Abstract
The mucosal epithelium secretes a variety of antimicrobial peptides that act as part of the innate immune system to protect against invading microbes. Here, we describe the functional properties of human defensin 5, the major antimicrobial peptide produced by Paneth cells in the ileum, in relation to its structure. The antimicrobial activity of human defensin 5 against Escherichia coli proved to be independent of its structure, whereas the unstructured peptide showed greatly reduced antimicrobial activity against Staphylococcus aureus. We find that human defensin 5 binds to the cell membrane of intestinal epithelial cells and induced secretion of the chemokine IL-8 in a concentration- and structure-dependent fashion. Incubation of human defensin 5 in the presence of tumor necrosis factor alpha further increased IL-8 secretion synergistically, suggesting that human defensin 5 may act as a regulator of the intestinal inflammatory response.
Keywords: human defensin 5, intestinal epithelium, interleukin 8, TNFα
Introduction
Epithelial cells of the mucosa form a barrier between the gut lumen and underlying host tissues. In addition to this barrier function, epithelial cells perform a key role in host innate and adaptive responses, secreting of a wide range of immunomodulatory molecules [1, 2]. Paneth cells are specialized ileal epithelial cells located at the crypt base in close vicinity of multipotent stem cells and fulfill a crucial role in innate immunity. They are a source of several antimicrobial enzymes such as lysozyme and group IIA phospholipase A2 (PLA2) as well as the antimicrobial peptides human defensin 5 and 6 (HD-5 and HD-6), which are stored in secretory granules [3, 4].
Defensins are small, cationic peptides with a characteristic β-sheet-rich structure stabilized by three internal disulfide bonds [5]. Based on the connectivity of the six cysteine residues, human defensins are classified into α and β subfamilies [6–8]. In humans, six α-defensins have been described: HD-5 and HD-6 and the human neutrophil peptides 1–4 (HNP1-4) expressed predominantly in neutrophils and natural killer cells [9]. Both the α- and β-defensin gene families evolved from an ancestral β-defensin gene and distinct clusters of both families are found adjacent on the chromosomal maps of all mammals expressing α- and β-defensins [10, 11].
Murine intestinal epithelium expresses at least 20 isoforms of α-defensins, termed cryptdins [12, 13]. Procryptdins are processed to mature peptides by the matrix metalloproteinase matrilysin, which is co-expressed within the Paneth cell secretory granules [14]. In contrast, HD-5 is stored in its pro-form and is further processed to the mature peptide after secretion. Pro-forms of HD-5 can be processed in vitro by trypsin, which is also expressed in Paneth cells [15]. In addition to their antimicrobial properties, defensins have chemokine-like activities [16]. Members of both α- and β-defensins act as chemotactic attractants for human monocytes and subsets of dendritic cells and T cells [17, 18], however, their effect on intestinal epithelial cells is not very well characterized. Here, we examine the antibacterial activity and the interaction with intestinal epithelial cells of HD-5 in relation to its structure.
Materials and methods
Materials
Chemicals used for solid phase peptide synthesis were obtained as described [19]. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 were from Microbiologics (St. Cloud, MN). Caco-2 cell line was obtained from the American Type Culture Collection (Manassas, Va).
Solid phase peptide synthesis
Chemical synthesis of HD-5 and HD-5Abu, a linear, unstructured form of HD-5 in which the six cysteine residues are replaced by isosteric α-aminobutyric acid (Abu) was carried out as described [19]. Folding of HD-5 was carried out as described [19]. The molecular mass of the peptides was verified by electrospray ionization mass spectrometry (ESI-MS) as described previously [19]. 5-carboxyltetramethylrhodamine (Molecular Probes, Eugene, Or) was coupled to HD-5 as follows: 2.0 mg HD-5 was dissolved in 1.0 ml 0.1 M NaHCO3, pH 8.3, 0.2 ml of 5-carboxyltetramethylrhodamine (10 mg/ml in DMSO) and 40 μl di-isopropyl-ethylamine (DIEA) were then added. After stirring for 2 hrs at room temperature, the reaction mixture was filtered and purified by reverse phase HPLC. The molecular mass was verified by ESI-MS as described above.
Antibacterial activity assay
The antibacterial activity of HD-5 and HD-5Abu against E. coli ATCC 25922 and S. aureus ATCC 29213 was carried out in a 96-well turbidimetric assay as described previously [20].
