Abstract
The objective of this study was to determine the effects of manipulating glucocorticoid negative feedback on acute ACTH and corticosterone responses to corticotrophin-releasing hormone (CRH) injection in 7-day old rats exposed to normoxia or hypoxia from birth. Chemical adrenalectomy was achieved with aminoglutethimide, and glucocorticoids were replaced with a low dose of dexamethasone. Hypoxia per se increased basal plasma corticosterone and attenuated the plasma ACTH response to CRH. Aminoglutethimide per se decreased plasma corticosterone and strongly increased basal plasma ACTH and anterior pituitary POMC gene expression. Dexamethasone partially attenuated elevations in basal plasma ACTH due to aminoglutethimide in both normoxic and hypoxic pups, but inhibited anterior pituitary POMC expression and CRH-induced plasma ACTH only in hypoxic pups. Despite this inhibition, hypoxic pups treated with both dexamethasone and aminoglutethimide still exhibited a significant CRH-induced increment in plasma ACTH that was lacking in hypoxic pups not treated with either dexamethasone or aminoglutethimide. We conclude that ACTH responses to acute stimuli in hypoxic neonatal rats are prevented by ACTH-independent increases in corticosterone rather than by intrinsic hypothalamic-pituitary hypoactivity.
Keywords: Adrenocorticotropin, corticosterone, negative feedback, adrenal
Introduction
Hypoxia is a common neonatal stress leading to significant short-term distress and long-term complications (Frankel & Stevenson, 1987; Friedman & Fahey, 1993; Low et al. 1993; Rubaltelli et al. 1998). Successful adaptation to neonatal hypoxia requires a coordinated physiological response, including an increase in the release of glucocorticoids from the adrenal cortex (Hanukoglu et al. 1995). Understanding the mechanisms by which the resulting increase in glucocorticoid secretion occurs, as well as the physiological impact of this increase in glucocorticoids, will aid in devising strategies to mitigate the short- and long-term effects of neonatal hypoxia. We have previously demonstrated that the neonatal rat exposed to hypoxia from birth has increased plasma corticosterone that is driven by sympathetic input to the adrenal cortex rather than by ACTH (Raff et al. 2003a; Raff et al. 2004). It is possible that this ACTH-independent increase in corticosterone in the neonate exposed to chronic hypoxia from birth is a mechanism to increase circulating glucocorticoids by bypassing the stress-hyporesponsive hypothalamus and/or pituitary.
We have demonstrated that the ACTH response to corticotrophin-releasing hormone (CRH) or ether stress was significantly attenuated in the 7-day old rat exposed to hypoxia from birth (Raff et al. 2003b). We hypothesized that this attenuated pituitary corticotroph response was due to the negative feedback effects of the aforementioned ACTH-independent, sympathetically-driven increase in corticosterone. This hypothesis is supported by evidence that increased sensitivity to glucocorticoid negative feedback is one of the possible mechanisms contributing to the stress-hyporesponsive period in the neonatal rat (Proulx et al. 2001; Schmidt et al. 2005; Walker et al. 1986b).
The current study evaluated the hypothesis that the attenuated ACTH response to CRH in the 7-day old neonatal rat pup exposed to hypoxia from birth is due to the ACTH-independent increase in corticosterone. Because it is virtually impossible to adrenalectomize hypoxic neonatal rats with any expectation of survival, we induced a chemical adrenalectomy with aminoglutethimide (Lerner et al. 1984) and then provided different levels of glucocorticoids by vehicle or low-dose dexamethasone injection (Proulx et al. 2001). We have used corticotroph responses to aminoglutethimide and CRH, in the presence or absence of dexamethasone, to assess the sensitivity of HPA axis in normoxic vs. hypoxic 7-day old rat pups to the removal or imposition of glucocorticoid negative feedback.
