Abstract
Previous experiments have demonstrated that the simultaneous presentation of independently established discriminative stimuli can control rates of operant responding substantially higher than the rates occasioned by the individual stimuli. This “additive summation” phenomenon has been shown with a variety of different reinforcers (e.g., food, water, shock avoidance, cocaine, and heroin). Discriminative stimuli previously used in such studies have been limited to the visual and auditory sensory modalities. The present experiment sought to (1) establish stimulus control on a free-operant baseline with an ambient olfactory discriminative stimulus, (2) compare olfactory control to that produced with an auditory discriminative stimulus, and (3) determine whether compounding independently established olfactory and auditory discriminative stimuli produces additive summation. Rats lever pressed for food on a variable-interval schedule in the presence of either a tone or an odor, with comparable control developed to each stimulus. In the absence of these stimuli responding was not reinforced. During stimulus compounding tests, the tone-plus-odor compound occasioned more than double the responses occasioned by either the tone or odor presented individually. Thus, the current study (1) established stimulus control with an ambient olfactory discriminative stimulus in a traditional free-operant setting and (2) extended the generality of stimulus-compounding effects by demonstrating additive summation when olfactory and auditory discriminative stimuli were presented simultaneously.
Keywords: stimulus control, additive summation, odor, olfactory, stimulus compounding, tone, lever press, rat
Odor has proven to be an especially useful stimulus to researchers due to its salience for rodents in comparison to other, more traditional stimuli, such as light and tone. For example, Taukulis and St. George (1982) demonstrated that a strong odor overshadowed other repeatedly paired environmental cues in an aversive conditioning paradigm with rats. This odor preference is apparent in many species commonly used in behavioral research such as rats and mice (Slotnick, 1984). This exceptional salience in rodents might be attributed in part to the large amount of brain area dedicated to olfaction in the central nervous system of these animals. The specialization of brain areas dedicated to the chemical senses is well documented (Moncrieff, 1967).
Several research paradigms have made use of odor as a stimulus. Some common examples are as a contextual cue in conditioned place preference (e.g., Barr & Rossi, 1992) and as a conditioned stimulus with a wide variety of species such as mice (e.g., Schellinck, Forestell, & LoLordo, 2001), rats (e.g., Slotnick, Hanford, & Hodos, 2000), and honey bees (e.g., Linster & Smith, 1997). Odor frequently has been used as a conditioned stimulus either because of the biological predisposition for olfactory cues to be especially effective when associated with foraging and food consumption (Palmerino, Rusiniak, & Garcia, 1980) and/or because of the ability of odor to fully saturate an environment. In many respects odor is particularly well suited for conditioned place preference due to the high associability of olfactory stimuli with gustation (Darling & Slotnick, 1994). However, odor can overshadow other associated stimuli (Taukulis & St. George, 1982).
Clearly there is a significant interest in the study and use of olfactory stimuli in more traditional animal learning areas. Linster and Smith's (1997) study was an attempt to create a model of associative learning with honey bees where olfactory stimuli were used to demonstrate phenomena such as blocking, unblocking, overshadowing, and generalization. However, many of these studies point towards a need to determine the properties of olfaction in relation to other, more well-studied conditioned stimuli, such as light and tone. Further, several of these studies describe in detail the ongoing issues of comparability between olfaction and other stimulus modalities (Durlach & Rescorla, 1980; Tapp & Long, 1968; Tapp, Mathewson, D'Encarnacao, & Long, 1970).