Evaluation of IL-8 secretion by Caco-2 cells
Subconfluent monolayers of Caco-2 cells were maintained in RPMI 1640 medium (Gibco), supplemented with 10% FBS (Valley Biomedical, Winchester, Va), 2 mM L-glutamine (Quality Biological, Gaithersburg, MD), 20 mM HEPES, 1 × nonessential amino acids, 1 mM sodium pyruvate and 5% Penicillin/Streptomycin (Sigma) in a humidified incubator at 37 °C with 5% CO2. Caco-2 cells were used between passages 35–42. Caco-2 cells were plated at a density of 4×104 cells/cm2 in a 96-well plate 48 hours before use. The cells were gently washed twice with serum-free medium and incubated for a further 18 hours in serum-free medium containing the peptides at a final concentration of 50 or 100 μg/ml. In the presence of serum, the induction of IL-8 secretion was not observed (not shown). Human recombinant tumor necrosis factor alpha (Sigma; 100 ng/ml) was included during incubation with the peptides (100 μg/ml) as indicated. The culture supernatant was collected for measurement of IL-8 using the Luminex-100 system (Bio-rad Laboratories).
Confocal Microscopy
Caco-2 cells (104 cells) were cultured on glass cover slips as described above for 24–48 hours. The cells were washed twice in serum-free medium and incubated with rhodamine-HD5 (10 μg/ml) for three hours. After incubation, cells were washed twice with Hanks’ Balanced Salt Solution (HBSS). The localization of rhodamine-HD5 on Caco-2 cells was visualized using a Zeiss Laser Scanning Microscope (LSM) 510 system (Carl Zeiss MicroImaging Inc., Thornwood, NY). Fluorescence was excited using a helium-neon laser (543 nm). Emission was passed through a 560 nm long-pass filter prior to acquisition. Optical sections were 1 μm thick.
Results
Chemical synthesis of HD-5 peptides
The HD-5 structure involves three intra-molecular disulfide bonds [19]. To determine the structure of HD-5 in relation to its function, a HD-5 derivative peptide was synthesized, in which the six cysteine residues were replaced with L-α-aminobutyric acid (HD-5Abu), thus preventing the formation of disulfide linkage while leaving the peptide sequence otherwise unaltered. Folded and purified HD-5 and purified HD-5Abu were analyzed on C18 RP-HPLC (Fig. 1, A and B). HD-5 was less hydrophobic than HD-5Abu, as indicated by their relative retention time on C18 RP-HPLC. The molecular mass of both peptides was confirmed by ESI-MS (Fig. 1, C and D). The observed molecular masses of 3582.0±0.5 Da for HD-5 and 3480.7±0.3 Da for HD-5Abu agree with the calculated average isotopic values of 3582.2 and 3480.2 Da respectively.
Figure 1.
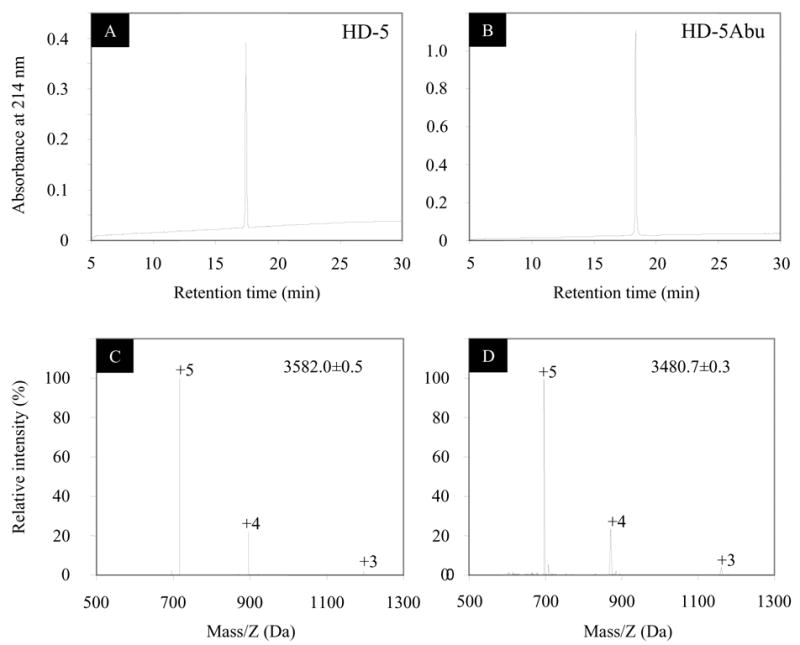
Folded and purified HD-5 and HD-5Abu analyzed by reversed phase high-performance liquid chromatography (RP-HPLC) and electrospray ionization mass spectrometry (ESI-MS). The HPLC analysis was carried out at 40 °C using a linear gradient of 15–60% (solvent A: water + 0.1% TFA; solvent B: acetonitrile + 0.1% TFA) at a flow rate of 1 ml/min over 30 min. The determined molecular masses were within experimental error of the expected values based on calculations of the average isotopic compositions.
