Abstract
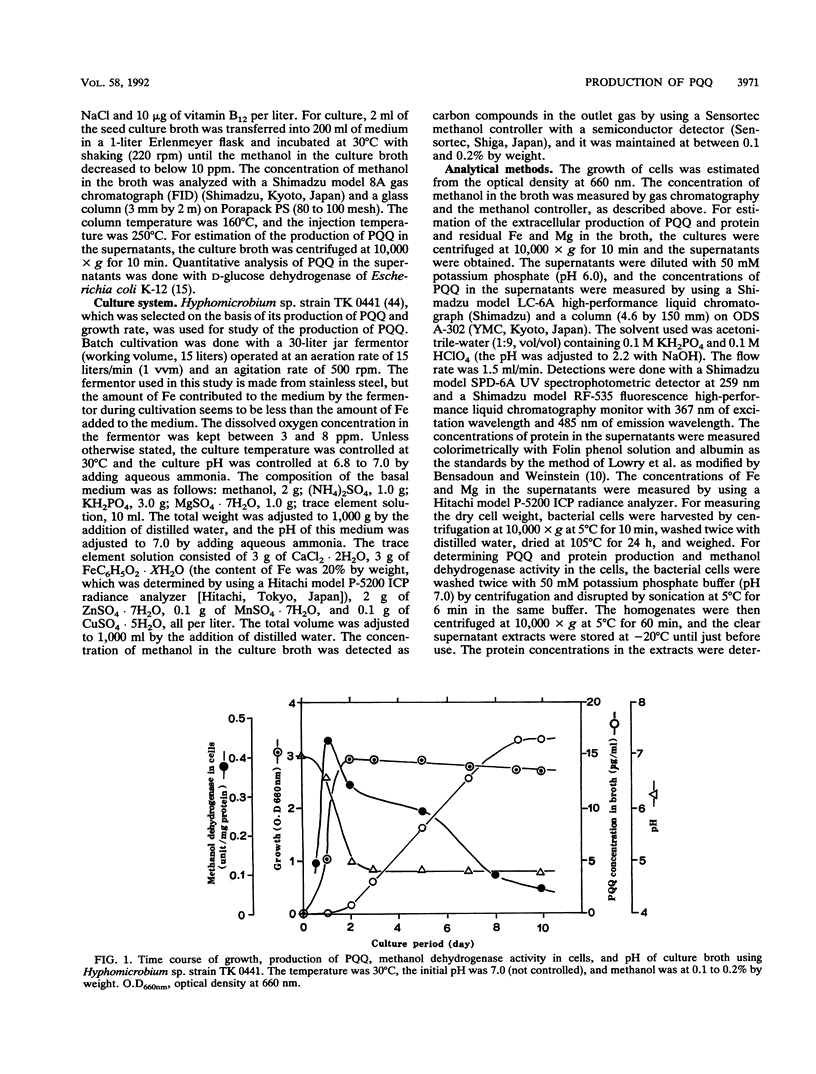
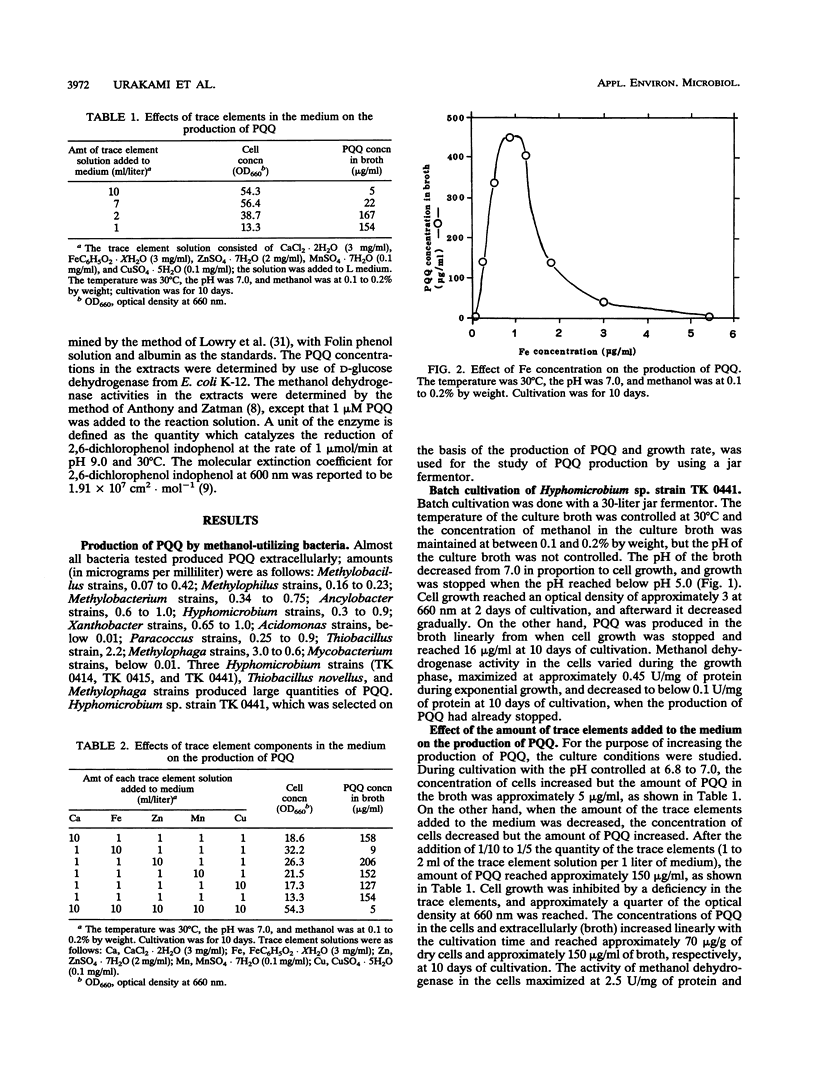
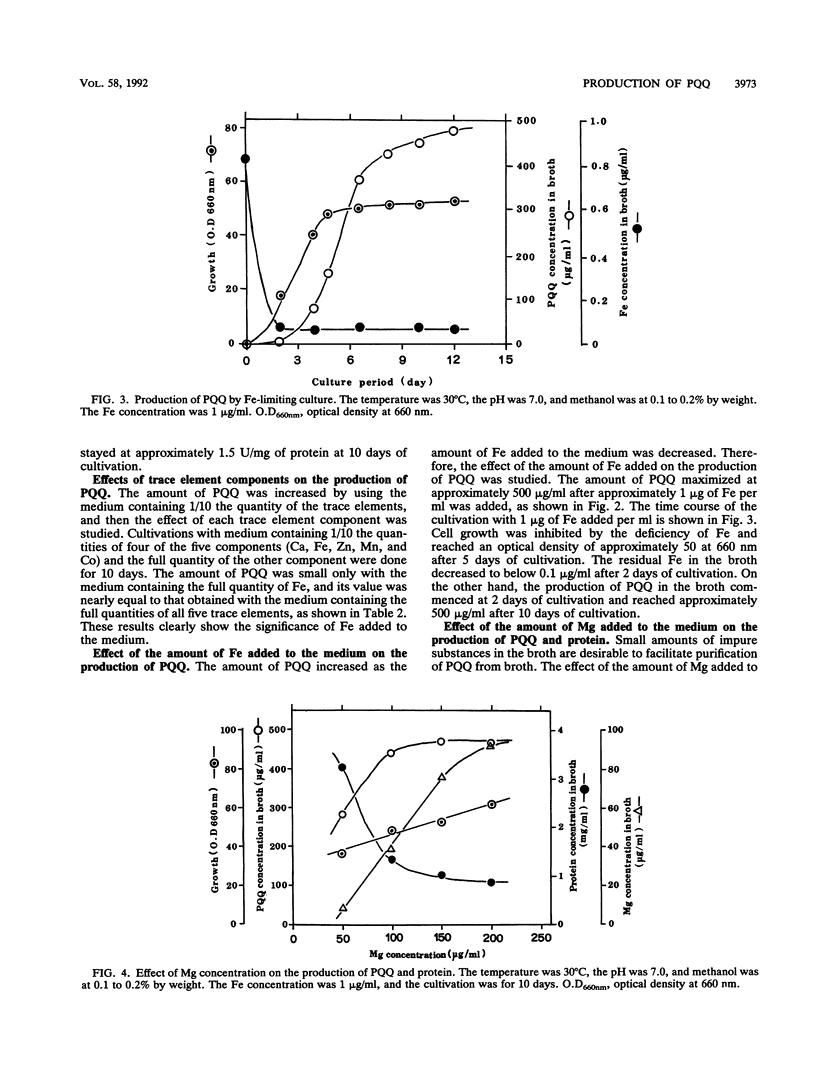
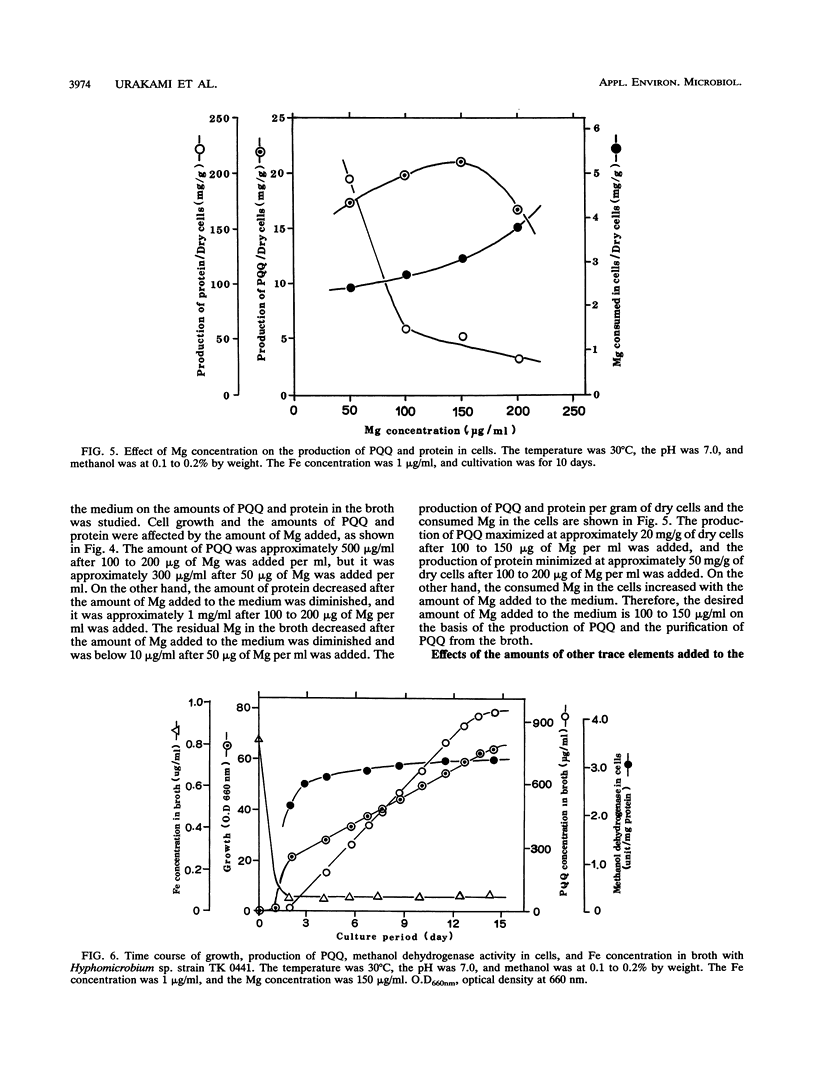
A large number of methanol-utilizing bacteria were screened for extracellular production of pyrroloquinoline quinone (PQQ) by using methanol as the carbon and energy sources. Of the bacteria selected, Hyphomicrobium sp. strain TK 0441 was examined for PQQ production by using a jar fermentor. The amount of PQQ in the broth and the level of methanol dehydrogenase activity in the cells were increased by simply decreasing the amount of Fe added to the medium. On the other hand, extracellularly produced protein which interfered with the purification of PQQ was decreased by simply increasing the amount of Mg added to the medium. A suitable medium that contained 1 μg of Fe per ml, 150 μg of Mg per ml, and trace elements was developed. In this medium, the production of PQQ reached approximately 1 mg/ml and protein formation was low.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ameyama M., Matsushita K., Ohno Y., Shinagawa E., Adachi O. Existence of a novel prosthetic group, PQQ, in membrane-bound, electron transport chain-linked, primary dehydrogenases of oxidative bacteria. FEBS Lett. 1981 Aug 3;130(2):179–183. doi: 10.1016/0014-5793(81)81114-3. [DOI] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. Purification and properties of the alcohol dehydrogenase of Pseudomonas sp. M27. Biochem J. 1967 Sep;104(3):953–959. doi: 10.1042/bj1040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J. Quinoprotein alcohol dehydrogenase from a non-methylotroph, Acinetobacter calcoaceticus. J Gen Microbiol. 