In the last half-century, little research using odor as a discriminative stimulus on a free-operant baseline has been conducted, with the research by Braun and Marcus (1969) being one of the only studies of this kind published to date. Moreover, it is important to note that in the Braun and Marcus study, a subject's movement was severely limited by the relatively small cylindrical container that was used as an operant chamber. A lack of research in this area may in large part be due to the many challenges posed when attempting to use ambient odor in a free-operant setting. These challenges are related to creating an apparatus that would permit the abrupt presentation, the maintenance, and the rapid removal of an odorant in a traditional operant chamber. Therefore, the first objective of the current study was to address these technical issues so that odor could be used as a discriminative stimulus (SD) on a free-operant baseline in a manner similar to other, more commonly used stimuli such as tones and a lights. Meeting this objective involved presenting an odor stimulus in a manner comparable to the way a light or a tone stimulus floods an operant chamber so that either is detectable throughout the entire chamber. Additionally, equipment needed to be developed that would quickly present (i.e., turn the odor “on”) and also quickly remove (i.e., turn “off”) the odorant from the chamber. Meeting these objectives requires more than just rapid and complete air exchange because of an odor's ability to linger within the operant chamber by sticking to the chamber walls, remaining in the air within the chamber, or sticking to the animal's fur (Pfaffmann, Goff, & Bare, 1958). This problem was addressed by using the highly evaporative odorant amyl acetate in a high-speed flow-through design that permitted the odor to be turned on and off rather abruptly in much the same manner as an auditory or visual stimulus.
A second major objective of this study was to achieve stimulus control by the tone and the odor stimuli that is comparable to that reported to tone and light discriminative stimuli in traditional stimulus-compounding studies. The baseline free-operant stimulus control that is a goal of this study relates to the area of stimulus compounding where two or more independently trained stimuli are presented simultaneously. Additive summation is defined as an increase in a behavioral measure when two stimuli, independently trained as discriminative or conditioned stimuli, are presented together in a stimulus compounding test (Weiss, 1964). The operant schedule most often employed in bringing animals' behavior under stimulus control in such studies is the three-ply multiple variable-interval variable-interval extinction schedule (mult VI VI EXT). On this schedule, lever pressing produces reinforcement on a variable-interval (VI) schedule when an auditory or visual discriminative stimulus (SD) is present, whereas no reinforcement is available in the SΔ condition (extinction; EXT) when these stimuli are absent. This arrangement produces stimulus control such that the subjects respond at stable, moderate rates in the SD (tone or light) on the VI schedule, with responding ceasing in SΔ components (no tone and no light). Additive summation has been most robustly observed when response rates in both SD conditions (tone or light) are comparable. Therefore, modifications to stimulus intensities could be made to create stimulus values that contribute to producing comparable rates.
After stimulus control has been established, a stimulus compounding test is administered in which the tone (T) and light (L) stimuli are presented together (T+L) for the first time. Under these conditions, T+L often controls double to triple the responding of either element (T or L) alone during testing (Weiss, 1978). These results have been produced with a variety of reinforcers (e.g., food, Weiss, 1964, 1969, 1971, Exp. 2; water, Weiss, Schindler, & Eason, 1988; food and water, Weiss et al., 1988; cocaine and food, Panlilio, Weiss & Schindler, 1998; shock avoidance, Emurian & Weiss, 1972; Weiss, 1976; cocaine, Panlilio, Weiss, & Schindler, 1996; and heroin, Panlilio, Weiss, & Schindler, 2000), as well as with different species (e.g., rats, Weiss, 1964, 1969, 1971; Wolf, 1963; and pigeons, Long & Allen, 1974; Meltzer & Hamm,1976). However, all stimulus-compounding research using operant schedules has used discriminative stimuli that stimulated the visual and auditory sensory modalities.
Since odor has rarely been used as a discriminative stimulus to control free-operant responding, the range of effective odor intensities was unknown. Determining a stimulus value that is intense enough to be detectable but not so intense as to be aversive, or so dominant that other cues were overpowered, required the manipulation of both the tone and odor intensity over the course of the training period. These efforts brought us to the final objective of the current study which was to determine if compounding tone and odor SDs that each controlled comparable responding would produce a magnitude of additive summation within the range observed after similar training when tone and light SDs were compounded. By testing a new stimulus modality (odor) within the context of the traditional stimulus-compounding paradigm, the degree of additive summation during compound testing should provide insight into the possibility of expanding the use of ambient odor as an SD in operant psychology while extending the scope of the summative process to another stimulus modality.
