Abstract
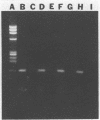
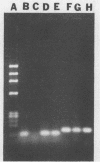
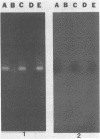
The human immunodeficiency virus type 1 (HIV-1) released by infected individuals or present in human and hospital wastes can potentially cause contamination problems. The presence of HIV-1 was investigated in 16 environmental samples, including raw wastewater, sludge, final effluent, soil, and pond water, collected from different locations. A method was developed to extract total nucleic acids in intact form directly from the raw samples or from the viral concentrates of the raw samples. The isolated nucleic acids were analyzed for the presence of HIV-1 by using in vitro amplification of the target sequences by the polymerase chain reaction (PCR) method. HIV-1-specific proviral DNA and viral RNA were detected in the extracted nucleic acids obtained from three wastewater samples by this method. The specificity of the PCR-amplified products was determined by Southern blot hybridization with an HIV-1-specific oligonucleotide probe, SK19. The isolated nucleic acids from wastewater samples were also screened for the presence of poliovirus type 1, representing a commonly found enteric virus, and simian immunodeficiency virus, representing, presumably, rare viruses. While poliovirus type 1 viral RNA was found in all of the wastewater samples, none of the samples yielded a simian immunodeficiency virus-specific product. No PCR-amplified product was yielded when wastewater samples were directly used for the detection of HIV-1 and poliovirus type 1. The wastewater constituents appeared to be inhibitory to the enzymes reverse transcriptase and DNA polymerase.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

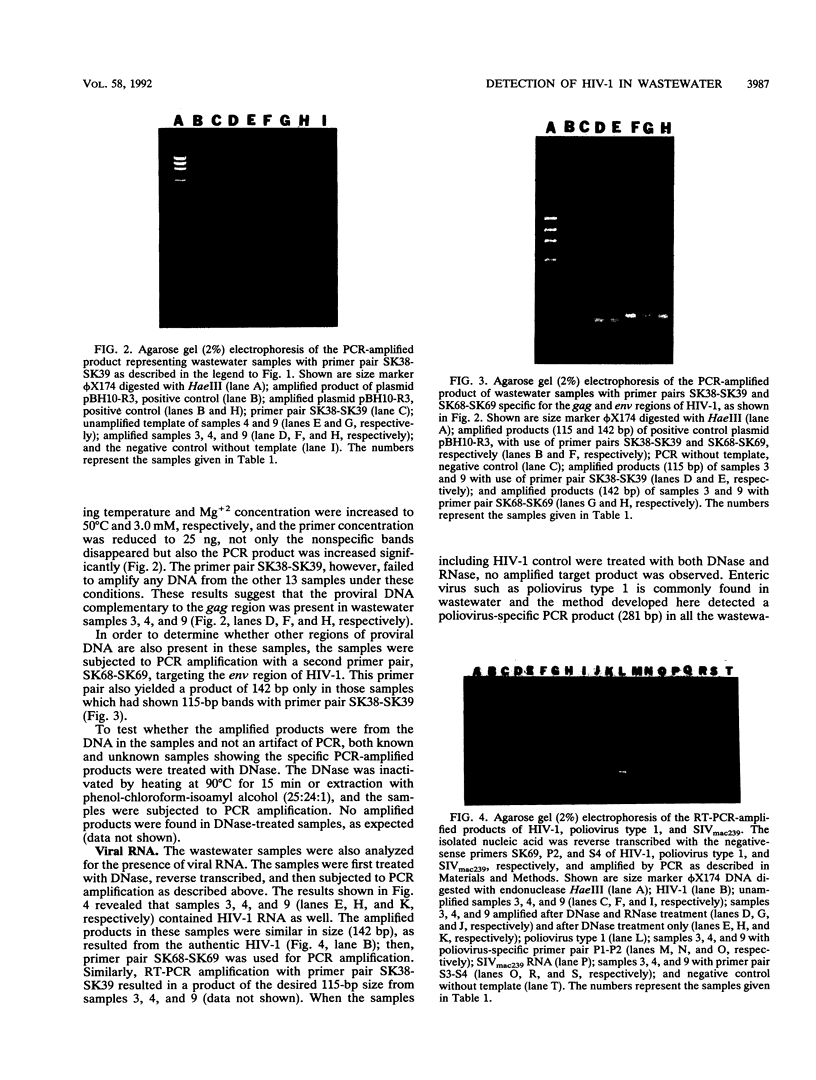



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari S. A., Springthorpe V. S., Sattar S. A. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis. 1991 May-Jun;13(3):448–461. doi: 10.1093/clinids/13.3.448. [DOI] [PubMed] [Google Scholar]
- Barre-Sinoussi F., Nugeyre M. T., Chermann J. C. Resistance of AIDS virus at room temperature. Lancet. 1985 Sep 28;2(8457):721–722. doi: 10.1016/s0140-6736(85)92955-1. [DOI] [PubMed] [Google Scholar]
- Fujikawa L. S., Salahuddin S. Z., Palestine A. G., Masur H., Nussenblatt R. B., Gallo R. C. Isolation of human T-lymphotropic virus type III from the tears of a patient with the acquired immunodeficiency syndrome. Lancet. 1985 Sep 7;2(8454):529–530. doi: 10.1016/s0140-6736(85)90464-7. [DOI] [PubMed] [Google Scholar]
- Groopman J. E., Salahuddin S. Z., Sarngadharan M. G., Markham P. D., Gonda M., Sliski A., Gallo R. C. HTLV-III in saliva of people with AIDS-related complex and healthy homosexual men at risk for AIDS. Science. 1984 Oct 26;226(4673):447–449. doi: 10.1126/science.6093247. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Byington R. E., Schooley R. T., Flynn T., Rota T. R., Hirsch M. S. Infrequency of isolation of HTLV-III virus from saliva in AIDS. N Engl J Med. 1985 Dec 19;313(25):1606–1606. doi: 10.1056/NEJM198512193132512. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Schooley R. T., Rota T. R., Kaplan J. C., Flynn T., Salahuddin S. Z., Gonda M. A., Hirsch M. S. HTLV-III in the semen and blood of a healthy homosexual man. Science. 1984 Oct 26;226(4673):451–453. doi: 10.1126/science.6208608. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Coombs R. W., Collier A. C., Ross S. O., Chaloupka K., Cummings D. K., Murphy V. L., Corey L. Recovery of human immunodeficiency virus type 1 from semen: minimal impact of stage of infection and current antiviral chemotherapy. J Infect Dis. 1991 Feb;163(2):386–388. doi: 10.1093/infdis/163.2.386. [DOI] [PubMed] [Google Scholar]