1981 Feb;122(2):201–209. doi: 10.1099/00221287-122-2-201. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Geiger O., Görisch H. Enzymatic determination of pyrroloquinoline quinone using crude membranes from Escherichia coli. Anal Biochem. 1987 Aug 1;164(2):418–423. doi: 10.1016/0003-2697(87)90513-6. [DOI] [PubMed] [Google Scholar]
- Glatz Z., Kovár J., Macholán L., Pec P. Pea (Pisum sativum) diamine oxidase contains pyrroloquinoline quinone as a cofactor. Biochem J. 1987 Mar 1;242(2):603–606. doi: 10.1042/bj2420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen B. W., van der Meer R. A., Duine J. A. Evidence for PQQ as cofactor in 3,4-dihydroxyphenylalanine (dopa) decarboxylase of pig kidney. FEBS Lett. 1988 Sep 12;237(1-2):98–102. doi: 10.1016/0014-5793(88)80179-0. [DOI] [PubMed] [Google Scholar]
- Hamagishi Y., Murata S., Kamei H., Oki T., Adachi O., Ameyama M. New biological properties of pyrroloquinoline quinone and its related compounds: inhibition of chemiluminescence, lipid peroxidation and rat paw edema. J Pharmacol Exp Ther. 1990 Dec;255(3):980–985. [PubMed] [Google Scholar]
- Janes S. M., Mu D., Wemmer D., Smith A. J., Kaur S., Maltby D., Burlingame A. L., Klinman J. P. A new redox cofactor in eukaryotic enzymes: 6-hydroxydopa at the active site of bovine serum amine oxidase. Science. 1990 May 25;248(4958):981–987. doi: 10.1126/science.2111581. [DOI] [PubMed] [Google Scholar]