Antimicrobial activity of HD-5 peptides
The antimicrobial activity of both peptides was examined against E. coli ATCC 25922 and S. aureus ATCC 29213 (Fig. 2A). As described previously [20], HD-5 efficiently killed both bacterial strains and, at comparable peptide concentration, proved more toxic towards S. aureus as compared to E. coli. At the highest peptide concentration tested (125 μg/ml), S. aureus appeared unable to recover from the 2 hour incubation with HD-5. HD-5Abu was comparable to HD-5 in antimicrobial activity towards E. coli, and was even slightly more efficient in killing at higher concentrations. Surprisingly, killing of S. aureus by HD-5Abu was four to five orders of magnitude less efficient than killing by HD-5 at comparable peptide concentration.
Figure 2.
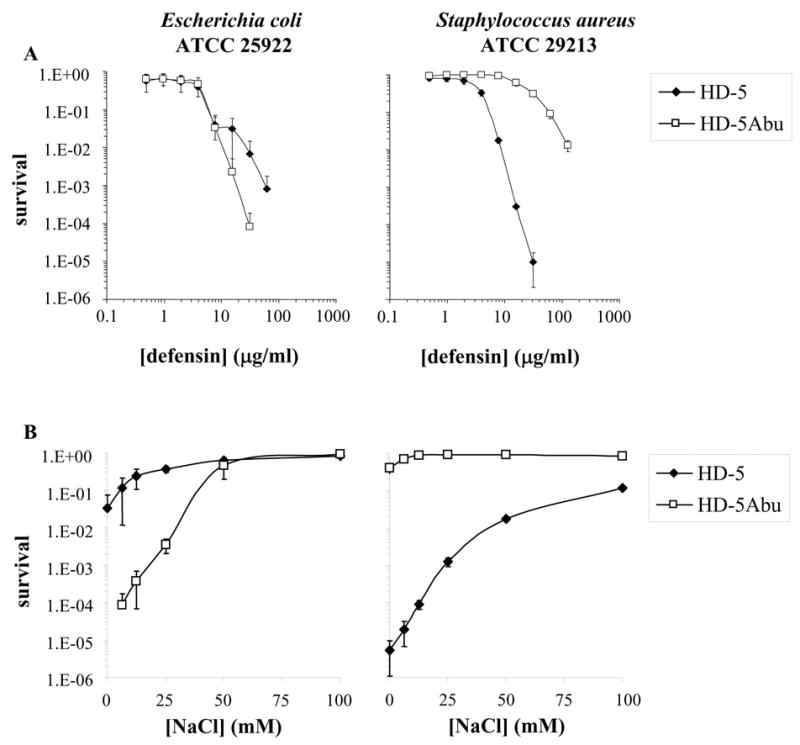
(A) Survival curves of E. coli ATCC 25922 (left) and S. aureus ATCC 29213 (right) exposed to HD-5 (filled symbols) or HD-5Abu (open symbols). Strains were exposed to the peptides at concentrations varying twofold from 0.12 to 125 μg/ml. (B) Strains were exposed to fixed peptide concentrations (100 μg/ml for E. coli; 50 μg/ml for S. aureus) in the absence or presence of the indicated concentrations of sodium chloride. Each curve is the mean of three separate experiments. Points scored as zero survival could not be plotted.
To evaluate the salt dependence of HD-5 bacterial killing, the antimicrobial assay was performed at increasing sodium chloride concentrations (Fig. 2B). For these experiments, a fixed peptide concentration of 100 μg/ml was used against E. coli and 50 μg/ml against S. aureus. Increasing salt concentration reduced the antimicrobial activity of HD-5. At the highest salt concentration tested (100 mM), HD-5 was still significantly toxic against S. aureus, whereas antibacterial activity against E. coli was almost completely inhibited, even at twice the concentration of peptide. Against E. coli, the bactericidal activity of HD-5Abu was significantly inhibited only at a concentration of 50 mM NaCl, inhibition was not increased further by higher salt concentration. Against S. aureus, the antibacterial activity of HD-5Abu was completely inhibited even at low salt concentrations. Taken together, these data indicate that effective killing of E. coli by HD-5 is structure-independent or alternatively, more sequence-driven. Efficient activity against S. aureus requires the peptide to be folded and was less inhibited by increasing salt concentrations.