Method
Subjects
Four experimentally naïve male Long-Evans Hooded rats, weighing approximately 380 g to 440 g, were food deprived to 75–80% (295 g–334 g) of their free-feeding weight. The rats were housed individually in a colony room and allowed free access to water. In addition to the approximately 80 to 100, 45-mg Noyes Formula A rodent pellets they received during training sessions, the rats received, on average, 10–12 g of supplemental food (Tekland Rat Diet) immediately following each training session to maintain a stable deprivation weight. Lights in the colony room were turned on at 0800 hr and off at 2000 hr. The experiments were conducted between 0800 hr and 2000 hr. The animal facilities and all procedures were in accordance with the guidelines set forth by the National Research Council in the Guide for the Care and Use of Laboratory Animals (1996). All procedures were approved by the American University Animal Care and Use Committee.
Apparatus
The custom-made operant-conditioning chamber built to accommodate the olfactory stimulus presentation arrangement developed for this experiment is shown in Figure 1. The chamber was 23 cm long by 15 cm wide by 23 cm high and was enclosed in a sound-attenuation chest similar to those described elsewhere (Weiss, 1970), but with additional ports to accommodate the air-flow tubes related to the olfactory equipment and lined with black and white checkerboard-patterned contact paper. The chamber walls, door, and ceiling were clear PlexiglasTM. The floor consisted of stainless steel rods that were 0.6 cm in diameter (rods spaced approximately1.25 cm apart from rod centers) running perpendicular to the length of the chamber. The left wall served as the chamber door. A Gerbrands microswitch lever (A in Figure 1) was located on the right side of the front wall of the chamber, approximately 7.5 cm above the floor (measured from the top of the lever). A force equivalent to 15–20 g (0.15–0.20 N) was required to operate the lever. A stainless steel feeder trough (B in Figure 1) (1.25 cm long by 1.25 cm wide by 1.25 cm high) located 2.5 cm above the chamber floor was situated on the left side of the front wall. Pellets were dispensed from a Gerbrands 45-mg feeder (C in Figure 1) (model #65120) located behind the front wall. A 10-W, 24-V light bulb located above the top of the front wall was used as a houselight.
Fig 1. The operant chamber contained within the sound-attenuation chest.

The Tygon tubing (D) used to deliver odorant into the air stream can be seen suspended between the sound-attenuation chest and the 10-cm dryer tubing (F) attached to the top of the operant chamber. See the Apparatus section for a description of the ambient odor presentation, maintenance, and elimination.
A 4000-Hz, 80- or 87-dB auditory stimulus was generated by a Med-Associates (Burlington, VT) audio stimulus generator card (model #ANL-926). The auditory stimulus generator was connected to a Radio Shack 8Ω speaker (model #14-8278B) centered 4.5 cm behind the rear side wall of the chamber and positioned facing upwards, 5.5 cm above the height of the floor. The speaker was free standing on a small pedestal so that it could be moved out of the way when cleaning the chamber.
The odor generator was a single dilution olfactometer (see Pfaffmann et al., 1958). Air, supplied by a 7-W Optima aquarium pump, was directed into a horizontally mounted cylindrical glass container that was filled approximately half way with amyl acetate. The surface area when half filled was approximately 39 cm2. This odor-saturated air was valve-limited to approximately 19 cc/min. The odor was then mixed with 2800 cc/min of room air. This produced a dilution ratio of approximately 0.7% of vapor-saturated amyl acetate odor. This diluted air was then directed from the olfactometer into the intake vent via 60 cm of Tygon tubing (D in Figure 1) (0.25 cm inner diameter). Tygon tubing of the same diameter also was used to connect the aquarium pump, valves, and chambers used in the olfactometer. Amyl acetate has a distinct chemical scent and was a novel odorant for the rats used in the experiment.
During the SD components, odor was directed into the operant chamber, and during the SΔ components, odor was directed out to an exhaust vent rather than into the intake vent of the chamber. This directing of the odorant into either the chamber or exhaust vent was accomplished with a three-way valve (General Valve Corp. #1-160-001) at the point of dilution. A second identical valve was turned on and off randomly at an average rate of once per min (range 40 s to 90 s) to mask the sound of the active valve.