- Killgore J., Smidt C., Duich L., Romero-Chapman N., Tinker D., Reiser K., Melko M., Hyde D., Rucker R. B. Nutritional importance of pyrroloquinoline quinone. Science. 1989 Aug 25;245(4920):850–852. doi: 10.1126/science.2549636. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Seno H., Urakami T., Suzuki O. Failure to verify the presence of pyrroloquinoline quinone (PQQ) in bovine plasma amine oxidase by gas chromatography/mass spectrometry. Arch Biochem Biophys. 1990 Dec;283(2):533–536. doi: 10.1016/0003-9861(90)90679-s. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- McIntire W. S., Weyler W. Factors affecting the production of pyrroloquinoline quinone by the methylotrophic bacterium W3A1. Appl Environ Microbiol. 1987 Sep;53(9):2183–2188. doi: 10.1128/aem.53.9.2183-2188.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Yamada H. Nitrile hydratase is a quinoprotein. A possible new function of pyrroloquinoline quinone: activation of H2O in an enzymatic hydration reaction. Biochem Biophys Res Commun. 1987 Sep 15;147(2):701–709. doi: 10.1016/0006-291x(87)90987-9. [DOI] [PubMed] [Google Scholar]
- Nishigori H., Yasunaga M., Mizumura M., Lee J. W., Iwatsuru M. Preventive effects of pyrroloquinoline quinone on formation of cataract and decline of lenticular and hepatic glutathione of developing chick embryo after glucocorticoid treatment. Life Sci. 1989;45(7):593–598. doi: 10.1016/0024-3205(89)90044-1. [DOI] [PubMed] [Google Scholar]
- Salisbury S. A., Forrest H. S., Cruse W. B., Kennard O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nature. 1979 Aug 30;280(5725):843–844. doi: 10.1038/280843a0. [DOI] [PubMed] [Google Scholar]