Interaction of HD-5 peptides with intestinal epithelial cells
To examine the interaction of HD-5 with Caco-2 cells, rhodamine-labeled HD-5 was synthesized. The molecular mass of the purified peptide was verified by ESI-MS to be 3994.6±0.6 Da, in good agreement with the calculated value of 3994.2 Da (not shown). Following incubation of Caco-2 cells with the labeled peptide, localization of HD-5 was visualized by fluorescence confocal microscopy (Fig. 3). While localization of HD-5 was predominantly seen at the surface of the Caco-2 cells, internalization of the probe was also observed.
Figure 3.
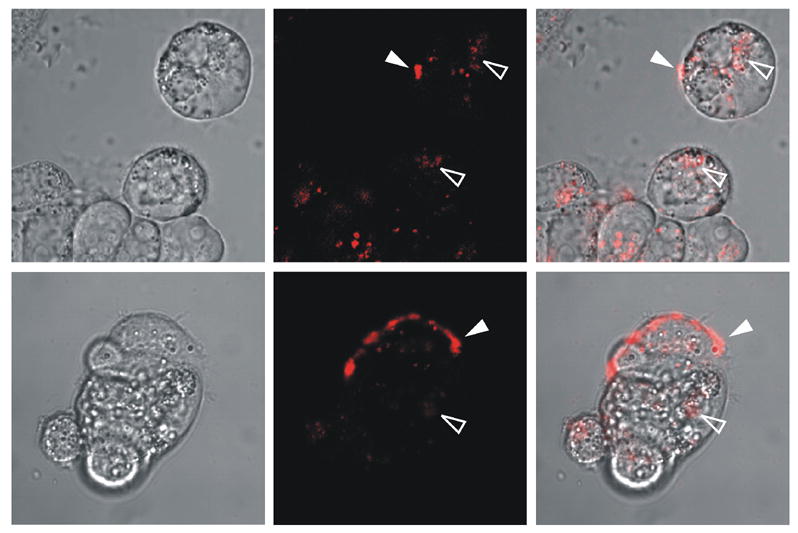
Confocal laser scanning microscopy images of Caco-2 cells incubated with rhodamine-HD-5. Cells were incubated in serum-free RPMI medium for 3 hours with 10 μg/ml of the peptide and were gently washed twice with HBSS prior to imaging. Left panels show the bright field image, middle panels show the fluorescence image of the rhodamine-labeled peptide, and right panels are a superposition of the two images. The fluorescence image is a 1-μm optical section acquired approximately at the equator of the largest cells, which are typically 14 – 15 μm in thickness. Filled arrowheads indicate examples of surface labeling; open arrowheads indicate examples of internalization.
To test whether HD-5 induces an intestinal inflammatory response, Caco-2 intestinal epithelial cells were incubated in the presence of increasing concentrations of the peptide. Steady-state quantities of secreted HD-5 have been estimated to be in the range of 50–250 μg/ml in the intestinal lumen [4, 15]. Therefore, we examined the effects of the peptides at concentrations of 50 and 100 μg/ml. Cells incubated with 100 ng/ml tumor necrosis factor α (TNFα) were used as a positive control. HD-5 induced IL-8 secretion in a dose-dependent fashion (Fig. 4, white bars). IL-8 levels increased ~10-fold at a peptide concentration of 100 μg/ml compared to control cells, similar to IL-8 levels observed after incubation with TNFα (Fig. 4, grey bar). To examine the effect of peptide structure, cells were incubated with the same concentration of the HD-5Abu peptide. Induction of IL-8 secretion was not observed in the presence of HD-5Abu at the same concentrations (Fig. 4, black bars), indicating the induction of IL-8 secretion depends on the structure of HD-5.
Figure 4.