To ventilate the chamber rapidly, a 7.5-cm fan (E in Figure 1) (Radio Shack) running continuously at 24 V was used to introduce additional room air into the chamber. This air was drawn from an adjacent room and ducted into the sound attenuation chest using 10-cm dryer hose (F in Figure 1). The odor-filled air then was mixed with this air stream at a point 2.5 cm above the intake fan. The air was dispersed within the chamber using a 7.5 cm square PlexiglasTM dispersion plate (G in Figure 1) mounted 1.25 cm below the center of the chamber ceiling directly under the 7.5-cm fan mounted above a port in the chamber ceiling.
Air was exhausted from the chamber using a 12.5-cm, 24-V fan (Radio Shack) mounted into the sound-attenuation chest directly below the center of the operant chamber floor. The exhaust fan also ran continuously and was protected from excrement and urine using a porous polyethylene sheet (Scotch-BriteTM commercial scouring pad). The properties of this sheet allowed the passage of chamber air into the exhaust vent but prevented feces from dropping directly onto the fan and directed urine away from the exhaust fan port. This vented air was carried away from the sound-attenuation chest by a 10-cm dryer hose connected directly to the ventilation exhaust in the ceiling of the running room. By supplying air from a room adjacent to the running room, and removing the odor-filled air from the room, recirculated odor-contaminated air was prevented from being reintroduced into the operant chamber. The entire apparatus produced the equivalent of a vertically oriented wind tunnel operating at essentially a constant pressure with a traditional operant chamber positioned in the center between the intake and exhaust fans.
When sampled at the point where the Tygon tubing joined with the 10-cm dryer vent, it was determined that the odor-filled air was eliminated (i.e., it was not detectable by the experimenter when sampled through a glass tube inserted into the opening) from the Tygon tubing and evacuated from the chamber in less than 1.5 s. The same was true for the introduction of odor into the chamber when similarly sampled inside the operant chamber. When odor was directed into the chamber, it was detectable in the chamber by the experimenter less than 1.5 s after the three-way valve was activated. As a result of this relatively short delay, no correction was made to account for this time lag as odor components were considered to start and stop when the three-way valve was activated.
All equipment was connected to a Med-Associates interface located in an adjacent room and was operated by a Dell Optiplex GXa Pentium II computer running Med-PC software. Data were recorded on the computer as well as on a Gerbrands (Arlington, MA) cumulative recorder located in the same room as the interface and computer. The operant chamber was thoroughly cleaned before each rat's session to reduce the buildup of odor on the equipment.
Procedure
Training
Rats O-1 and O-2 received initial training using the odor (O) stimulus and Rats O-3 and O-4 received initial training in the tone (T) stimulus. Initial training was conducted using a conjoint continuous-reinforcement (CRF) fixed-time (FT) 120-s schedule in which every response was reinforced and the rat would automatically receive a food pellet every 120 s. This procedure pairs the feeder click with food presentation (“magazine training”) and produces lever pressing without hand shaping. During this initial training, either T or O remained on during the entire session. Once the rat emitted eight reinforced responses in a single session, the FT schedule was discontinued, and the rat progressed from a fixed-ratio (FR) 1 to an FR 10 over the course of approximately 1 to 2 weeks.
When a rat was responding consistently on an FR 10 schedule, the schedule was changed to a two-ply multiple (mult) schedule with a VI contingency substituted for the FR. The SD used during this phase of multiple-schedule training was the same stimulus used during initial training (either T or O). During the SD (T or O), subjects progressed over 3 days (7 days for Rat O-1) from a VI 15-s (range 0.5 s to 39.5 s) to a VI 30-s schedule (range 1 s to 79 s). During SΔ components, neither T nor O was present and responding was not reinforced (EXT). The SD and SΔ components alternated, and averaged 90 s (range 45 s to 180 s).
