lancet Infect Dis. 2004;4:278–286. doi: 10.1016/S1473-3099(04)01003-5. [DOI] [PubMed] [Google Scholar]
- 6.Schultz KR, Miklos DB, Fowler D, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12:126–137. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Mandel ID. The role of saliva in maintaining oral homeostasis. J Am Dent Assoc. 1989;119:298–304. doi: 10.14219/jada.archive.1989.0211. [DOI] [PubMed] [Google Scholar]
- 8.Amerongen AV, Veerman EC. Saliva--the defender of the oral cavity. Oral Dis. 2002;8:12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- 9.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 11.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 12.Dens F, Boogaerts M, Boute P, Declerck D, Demuynck H, Vinckier F. Quantitative determination of immunological components of salivary gland secretion in transplant recipients. Bone Marrow Transplant. 1996;17:421–423. [PubMed] [Google Scholar]
- 13.Fox PC, van der Ven PF, Sonies BC, Weiffenbach JM, Baum BJ. Xerostomia: evaluation of a symptom with increasing significance. J Am Dent Assoc. 1985;110:519–525. doi: 10.14219/jada.archive.1985.0384. [DOI] [PubMed] [Google Scholar]
- 14.Ryu OH, Atkinson JC, Hoehn GT, Illei GG, Hart TC. Identification of parotid salivary biomarkers in Sjogren’s syndrome by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatology (Oxford) 2006 doi: 10.1093/rheumatology/kei212. [DOI] [PubMed] [Google Scholar]
- 15.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 16.Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006;110:21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- 17.Child JA, Kushwaha MR. Serum beta 2-microglobulin in lymphoproliferative and myeloproliferative diseases. Hematol Oncol. 1984;2:391–401. doi: 10.1002/hon.2900020409. [DOI] [PubMed] [Google Scholar]
- 18.Michalski JP, Daniels TE, Talal N, Grey HM. Beta2 microglobulin and lymphocytic infiltration in Sjogren’s syndrome. N Engl J Med. 1975;293:1228–1231. doi: 10.1056/NEJM197512112932404. [DOI] [PubMed] [Google Scholar]
- 19.Sale GE, Shulman HM, Schubert MM, et al. Oral and ophthalmic pathology of graft versus host disease in man: predictive value of the lip biopsy. Hum Pathol. 1981;12:1022–1030. doi: 10.1016/s0046-8177(81)80260-2. [DOI] [PubMed] [Google Scholar]
- 20.Tang N, Tornatore P, Weinberger SR. Current developments in SELDI affinity technology. Mass Spectrom Rev. 2004;23:34–44. doi: 10.1002/mas.10066. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan R, Daniels J, Fusaro V, et al. Accurate diagnosis of acute graft-versus-host disease using serum proteomic pattern analysis. Exp Hematol. 2006;34:796–801. doi: 10.1016/j.exphem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser T, Kamal H, Rank A, et al. Proteomics applied to the clinical follow-up of patients after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:340–349. doi: 10.1182/blood-2004-02-0518. [DOI] [PubMed] [Google Scholar]
- 23.Van den Bergh G, Arckens L. Fluorescent two-dimensional difference gel electrophoresis unveils the potential of gel-based proteomics. Curr Opin Biotechnol. 2004;15:38–43. doi: 10.1016/j.copbio.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Ward PP, Uribe-Luna S, Conneely OM. Lactoferrin and host defense. Biochem Cell Biol. 2002;80:95–102. doi: 10.1139/o01-214. [DOI] [PubMed] [Google Scholar]
- 25.Kimber I, Cumberbatch M, Dearman RJ, Headon DR, Bhushan M, Griffiths CE. Lactoferrin: influences on Langerhans cells, epidermal cytokines, and cutaneous inflammation. Biochem Cell Biol. 2002;80:103–107. doi: 10.1139/o01-227. [DOI] [PubMed] [Google Scholar]
- 26.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 27.Konttinen YT, Kulomaa M, Malmstrom M, Kilpi A, Reitamo S. Lactoferrin in Sjogren’s syndrome. Arthritis Rheum. 1984;27:462–467. doi: 10.1002/art.1780270416. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson DP. Salivary (SD-type) cystatins: over one billion years in the making--but to what purpose? Crit Rev Oral Biol Med. 2002;13:485–508. doi: 10.1177/154411130201300606. [DOI] [PubMed] [Google Scholar]
- 29.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashcroft GS, Lei K, Jin W, et al. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–1153. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- 31.Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura A, Mori Y, Hagiwara K, et al. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J Exp Med. 2003;197:669–674. doi: 10.1084/jem.20021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor- alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 35.Johansen FE, Pekna M, Norderhaug IN, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bortner CA, Miller RD, Arnold RR. Effects of alpha-amylase on in vitro growth of Legionella pneumophila. Infect Immun. 1983;41:44–49. doi: 10.1128/iai.41.1.44-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jespersgaard C, Hajishengallis G, Russell MW, Michalek SM. Identification and characterization of a nonimmunoglobulin factor in human saliva that inhibits Streptococcus mutans glucosyltransferase. Infect Immun. 2002;70:1136–1142. doi: 10.1128/IAI.70.3.1136-1142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 39.Wehkamp J, Fellermann K, Herrlinger KR, et al. Human beta-defensin 2 but not beta-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2002;14:745–752. doi: 10.1097/00042737-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Holler E, Rogler G, Brenmoehl J, et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood. 2006;107:4189–4193. doi: 10.1182/blood-2005-09-3741. [DOI] [PubMed] [Google Scholar]
- 41.Holler E, Rogler G, Herfarth H, et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104:889–894. doi: 10.1182/blood-2003-10-3543. [DOI] [PubMed] [Google Scholar]