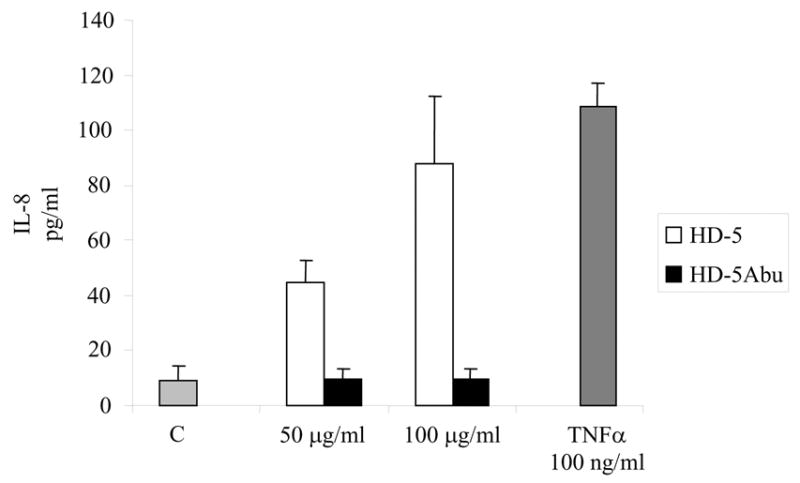
IL-8 secretion by Caco-2 cells in the absence (light grey bar) or presence of HD-5 (white bars) or HD-5Abu (black bars) at final concentrations of 50 or 100 μg/ml. TNFα (100 ng/ml; dark grey bar) served as a positive control. Following incubation for 18 hours, culture supernatants were analyzed for IL-8 using the Luminex-100 system in duplicate. Data represent mean and standard deviation of three individual experiments.
In addition to HD-5, TNFα is also expressed by Paneth cells [3]. Therefore, we examined the IL-8 secretion upon co-incubation of intestinal epithelial cells with HD-5 and TNFα (Fig. 5). Incubation with HD-5 or TNFα alone increased IL-8 secretion ~10-fold relative to the control. Co-incubation of HD-5 and TNFα resulted in an additional 6-fold increase in IL-8 secretion as compared to the levels observed when the two agents were applied separately. In contrast, HD-5Abu was completely ineffective in stimulating IL-8 secretion. Together, these data suggest that HD-5 and TNFα enhance IL-8 secretion by intestinal epithelial cells synergistically, and that HD-5 must be structured to stimulate secretion.
Figure 5.
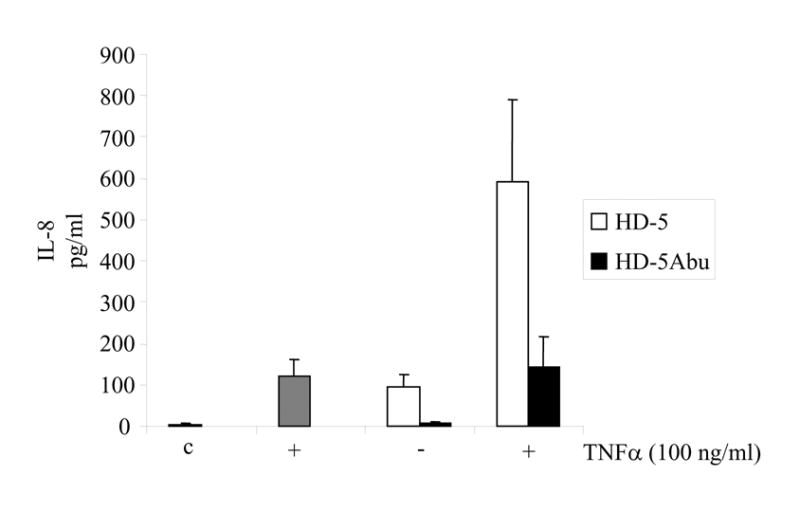
IL-8 secretion by Caco-2 cells in the presence of HD-5 (100 μg/ml; white bars) or HD-5Abu (100 μg/ml; black bars), with and without TNFα (100 ng/ml) as indicated. No peptides (c) and TNFα alone (dark grey bar) served as controls. Following incubation for 18 hours, culture supernatants were analyzed for IL-8 using the Luminex-100 system in duplicate. Data represent mean and standard deviation of three individual experiments.