Material and Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of Aurora Health Care. Timed pregnant, Sprague-Dawley rats (Harlan, Indianapolis, N=24) were obtained at 14 days gestation and maintained on a standard diet and water ad libitum (0600–1800 lights on). Immediately after parturition (day 21–22), dams and their pups were continuously exposed to either normoxia (21% O2) or hypoxia (12% O2) in an environmental chamber as described in detail previously (Raff et al. 2000; Raff et al. 2003a; Thomas & Marshall, 1995). The experimental design is diagrammed in Figure 1. On postnatal day (PND) 6 at 1600 hr, pups were separated into 4 pre-treatment groups, with all pups from a given litter assigned to the same pre-treatment. The 4 pre-treatments, each consisting of 2 ip injections 14h apart, were as follows: (1) aminoglutethimide (400 mg/kg aminoglutethimide tartrate in 5 ml/kg saline at 1600 h of PND 6, followed by 5 ml/kg saline at 0600 h of PND 7); (2) dexamethasone (5 ml/kg saline at 1600 h of PND 6, followed by 5 ug/kg dexamethasone phosphate in 5 ml/kg saline at 0600h of PND 7), (3) aminoglutethimide plus dexamethasone (each given at the time and dose indicated above), or (4) vehicle (5 ml/kg saline at both times). Pups were weighed before each pre-treatment injection. Aminoglutethimide and dexamethasone were obtained from Sigma (St. Louis, MO).
Figure 1.
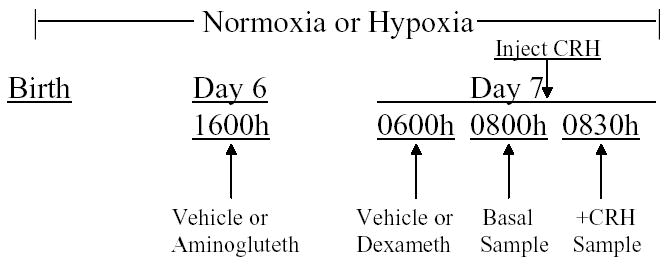
Experimental protocol. At birth, pups were exposed to normoxia or hypoxia for the entire experiment. On Day 6 at 0600h, pups were injected with vehicle or aminoglutethimide. On Day 7 at 0600h, pups were injected with vehicle or dexamethasone. Two hours later, some pups within a litter were sampled (Basal). The remaining pups were injected with CRH and sampled 30 min later.
At 0800 (2 hrs after dexamethasone or saline [vehicle] injection), some pups within each litter (Basal) were decapitated and trunk blood was pooled (3 pups/sample). Each pool was considered N=1 for statistical analysis. Pituitary glands were quickly removed, and the anterior lobe was dissected from the neurointermediate lobe. The anterior pituitary lobes of 3 pups were pooled for each sample and frozen in liquid nitrogen. Each pool was considered N=1 for statistical analysis. The remaining pups (+CRH) within each litter were weighed and injected ip with 10 ug/kg of CRH (Bachem; diluted in phosphate-buffered saline; 10 uL per gram body weight). The CRH-injected pups were decapitated 30 min later. Each litter, therefore, provided four pooled plasma samples (two basal; two +CRH) and 1–2 pooled basal anterior pituitary samples. (Not every anterior pituitary was successfully retrieved.) A vehicle control for CRH injection was omitted because injection stress does not activate the neonatal HPA axis (Arai & Widmaier, 1991; Walker et al. 1986a).
Plasma ACTH and corticosterone were measured by RIA as described previously (Raff et al. 2003a; Raff et al. 2003b). Pituitary pro-opiomelanocortin (POMC) mRNA was assessed by Northern analysis as described previously (Raff et al. 2003b; Jacobson et al. 1997). ACTH data were log-transformed before analysis of variance to achieve a normal distribution. Data were analyzed by 3-factor analysis of variance followed by Duncan’s multiple range test.