To promote response cessation in the SΔ, a response correction (RC) contingency was introduced in the SΔ component. The RC delayed the onset of the SD until the period of the RC had elapsed without a response. Functionally, the RC was programmed to begin timing RC-seconds before the end of each SΔ component. Responses emitted during the RC period delayed SD onset and reset the RC, preventing a response at the end of SΔ from being effectively reinforced by presentation of the SD. The value of the response correction was increased from RC = 0 s to an average of RC = 20 s during this part of the training phase. All progression in multiple-schedule training was performance based. Rats were maintained on the two-ply mult schedule until they achieved at least a 2∶1 SD to SΔ (i.e., tone∶no-tone or odor∶no-odor) discrimination ratio. After an average of 9 days (range 8–10 days) of mult training described above, the rats were switched to the other stimulus (either T or O) for an additional 5 days on the mult VI 30-s EXT schedule. All rats demonstrated approximately equal discrimination ratios for each of the two discriminative stimuli (approximately 2∶1) going into the three-ply phase of the training.
During the three-ply multiple schedule sessions, the rats received presentations of tone components and odor components in random order, separated by SΔ wherein neither tone nor odor was present (T̄Ō). The tone and the odor components were presented with equal probability with the limitation that no more than three consecutive SD components could be the same. The terminal baseline schedule for Rat O-2 on the three-ply multiple schedule was a mult VI 45-s (Tone) VI 45-s (Odor) EXT (T̄Ō) with SDs averaging 105 s (range 45 s to 210 s) and SΔs averaging 120 s (range 60 s to 240 s) plus any additional time spent in the 45-s RC that extended the SΔ component. The VI range was 1.5 s to 118.5 s in both the T and O stimulus. For Rats O-1, O-3, and O-4 the terminal baseline schedule was a mult VI 60-s (Tone) VI 60-s (Odor) EXT (T̄Ō) with, on average, 90-s SDs and 90-s SΔs that included a 50-s RC. The VI ranged from 2 s to 178 s, and SD as well as SΔ components ranged from 45 s to 180 s (plus any additional time spent in the RC during SΔ). Total daily session duration was approximately 3 to 4 hr.
Due to the persistent dominance of the odor stimulus in maintaining higher response rates during three-component multiple schedule training, the dilution ratio of amyl acetate was gradually reduced throughout training until discrimination ratios between O and T̄Ō became adversely affected. At this point the odor level was between approximately 15 cc/min and 25 cc/min, but varied across animals and across days. The flow rate of amyl acetate was then increased slightly (approximately 2 cc/min) until the discrimination between O and T̄Ō improved. Thus, the level of amyl acetate used in this preparation was just slightly greater than the observed threshold for the odor discrimination task, as further reductions in the concentration of amyl acetate impaired the discrimination.
Determining the odor threshold proved challenging due to the variable nature of the odor discrimination threshold. Potential factors contributing to this variability included (1) onset and duration of each individual odor stimulus, (2) length of time in the experimental chamber, (3) variability of the flow rate due to limitations of the equipment, and (4) other factors which affected the “stickiness” of the smell (e.g., humidity, temperature in home cage, and cleanliness of the animal's fur). Therefore, the value given for the dilution ratio (19 cc/min) is an estimate of the effective value. In addition, small daily manipulations in this dilution ratio (2–5 cc/min) within what could be described as boundary values (15 cc/min to 25 cc/min) were used in order to maximize discrimination while keeping response rates between O and T comparable.
In spite of these efforts, the rats continued to produce greater response rates in the odor component as compared to the tone component. Since odor levels could not be reduced further without affecting discrimination ratios, all subjects were given additional training in tone. This was accomplished by increasing the probability of tone components from 50% to 80% and often to 100% for several days of training. However, the final 3 days of training prior to testing always were conducted with T and O presented with equal probability (50%). Even with this additional training with the tone, all the rats except O-2 continued to respond at a higher rate in the odor component. As a result, the intensity of the tone was increased from 80 dB to 87 dB in an effort to create conditions where tone and odor produced equivalent response rates.
The criteria for testing were: (1) a discrimination ratio greater than 10∶1 for 3 out of 4 consecutive days when combined rates in T and in O were compared to those in T̄Ō; (2) a discrimination ratio greater than 10∶1 for the average of the ratios in T and in O, compared to those in T̄Ō, during the final 2 days of training; (3) average response rates in SD components within 20% of those in the higher of the two stimuli (T or O) for 3 out of 4 consecutive days; (4) initiation of responding at T or O onset; and (5) abrupt response cessation when T or O terminated. The fourth and fifth of these criteria were judged by review of cumulative records for the final 4 days of training. An exceptionally long training period was required in order to meet the criterion for testing as Rats O-1, O-2, O-3, and O-4 were trained for 222, 137, 232, and 194 days, respectively.