Discussion
The role of antibacterial peptides in mucosal biology and in particular how they link innate to adaptive immunity has gained increasing interest. Several studies show that both the alpha and the beta subfamilies of defensins display immunomodulatory or chemotactic properties in addition to their antimicrobial activity. We investigated the antimicrobial activity and the immunomodulatory properties of HD-5 in relation to the structure of the peptide. Here, we find that killing of E. coli by HD-5 is independent of peptide structure, whereas antimicrobial activity against S. aureus requires the native structure. Strain-specific as well as defensin-specific activity has been observed previously in bacterial killing assays. For example, HD-5 appeared more efficient in killing S. aureus ATCC 29213 than human neutrophil peptide 1–3 (HNP1-3), whereas HNP1 and HNP2 were more efficient in killing S. aureus ATCC 25923 [20]. Interestingly, the antimicrobial activity of human β-defensin 3 (HBD3) was affected neither by rearranging the cysteine connectivity of the molecule nor by linearization of the peptide [21]. This variation in potency against various bacterial strains including E. coli and S. aureus has also been observed for murine cryptdins [12, 22]. Most likely, these differences reflect differences in the nature of interactions between defensins and microbial membrane components present in different bacteria. One possibility is that, in the case of HD-5, the interaction with for example lipopolysaccharide (LPS), exclusively present in gram-negative organisms, is more dependent on electrostatic interactions, and hence more sensitive to increasing ionic strength.
We find that HD-5 induces secretion of IL-8 by epithelial cells in a structure-dependent manner. Furthermore, HD-5 and TNFα act synergistically to induce secretion. Murine cryptdin 3 was shown to induce secretion of IL-8 by a human intestinal epithelial cell line [23], most likely through a receptor-independent mechanism. Interestingly, the observed induction of IL-8 secretion was linked to the ability of cryptdin 3 to form anion-conducting pores in mammalian membranes. Cryptdin 4, which is unable to form pores in mammalian cells, did not induce IL-8 secretion [23]. Whether HD-5 has the ability to form channels in mammalian membranes is not known. However, at concentrations up to 100 μg/ml, HD-5 was not cytotoxic against Caco-2 cells, whereas the pore-forming toxin melittin did [24]. Chemotactic properties involving specific interactions with receptors have been described for various defensins. Both HBD-2 and-3 have been shown to bind specifically with the chemokine receptor CCR6 [18, 21]. More recently, interactions between HBD3 and CXCR4 [25] as well as between HBD-2 and Toll-like receptor 4 (TLR4) have been described [26]. Given the increasing evidence that defensin expression is regulated in part by Toll-like receptor mediated pathways [27] and that chemotactic properties of HD5 have not been described, it is tempting to speculate that the interaction between HD-5 and epithelial cells we observe specifically involves a member of the TLR family.
Recently, a specific deficiency of HD-5 was observed in patients with ileal Crohn’s disease, a chronic disease of the intestine characterized by inflammation of the gut [28]. A number of gene polymorphisms in several cellular receptors, including tumor necrosis factor receptors, Toll-like receptor 4 and NOD2/CARD15 have been found associated with this disease. [29–31]. In particular, NOD2/CARD15 has been identified to be tightly linked with susceptibility to Crohn’s disease [32, 33]. NOD2 is an intracellular receptor for the bacterial peptidoglycan component muramyl dipeptide and is expressed in Paneth cells [34]. Importantly, approximately one-third of Crohn’s disease patients carry loss-of-function mutations in NOD2/CARD15 and show a further reduced expression of HD-5 [28]. These observations have led to the suggestion that decreased levels of α-defensin secreted by Paneth cells weaken the antimicrobial defense of the ileal mucosa [35]. Based on these observations and on our results, we speculate that lower levels of HD-5 observed in Crohn’s disease patients compared to normal conditions may compromise the ability of the intestinal epithelium to respond to immune challenges by weakening the antibacterial activity as well as by weakening the immune response of the epithelium.
Acknowledgments
Erin Gutierrez and Darryl A. Auston are gratefully acknowledged for expert technical assistance. This work was supported by the National Institute of Health grants AI058939 and AI061482 to W.L.