Results
Figure 2 shows the ACTH and corticosterone levels achieved before (Basal) or 30 min after CRH injection (+CRH) in 7-day old rats exposed to normoxia vs. hypoxia from birth and treated with vehicle, aminoglutethimide, and/or dexamethasone. ACTH and corticosterone responded significantly to CRH injection in normoxic vehicle-treated rat pups (first pair of bars, top and bottom panels of Fig. 2). As we have previously reported (Raff et al. 2003a, Raff et al. 2004), hypoxic vehicle-treated pups had elevated basal levels of corticosterone without an increase in basal plasma ACTH. Unlike normoxic pups, hypoxic pups did not exhibit a significant, CRH-induced increment in either hormone over basal levels (second pair of bars in both panels of Fig. 2). Aminoglutethimide treatment per se significantly reduced corticosterone and increased basal ACTH; levels of both hormones were comparable between normoxic and hypoxic pups. In contrast to normoxic pups, hypoxic pups demonstrated a significant increase in ACTH in response to CRH after aminoglutethimide treatment (third and fourth pairs of bars, Fig. 2). Dexamethasone per se lowered plasma corticosterone to similar levels and blocked the ACTH response to CRH in both normoxic and hypoxic pups (fifth and sixth pairs of bars, Fig. 2). Administration of dexamethasone to aminoglutethimide-treated pups resulted in corticosterone levels that were not significantly different from those in pups treated with either drug alone. Aminoglutethimide plus dexamethasone significantly decreased but did not completely normalize basal plasma ACTH relative to levels in corresponding Vehicle controls. CRH induced significant increases over basal levels of ACTH in normoxic and hypoxic pups that had been treated with both dexamethasone and aminoglutethimide (seventh and eight pairs of bars, Fig. 2). The seemingly small difference in plasma ACTH between Basal and +CRH in the hypoxic pups was indeed significant, most likely because the post-hoc comparisons factor in rank order as well as differences among means. However, CRH-induced ACTH levels in aminoglutethimide-treated pups were significantly reduced by dexamethasone only in hypoxic pups (seventh and eight pairs of bars, Fig. 2).
Figure 2.
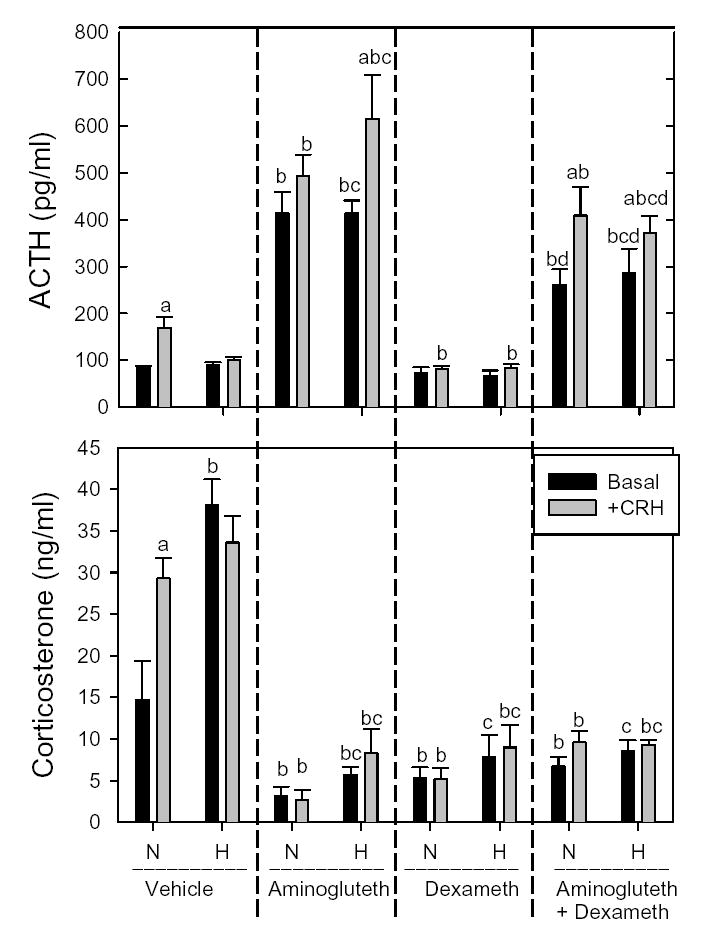
Plasma ACTH (top) and corticosterone (bottom) before (Basal - 0800 hr) and 30 minutes after ip injection of 10 μg/kg CRH (+CRH) in 7-day old rat pups exposed to normoxia (N) or hypoxia (H) from birth. 4 treatment groups of normoxic and hypoxic pups were studied: Vehicle (2 saline injections [1600 h the day before and 0600 that day]), Aminoglutethimide injection [400 mg/kg at 1600 the day before] followed by saline injection at 0600 that day), Dexamethasone (saline injection at 1600 hr the day before followed by dexamethasone at 0600 that day), and Aminoglutethimide+Dexamethasone (aminoglutethimide injection at 1600 the day before followed by dexamethasone injection at 0600 that day). Plasma from 3 pups was pooled and considered N=1. Each bar is the mean±SEM of N=5–9 pooled plasma samples..