Testing
After the training described above, the rats were given a stimulus compounding test where T and O were presented simultaneously for the first time. Testing commenced after an approximately 1.5-hr warm-up period on the terminal baseline schedule. The compounding test was conducted in extinction (i.e., food was discontinued) and consisted of 60-s presentations of T, O, and T+O, each separated by a 60-s presentation of T̄Ō. Presentations of T, O, and T+O were block-randomized.
Rat O-2 was the first subject tested. This test was discontinued after 18 block-randomized presentations of T, O, and T+O because the rat emitted only one response to either of the two stimulus elements over 30 min and only eight responses to T+O during the same time period. However, the remaining rats showed a minimal extinction effect to the compound and continued to respond well beyond the first 12 block-randomized presentations of T, O, and T+O. All 3 remaining rats received 24 block-randomized presentations of T, O, and T+O.
Results
Terminal Baseline Training Data
Responses per min in T, O, and T̄Ō for each rat from the 4 final days of baseline training are shown in Table 1 along with mean response rates over these criterion days. All rats, regardless of their overall response rate, responded to the tone and the odor discriminative stimuli at approximately equal rates during at least 3 of the 4 final days, and the average of these rates was at least 10 times their rate in the SΔ (T̄Ō).
Table 1. Response rates and discrimination ratios for each of the final 4 days of baseline training for each rat. Mean data for the final 4 days is located to the right of the training data. Note that for Rat O-3 there was an equipment malfunction on day 231. Data from day 231, therefore, were omitted from the Table and the rat was run for an additional day before being tested (day 232).
| RAT | DAYS PRIOR TO TEST |
|||||||||
| 4 |
3 |
2 |
||||||||
| Odor | Tone | T̄Ō* | Odor | Tone | T̄Ō | Odor | Tone | T̄Ō | ||
| O-1 | Resps/min (disc. ratio) | 22.1 (9.0) | 26.6 (10.9) | 2.4 | 32.8 (12.5) | 38.0 (14.5) | 2.6 | 39.8 (12.1) | 32.7 (13.3) | 2.5 |
| O-2 | Resps/min (disc. ratio) | 26.6 (13.1) | 23.7 (11.6) | 2.2 | 30.3 (9.0) | 27.1 (8.0) | 3.4 | 26.0 (14.0) | 18.6 (10.0) | 1.9 |
| O-3 | Resps/min (disc. ratio) | 41.0 (9.1) | 47.9 (10.6) | 4.5 | 40.5 (10.5) | 40.7 (10.6) | 3.8 | 24.3 (13.2) | 29.4 (16.1) | 1.8 |
| O-4 | Resps/min (disc. ratio) | 21.1 (16.9) | 22.1 (17.6) | 1.3 | 16.7 (6.4) | 23.2 (8.9) | 2.6 | 33.5 (9.7) | 33.3 (9.7) | 3.4 |
| RAT | DAYS PRIOR TO TEST |
Mean Resps/Min (final 4 days) |
Total Sessions | |||||
| 1 |
Odor | Tone | T̄Ō | |||||
| Odor | Tone | T̄Ō | ||||||
| O-1 | Resps/min (disc. ratio) | 39.0 (11.2) | 35.9 (10.3) | 3.5 | 33.4 (11.2) | 33.3 (12.3) | 2.8 | 222 |
| O-2 | Resps/min (disc. ratio) | 22.6 (11.7) | 20.7 (10.7) | 1.9 | 26.4 (12.0) | 22.5 (10.1) | 2.4 | 137 |
| O-3 | Resps/min (disc. ratio) | 27.8 (9.0) | 26.2 (11.8) | 3.1 | 33.4 (10.5) | 36.1 (12.3) | 3.3 | 232 |
| O-4 | Resps/min (disc. ratio) | 33.9 (13.6) | 33.1 (13.3) | 2.5 | 26.3 (11.7) | 28.0 (12.4) | 2.5 | 194 |
T̄Ō = the absence of tone and odor
Cumulative records from each rat showing representative performance on the terminal baseline schedule are presented in Figure 2. These records reveal that all rats typically (1) began responding shortly after the onset of the tone or odor SD, (2) responded throughout the T and O SDs, and (3) ceased responding shortly after the start of the SΔ component, with this cessation maintained during the absence of the T and O stimuli.