List of abbreviations
- HD
human defensin
- HNP
human neutrophil peptide
- IL
interleukin
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol. 1999;277:C351–358. doi: 10.1152/ajpcell.1999.277.3.C351. [DOI] [PubMed] [Google Scholar]
- 3.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouellette AJ. IV. Paneth cell antimicrobial peptides and the biology of the mucosal barrier. Am J Physiol. 1999;277:G257–261. doi: 10.1152/ajpgi.1999.277.2.G257. [DOI] [PubMed] [Google Scholar]
- 5.Szyk A, Wu Z, Tucker K, Yang D, Lu W, Lubkowski J. Crystal structures of human {alpha}-defensins HNP4, HD5, and HD6. Protein Sci. 2006 doi: 10.1110/ps.062336606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 7.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14:96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 8.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 9.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 10.Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, Casavant TL, McCray PB., Jr Discovery of five conserved beta -defensin gene clusters using a computational search strategy. Proc Natl Acad Sci U S A. 2002;99:2129–2133. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil A, Hughes AL, Zhang G. Rapid evolution and diversification of mammalian alpha-defensins as revealed by comparative analysis of rodent and primate genes. Physiol Genomics. 2004;20:1–11. doi: 10.1152/physiolgenomics.00150.2004. [DOI] [PubMed] [Google Scholar]
- 12.Ouellette AJ, Hsieh MM, Nosek MT, Cano-Gauci DF, Huttner KM, Buick RN, Selsted ME. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun. 1994;62:5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer PB, Harwig SS, Lehrer RI. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992;60:3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, Yadav SP, Crabb JW, Ganz T, Bevins CL. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 17.Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 18.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, Ericksen B, Tucker K, Lubkowski J, Lu W. Synthesis and characterization of human alpha-defensins 4–6. J Pept Res. 2004;64:118–125. doi: 10.1111/j.1399-3011.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 20.Ericksen B, Wu Z, Lu W, Lehrer RI. Antibacterial activity and specificity of the six human {alpha}-defensins. Antimicrob Agents Chemother. 2005;49:269–275. doi: 10.1128/AAC.49.1.269-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci U S A. 2003;100:8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selsted ME, Miller SI, Henschen AH, Ouellette AJ. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992;118:929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin PW, Simon PO, Jr, Gewirtz AT, Neish AS, Ouellette AJ, Madara JL, Lencer WI. Paneth cell cryptdins act in vitro as apical paracrine regulators of the innate inflammatory response. J Biol Chem. 2004;279:19902–19907. doi: 10.1074/jbc.M311821200. [DOI] [PubMed] [Google Scholar]
- 24.Porter EM, Liu L, Oren A, Anton PA, Ganz T. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun. 1997;65:2389–2395. doi: 10.1128/iai.65.6.2389-2395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Z, Dubyak GR, Lederman MM, Weinberg A. Cutting Edge: Human beta defensin 3--a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol. 2006;177:782–786. doi: 10.4049/jimmunol.177.2.782. [DOI] [PubMed] [Google Scholar]
- 26.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 27.Froy O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol. 2005;7:1387–1397. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 28.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H, Jr, Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci U S A. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waschke KA, Villani AC, Vermeire S, Dufresne L, Chen TC, Bitton A, Cohen A, Thomson AB, Wild GE. Tumor necrosis factor receptor gene polymorphisms in Crohn’s disease: association with clinical phenotypes. Am J Gastroenterol. 2005;100:1126–1133. doi: 10.1111/j.1572-0241.2005.40534.x. [DOI] [PubMed] [Google Scholar]
- 30.Pierik M, Vermeire S, Steen KV, Joossens S, Claessens G, Vlietinck R, Rutgeerts P. Tumour necrosis factor-alpha receptor 1 and 2 polymorphisms in inflammatory bowel disease and their association with response to infliximab. Aliment Pharmacol Ther. 2004;20:303–310. doi: 10.1111/j.1365-2036.2004.01946.x. [DOI] [PubMed] [Google Scholar]
- 31.Sashio H, Tamura K, Ito R, Yamamoto Y, Bamba H, Kosaka T, Fukui S, Sawada K, Fukuda Y, Tamura K, Satomi M, Shimoyama T, Furuyama J. Polymorphisms of the TNF gene and the TNF receptor superfamily member 1B gene are associated with susceptibility to ulcerative colitis and Crohn’s disease, respectively. Immunogenetics. 2002;53:1020–1027. doi: 10.1007/s00251-001-0423-7. [DOI] [PubMed] [Google Scholar]
- 32.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 33.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 34.Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins:role in host-microbial interactions and inflammatory disease. Annual Reviews of Biochemistry. 2004;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 35.Fellermann K, Wehkamp J, Herrlinger KR, Stange EF. Crohn’s disease: a defensin deficiency syndrome? Eur J Gastroenterol Hepatol. 2003;15:627–634. doi: 10.1097/00042737-200306000-00008. [DOI] [PubMed] [Google Scholar]