a, significantly different from basal within the same treatment and the same normoxia or hypoxia group.
b, significantly different from the Vehicle normoxia group within the same basal or CRH group
c, significantly different from Vehicle basal or Vehicle CRH, respectively, within hypoxia.
d, significantly different from Aminoglutethimide basal or Aminoglutethimide CRH, respectively, within normoxia or hypoxia
Figure 3 shows anterior pituitary POMC mRNA levels in the Basal groups of normoxic and hypoxic pups given vehicle, aminoglutethimide, dexamethasone, or aminoglutethimide and dexamethasone. Despite differences in basal corticosterone levels (Fig. 2), POMC mRNA was similar between vehicle-treated normoxic and hypoxic pups, and increased to equivalent levels after aminoglutethimide administration (first and second pairs of bars, Fig. 3). Dexamethasone per se did not lower POMC mRNA below the levels in vehicle-treated pups (third pair of bars, Fig. 3). Administration of dexamethasone to aminoglutethimide-treated pups decreased POMC mRNA in hypoxic, but not normoxic, pups (fourth pair of bars, Fig. 3).
Figure 3.
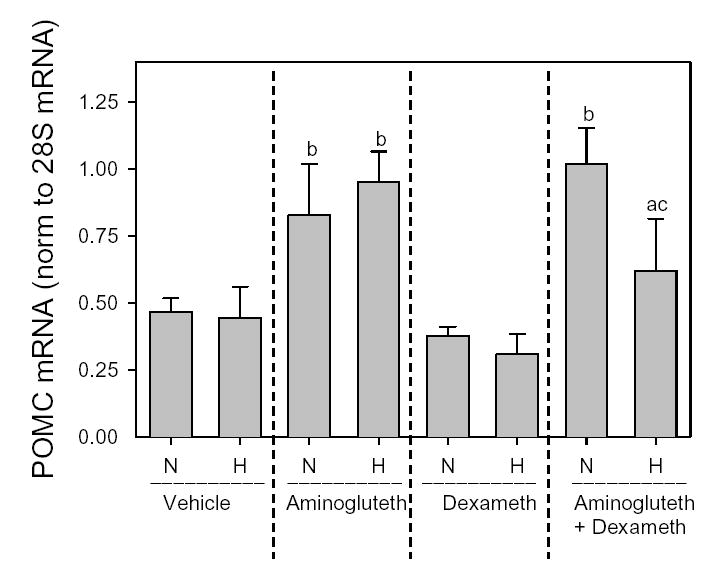
Anterior pituitary POMC mRNA normalized to 28S mRNA in 7-day old rat pups exposed to normoxia (N) or Hypoxia (H) from birth and treated with vehicle, aminoglutethimide, and/or dexamethasone as described in Figure 1 legend and Methods. Pituitaries from 3 pups were pooled and considered N=1. Each bar is mean±SEM of 4 anterior pituitary pools collected from pups in the Basal group.
a, different from normoxic pups within same treatment group.
b, different from corresponding Vehicle controls within normoxia or hypoxia.
c, different from aminoglutethimide treatment alone within normoxia or hypoxia.