Fig 2. Representative 30-min segments of cumulative records of Rats O-1, O-2, O-3, and O-4 from a terminal criterion baseline training session on the three-ply multiple VI VI EXT schedule.
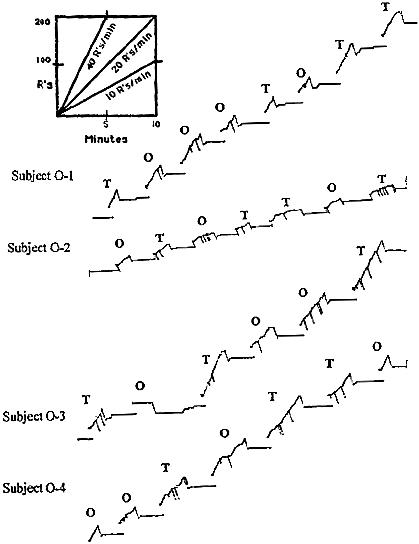
For Rat O-1 the record contains data collected 3 days prior to testing (session 220) For Rat O-2 the record contains data collected on session 136, 2 days prior to testing. For Rats O-3 and O-4, the record contains data collected on the last day of terminal baseline training (session 232 and 194, respectively). Tone (T) or odor (O) components are indicated by corresponding letters above the record. When the pen is depressed neither stimulus was present, and responding was on extinction. The slash marks by the response pen denote the delivery of a reinforcer.
Stimulus Compounding Tests
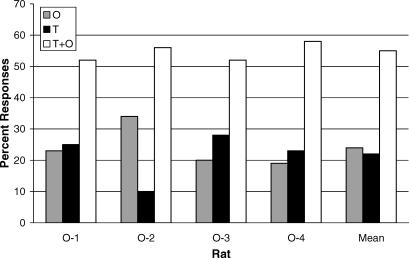
Figure 3 presents the results of the stimulus compounding tests. Percentages were calculated by taking the total number of responses made to each stimulus element as a percentage of the total responses emitted during the test by that subject. This enabled each animal's test results to be weighted equally rather than having the mean data skewed by animals that emitted more overall responses during testing. For example, the total number of responses from Rat O-2 during testing was less than half the number of responses emitted by both Rats O-1 and O-3. However, by taking the mean percentage of responses to T, O, and T+O, the data from Rats O-1, O-2, and O-3 were all weighted equally.
Fig 3. The distribution of tone, odor, and tone-plus-odor (T+O) responses on the stimulus compounding test.
Percentages were calculated for each subject by dividing the responses emitted to each test condition by the total responses emitted to T, O, and T+O summed over the test, and then multiplying by 100. Mean test percentages are presented to the right.
All subjects responded more in T+O than in either T or O presented alone, with the mean number of responses in T and in O equal. The T and O SDs controlled less than half the number of responses emitted to the T+O compound. This robust additive summation observed for the group was approximately 2.3∶1 using the mean percentage of responses in the compound compared to the greater of the two elements. The total number of test responses emitted in T, O, and T+O during the test conditions differed significantly [F (2, 6) = 19.47, p < .01]. Significantly more responses were emitted in T+O than in T (p < .05) and in O (p < .05), whereas responding in T and in O was comparable (p = .98). However, this overall comparability is not representative of each subject. Rates in T and in O were approximately equivalent for Rats O-1 and O-4. However, Rat O-2 emitted 94 more responses in O than in T, whereas Rat O-3 emitted 81 more responses in T than in O. Therefore, despite the fact that there were individual differences, there was clearly no systematic difference in control by the elements (T and O) during the test. Responses in T̄Ō accounted for less than 5% of the total responses emitted over the course of the test. Further, most of the responses in T̄Ō occurred within 2 s of a component switch (i.e., response overrun) and were therefore excluded from further analysis for the purposes of clarity.