- Lapointe N., Michaud J., Pekovic D., Chausseau J. P., Dupuy J. M. Transplacental transmission of HTLV-III virus. N Engl J Med. 1985 May 16;312(20):1325–1326. doi: 10.1056/NEJM198505163122012. [DOI] [PubMed] [Google Scholar]
- Lepage P., Van de Perre P., Caraël M., Nsengumuremyi F., Nkurunziza J., Butzler J. P., Sprecher S. Postnatal transmission of HIV from mother to child. Lancet. 1987 Aug 15;2(8555):400–400. doi: 10.1016/s0140-6736(87)92423-8. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Human immunodeficiency viruses and the pathogenesis of AIDS. JAMA. 1989 May 26;261(20):2997–3006. [PubMed] [Google Scholar]
- Levy J. A., Kaminsky L. S., Morrow W. J., Steimer K., Luciw P., Dina D., Hoxie J., Oshiro L. Infection by the retrovirus associated with the acquired immunodeficiency syndrome. Clinical, biological, and molecular features. Ann Intern Med. 1985 Nov;103(5):694–699. doi: 10.7326/0003-4819-103-5-694. [DOI] [PubMed] [Google Scholar]
- Lifson A. R. Do alternate modes for transmission of human immunodeficiency virus exist? A review. JAMA. 1988 Mar 4;259(9):1353–1356. [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfai T., Kurtz P. Time-dependent effects of metrazol on memory. Pharmacol Biochem Behav. 1973 Jan-Feb;1(1):55–59. doi: 10.1016/0091-3057(73)90055-5. [DOI] [PubMed] [Google Scholar]
- Preston D. R., Farrah S. R., Bitton G., Chaudhry G. R. Detection of nucleic acids homologous to human immunodeficiency virus in wastewater. J Virol Methods. 1991 Aug;33(3):383–390. doi: 10.1016/0166-0934(91)90038-2. [DOI] [PubMed] [Google Scholar]
- Regier D. A., Desrosiers R. C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- Resnick L., Veren K., Salahuddin S. Z., Tondreau S., Markham P. D. Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. JAMA. 1986 Apr 11;255(14):1887–1891. [PubMed] [Google Scholar]
- Thiry L., Sprecher-Goldberger S., Jonckheer T., Levy J., Van de Perre P., Henrivaux P., Cogniaux-LeClerc J., Clumeck N. Isolation of AIDS virus from cell-free breast milk of three healthy virus carriers. Lancet. 1985 Oct 19;2(8460):891–892. doi: 10.1016/s0140-6736(85)90156-4. [DOI] [PubMed] [Google Scholar]
- Vogt M. W., Witt D. J., Craven D. E., Byington R., Crawford D. F., Schooley R. T., Hirsch M. S. Isolation of HTLV-III/LAV from cervical secretions of women at risk for AIDS. Lancet. 1986 Mar 8;1(8480):525–527. doi: 10.1016/s0140-6736(86)90884-6. [DOI] [PubMed] [Google Scholar]
- Wofsy C. B., Cohen J. B., Hauer L. B., Padian N. S., Michaelis B. A., Evans L. A., Levy J. A. Isolation of AIDS-associated retrovirus from genital secretions of women with antibodies to the virus. Lancet. 1986 Mar 8;1(8480):527–529. doi: 10.1016/s0140-6736(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Li S., Perman J., Viscidi R. Persistent diarrhea and fecal shedding of retroviral nucleic acids in children infected with human immunodeficiency virus. J Infect Dis. 1991 Jul;164(1):61–66. doi: 10.1093/infdis/164.1.64. [DOI] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Leibowitch J., Safai B., Groopman J. E., Feldman M., Sarngadharan M. G., Gallo R. C. HTLV-III in cells cultured from semen of two patients with AIDS. Science. 1984 Oct 26;226(4673):449–451. doi: 10.1126/science.6208607. [DOI] [PubMed] [Google Scholar]
- Ziegler J. B., Cooper D. A., Johnson R. O., Gold J. Postnatal transmission of AIDS-associated retrovirus from mother to infant. Lancet. 1985 Apr 20;1(8434):896–898. doi: 10.1016/s0140-6736(85)91673-3. [DOI] [PubMed] [Google Scholar]