Discussion
This study demonstrated in 7-day old rat pups that 1) aminoglutethimide-induced reductions in corticosterone reveal elevated basal plasma ACTH and ACTH responses to CRH in hypoxic pups and 2) providing glucocorticoid feedback by dexamethasone administration to aminoglutethimide-treated pups resulted in equivalent basal and CRH-stimulated ACTH levelsin normoxic vs. hypoxic pups, despite differential inhibition of anterior pituitary POMC gene expression.
We have previously demonstrated that hypoxia from birth induced an ACTH-independent increase in corticosterone in 7-day old rat pups (Raff et al. 2003a). This appeared to be mediated by sympathetic input to the adrenal cortex (Raff et al. 2004) and might be enhanced by the development of splanchnic innervation of the medulla at this age (Mikhail & Mahran, 1965; Slotkin and Seidler, 1988). We also previously demonstrated a significantly attenuated ACTH response to CRH and ether stress in 7-day old rat pups exposed to hypoxia from birth (Raff et al. 2003b). These differences are not due to differences in corticosteroid-binding globulin and, hence, free corticosterone levels between hypoxic and normoxic pups (Raff et al. 2003a). We confirmed the diminished ACTH response to CRH in hypoxic pups in the present study. We hypothesized that the ACTH-independent increases in corticosterone suppressed ACTH responses to acute stimuli via negative feedback inhibition. The current study supports that hypothesis.
First, the present study clearly showed that chemical adrenalectomy with aminoglutethimide resulted in large increases in basal ACTH in a manner similar to those observed in older rats (Jacobson et al. 1989). The effects of aminoglutethimide are consistent with prior evidence that glucocorticoid negative feedback is operational in neonatal rats and may be a component of the etiology of the stress-hyporesponsive period (Proulx et al. 2001; Schmidt et al. 2005; Walker et al. 1986b). The functionality of glucocorticoid feedback in neonates was further confirmed by dexamethasone administration per se, which inhibited basal corticosterone and CRH-stimulated ACTH release. Moreover, hypoxic pups responded at least as well as normoxic pups to aminoglutethimide-induced decreases in corticosterone, exhibiting increases in basal plasma ACTH, anterior pituitary POMC gene expression, and CRH-induced ACTH secretion that were as great or greater than those in normoxic pups. Since normal corticotroph responses to the removal of glucocorticoid feedback require hypothalamic input (Levin et al. 1988; Walker & Dallman, 1993), these results indicate that the attenuated ACTH responses to the stimuli of CRH or ether stress that we have previously demonstrated in the hypoxic neonatal rat pup are not due to inherent hypothalamic-pituitary hypoactivity. It is also interesting to note that, despite the prior increases in plasma corticosterone, the plasma ACTH rapidly increased after overnight aminoglutethimide, suggesting a rapid recovery from inhibition by chronically elevated glucocorticoids in hypoxic pups.
A relatively low dose of dexamethasone was chosen (Proulx et al. 2001) so as to reduce but not eliminate aminoglutethimide-induced increases in basal ACTH. When pups were treated with both aminoglutethimide and dexamethasone, CRH administration resulted in equivalent ACTH levels in normoxic vs. hypoxic pups. This result suggests that in the absence of differences in circulating glucocorticoids, hypoxia does not alter the neonatal ACTH response to CRH. Our findings also indicate that hypoxia does not specifically decrease responsiveness of the corticotroph to CRH, which is consistent with previous microanatomical studies showing an increase in number and size of the corticotroph population (Gosney, 1984; Kaur et al. 2002). In fact, with aminoglutethimide alone, the ACTH response to CRH was larger in hypoxic compared to normoxic pups.