- Shimao M., Fujita I., Kato N., Sakazawa C. Enhancement of Pyrroloquinoline Quinone Production and Polyvinyl Alcohol Degradation in Mixed Continuous Cultures of Pseudomonas putida VM15A and Pseudomonas sp. Strain VM15C with Mixed Carbon Sources. Appl Environ Microbiol. 1985 Jun;49(6):1389–1391. doi: 10.1128/aem.49.6.1389-1391.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimao M., Ninomiya K., Kuno O., Kato N., Sakazawa C. Existence of a novel enzyme, pyrroloquinoline quinone-dependent polyvinyl alcohol dehydrogenase, in a bacterial symbiont, Pseudomonas sp. strain VM15C. Appl Environ Microbiol. 1986 Feb;51(2):268–275. doi: 10.1128/aem.51.2.268-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kleef M. A., Duine J. A. Factors relevant in bacterial pyrroloquinoline quinone production. Appl Environ Microbiol. 1989 May;55(5):1209–1213. doi: 10.1128/aem.55.5.1209-1213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kleef M. A., Duine J. A. L-tyrosine is the precursor of PQQ biosynthesis in Hyphomicrobium X. FEBS Lett. 1988 Sep 12;237(1-2):91–97. doi: 10.1016/0014-5793(88)80178-9. [DOI] [PubMed] [Google Scholar]
- van der Meer R. A., Duine J. A. Covalently bound pyrroloquinoline quinone is the organic prosthetic group in human placental lysyl oxidase. Biochem J. 1986 Nov 1;239(3):789–791. doi: 10.1042/bj2390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer R. A., Jongejan J. A., Duine J. A. Dopamine beta-hydroxylase from bovine adrenal medulla contains covalently-bound pyrroloquinoline quinone. FEBS Lett. 1988 Apr 25;231(2):303–307. doi: 10.1016/0014-5793(88)80838-x. [DOI] [PubMed] [Google Scholar]
- van der Meer R. A., Jongejan J. A., Frank J., Duine J. A. Hydrazone formation of 2,4-dinitrophenylhydrazine with pyrroloquinoline quinone in porcine kidney diamine oxidase. FEBS Lett. 1986 Sep 29;206(1):111–114. doi: 10.1016/0014-5793(86)81350-3. [DOI] [PubMed] [Google Scholar]