We also demonstrated that anterior pituitary POMC mRNA levels are increased by aminoglutethimide in the neonatal rat, and that these increases are comparable between hypoxic and normoxic pups. Interestingly, administration of dexamethasone to aminoglutethimide-treated pups, which decreased basal ACTH significantly to similar levels in both normoxic and hypoxic pups, decreased POMC mRNA only in hypoxic, and not in normoxic pups. It may be that decreases in POMC mRNA would have been evident in normoxic pups if we had used sampling times later than 2 hours. However, at the time points we used, our data clearly show more rapid inhibition of ACTH and POMC in hypoxic pups after aminoglutethimide and dexamethasone treatment. Consistent with the POMC mRNA data, the ACTH response to CRH in aminoglutethimide-treated pups was also only inhibited by dexamethasone in hypoxic pups. The differential suppression of POMC and CRH-induced ACTH secretion in hypoxic pups is unlikely to be due to differences in circulating levels or clearance of dexamethasone, since basal plasma ACTH showed similar inhibition by dexamethasone in both normoxic and hypoxic pups. The apparently greater sensitivity of POMC and ACTH responses to CRH to dexamethasone in aminoglutethimide-treated, hypoxic pups is particularly intriguing given the lack of inhibition of POMC expression by the elevated corticosterone levels in vehicle-treated hypoxic pups. We currently cannot distinguish whether this enhanced sensitivity occurs at the corticotroph, hypothalamus, or higher levels in the HPA axis of the hypoxic neonatal rat.
It is important to point out that the use of chemical adrenalectomy with aminoglutethimide does introduce potential confounds. The primary use of aminoglutethimide in this study was as an inhibitor of P450scc, the first step in the steroidogenic pathway (Chabner et al. 1996). However, in addition to inhibiting adrenal steroidogenesis, aminoglutethimide also decreases gonadal steroidogenesis and inhibits aromatase (Chabner et al. 1996). Despite these confounds, aminoglutethimide has been used for experimental adrenalectomy in previous studies (Jacobson et al. 1989; Lerner et al. 1984). Its advantages are several. First, our model is exposure of neonatal rat pups to hypoxia from birth. General anesthesia and adrenalectomy of neonatal rats under hypoxic conditions is not a viable experimental model. Second, aminoglutethimide allows the maintenance of the integrity of the adrenal medulla (Kent and Parker, 1993), which is important in the neonatal adaptation to hypoxia (Hedner et al. 1980; Slotkin & Seidler, 1988). Therefore, the theoretical downsides to the use of aminoglutethimide are outweighed by its advantages in this particular experimental model.
In conclusion, we have demonstrated that the attenuation of the ACTH response to acute stimulation in 7-day-old rat pups exposed to hypoxia from birth is most likely due to glucocorticoid negative feedback. Although total corticosterone levels are low in 7-day-old rats compared to adults, this is most likely due to low corticosteroid-binding globulin levels (Raff et al. 2003a; Viau et al. 1996). In fact, we propose that free (biologically active) corticosterone is actually normal or even increased, accounting for at least a component of the stress-hyporesponsiveness observed by others (Proulx et al. 2001; Schmidt et al. 2005; Walker et al. 1986b). The current findings demonstrate that even if low, the ACTH-independent increases in glucocorticoid levels in hypoxic neonates are capable of suppressing the ACTH response to acute stimuli such as CRH administration or ether stress (Raff et al. 2003b). Since glucocorticoid therapy is used to treat pulmonary disease and hypoxia in premature and term neonates (Tzukahara et al. 1999), inhibitory effects of exogenous glucocorticoids, in addition to enhanced feedback due to elevated endogenous glucocorticoid secretion, could impair the ability of the neonatal HPA axis to respond to other stresses in the post-natal period. Because glucocorticoid excess in the perinatal period can also permanently alter the regulation of the HPA axis and glucocorticoid-sensitive endpoints (Raff 2004), elucidating the mechanisms of glucocorticoid feedback in the normal and hypoxic neonate will help to avoid adverse long-term sequellae of glucocorticoid therapy.
Acknowledgments
The authors thank Eric Bruder, Peter Homar, Barbara Jankowski and Rebecca Rokow-Kittell for their expert technical assistance.
Funding
This study was supported by NIH DK-54685 to H Raff and by intramural funds from Albany Medical College to LJ. There are no conflicts of interest to disclose.
References
- Arai M, Widmaier EP. Activation of the pituitary-adrenocortical axis in day-old rats by insulin-induced hypoglycemia. Endocrinology. 1991;129:1505–1512. doi: 10.1210/endo-129-3-1505. [DOI] [PubMed] [Google Scholar]
- Chabner BA, Allegra CJ, Curt GA, Calabresi P. Antineoplastic agents. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s The Pharmacological Basic of Therapeutics. edn 9. New York: McGraw-Hill; 1996. pp. 1233–1287. [Google Scholar]
- Frankel L, Stevenson DK. Metabolic emergencies of the newborn: hypoxemia and hypoglycemia. Compreh Therap. 1987;13:14–19. [PubMed] [Google Scholar]
- Friedman AH, Fahey JT. The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Seminars in Perinatology. 1993;17:196–121. [PubMed] [Google Scholar]
- Gosney JR. The effects of hypobaric hypoxia on the corticotroph population of the adenohypophysis of the male rat. J Pathol. 1984;142:163–168. doi: 10.1002/path.1711420303. [DOI] [PubMed] [Google Scholar]
- Hanukoglu A, Fried D, Nakash I, Hanukoglu I. Selective increases in adrenal steroidogenic capacity during acute respiratory disease in infants. Eur J Endocrinol. 1995;133:552–556. doi: 10.1530/eje.0.1330552. [DOI] [PubMed] [Google Scholar]
- Hedner T, Bergman B, Holmgren M. Adrenal catecholamines during and following hypoxia in neonatal rats. Med Biol. 1980;58:228–231. [PubMed] [Google Scholar]
- Jacobson L, Akana SF, Cascio CS, Scribner K, Shinsako J, Dallman MF. The adrenocortical system responds slowly to removal of corticosterone in the absence of concurrent stress. Endocrinology. 1989;124:2144–52. doi: 10.1210/endo-124-5-2144. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Zurakowski D, Majzoub JA. Protein malnutrition increases plasma ACTH and anterior pituitary pro-opiomelanocortin messenger ribonucleic acid in the rat. Endocrinology. 1997;138:1048–57. doi: 10.1210/endo.138.3.5011. [DOI] [PubMed] [Google Scholar]
- Kaur C, Singh J, Peng CM, Ling EA. Upregulation of adrenocorticotrophic hormone in the corticotrophs and downregulation of surface receptors and antigens on the macrophages in the adenohypophysis following exposure to high altitude. Neurosci Let. 2002;318:125–128. doi: 10.1016/s0304-3940(01)02474-0. [DOI] [PubMed] [Google Scholar]
- Kent C, Parker KG. Effects of ACTH and aminoglutethimide on the catecholamine content and chromaffin cell morphology of the adrenal medulla in the neonatal rat. J Anat. 1993;183:601–607. [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Jelinek B, Struve M, Bruder ED, Raff H. Effect of neonatal hypoxia on the development of hepatic lipase in the rat. Am J PhysiolRegulat Integrat Compar Physiol. 2000;279:R1341–R1347. doi: 10.1152/ajpregu.2000.279.4.R1341. [DOI] [PubMed] [Google Scholar]
- Lerner A, Lee PC, Lebenthal E. Chemical adrenalectomy by aminoglutethimide and the pancreas in suckling rats. Am J Physiol Gastrointest Liver Physiol. 1984;247:G346–51. doi: 10.1152/ajpgi.1984.247.4.G346. [DOI] [PubMed] [Google Scholar]
- Levin N, Shinsako J, Dallman MF. Corticosterone acts on the brain to inhibit adrenalectomy-induced adrenocorticotropin secretion. Endocrinology. 1988;122:694–701. doi: 10.1210/endo-122-2-694. [DOI] [PubMed] [Google Scholar]
- Low JA, Froese AB, Galbraith RS, Smith JT, Sauerbrei EE, Derrick EJ. The association between preterm newborn hypotension and hypoxemia and outcome during the first year. Acta Paediatr. 1993;82:433–437. doi: 10.1111/j.1651-2227.1993.tb12717.x. [DOI] [PubMed] [Google Scholar]
- Mikhail Y, Mahran Z. Innervation of the cortical and medullary portions of the adrenal gland of the rat during postnatal life. Anat Rec. 1965;152:431–437. doi: 10.1002/ar.1091520402. [DOI] [PubMed] [Google Scholar]
- Proulx K, Clavel S, Nault G, Richard D, Walker CD. High neonatal leptin exposure enhances brain GR expression and feedback efficacy on the adrenocortical axis of developing rats. Endocrinology. 2001;142:4607–4616. doi: 10.1210/endo.142.11.8512. [DOI] [PubMed] [Google Scholar]
- Raff H. Neonatal dexamethasone therapy: short and long-term consequences. Trends Endo Metab. 2004;15:351–352. doi: 10.1016/j.tem.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Raff H, Bruder ED, Jankowski BM, Goodfriend TL. Neonatal hypoxic hyperlipidemia in the rat: effects on aldosterone and corticosterone synthesis in vitro. Am J Physiol Regulat Integrat Compar Physiol. 2000;278:R663–R668. doi: 10.1152/ajpregu.2000.278.3.R663. [DOI] [PubMed] [Google Scholar]
- Raff H, Hong JJ, Oaks MK, Widmaier EP. Adrenocortical responses to ACTH in the neonatal rat: The effect of hypoxia from birth on corticosterone, StAR, and PBR. Am J Physiol Regul Integr Comp Physiol. 2003a;284:R78–R85. doi: 10.1152/ajpregu.00501.2002. [DOI] [PubMed] [Google Scholar]
- Raff H, Jacobson L, Cullinan WE. Elevated corticosterone and inhibition of ACTH responses to CRH and ether in the neonatal rat: effect of hypoxia from birth. Am J Physiol Regul Integr Comp Physiol. 2003b;285:R1224–R1230. doi: 10.1152/ajpregu.00259.2003. [DOI] [PubMed] [Google Scholar]
- Raff H, Lee JJ, Widmaier EP, Oaks MK, Engeland WC. Basal and ACTH-stimulated corticosterone in the neonatal rat exposed to hypoxia from birth: modulation by chemical sympathectomy. Endocrinology. 2004;145:79–86. doi: 10.1210/en.2003-1130. [DOI] [PubMed] [Google Scholar]
- Rubaltelli FF, Bonafe L, Tangucci M, Spagnolo A, Dani C. Epidemiology of neonatal acute respiratory disorders. Biol Neonate. 1998;74:7–15. doi: 10.1159/000014005. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Levin S, Oitzl MS, van der Mark M, Muller MB, Holsboer F, de Kloet ER. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology. 2005;146:1458–1464. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Adrenomedullary catecholamine release in the fetus and newborn: secretory mechanisms and their role in stress and survival. J Develop Physiol. 1988;10:1–16. [PubMed] [Google Scholar]
- Thomas T, Marshall JM. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. J Physiol. 1995;487:513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzukahara H, Watanabe Y, Yasutomi M, Kovata R, Tamura S, Kimura K, Hiraoka M, Mayumi M. Early (4–7 days of age) dexamethasone therapy for prevention of chronic lung disease in preterm infants. Biol Neonate. 1999;76:283–290. doi: 10.1159/000014170. [DOI] [PubMed] [Google Scholar]
- Viau V, Sharma S, Meaney MJ. Changes in plasma adrenocorticotropin, corticosterone, corticosterone-binding globulin, and hippocampal glucocorticoid receptor occupancy/translocation in rat pups in response to stress. J Neuroendocrinol. 1996;8:1–8. doi: 10.1111/j.1365-2826.1996.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Walker CD, Dallman MF. Neonatal facilitation of stress-induced adrenocorticotropin secretion by prior stress: evidence for increased central drive to the pituitary. Endocrinology. 1993;132:1101–1107. doi: 10.1210/endo.132.3.8382596. [DOI] [PubMed] [Google Scholar]
- Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Walker CD, Sapolsky RM, Meaney MJ, Vale WW, Rivier CL. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology. 1986;119:1816–1821. doi: 10.1210/endo-119-4-1816. [DOI] [PubMed] [Google Scholar]