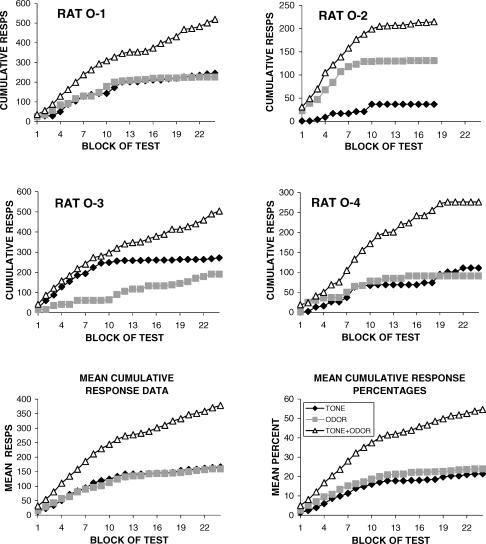
The top four graphs of Figure 4 show the responses emitted by each rat to each stimulus during the stimulus compounding test accumulated over each block of the test. In addition, the mean cumulative response data for all the rats (lower left graph) and the mean cumulative distribution of individual response data converted to percentages of total test responses (lower right graph) are presented. Figure 4 reveals that additive summation was observed in all the animals, with Rats O-1 and O-4 showing the greatest effect. In the lower right graph, each point was calculated as follows: (1) the cumulative number of responses emitted in each condition to that point in the test by each animal was divided by the total number of responses that animal emitted over the entire test and multiplied by 100, then (2) these cumulative response percentages were averaged over all 4 animals for each point in every test block. Therefore, each animal was comparably weighted irrespective of its response rate.
Fig 4. The upper four graphs present the total test responses emitted by individual rats accumulated over each block-randomized presentation of Tone, Odor, and Tone+Odor.
Mean cumulative response data are presented in the lower left graph. The lower right graph presents the mean data in terms of mean cumulative response percentages that weights all animals comparably irrespective of their absolute response rate (see text for details of how these percentages were calculated).
As described above, the data presented for Rat O-2 contain only 18 block-randomized presentations of T, O, and T+O. In order to include the data from Rat O-2 into the mean cumulative response graph and response percentage graph it was conservatively assumed that the response rate in T, O, and T+O for the remaining 30 min of the test for Rat O-2 would be zero.
Discussion
Discrimination between the odor SD and the SΔ conditions was apparent early in training. Discrimination differences of greater than 2∶1 between odor and no odor appeared within approximately 9 days of multiple-schedule discrimination training for Rats O-1 and O-2. On the three-ply multiple schedule, with presentations of tone and of odor separated by T̄Ō, differences in response rate emerged between the two discriminative stimuli, with subjects responding faster in the Odor component than in the Tone component. By reducing the intensity of the discriminative odor stimulus, increasing the tone intensity, and providing additional training with the tone stimulus, comparable response rates emerged. Other aspects of subjects' response patterns were generally comparable.
Once the first two objectives of the study (discriminative control by odor plus equivalent response rates and topography in tone and odor) were achieved, the stimulus compounding test was administered. As predicted, and in agreement with research using similar schedules and the more traditional discriminative stimuli of tone and light, the present study demonstrated robust additive summation when the T and the O SDs were presented simultaneously. The T+O compound controlled more than twice the responding of either stimulus element (T or O) presented individually during testing. This degree of additive summation is in the range of that obtained using schedule parameters similar to those used in the present study when stimuli from different sensory modalities were compounded (e.g., Weiss, 1964, 1969, 1971; Panlilio et al., 1996, 1998, 2000) and has never been observed with two discriminative stimuli from the same sensory modality.
Of particular interest are the slopes of the cumulative response lines in the bottom two graphs in Figure 4 that represent the mean rates occasioned by T, O, and T+O over the test. The decreasing slope of the response rate in T and in O is indicative of extinction, and few, if any, responses were emitted in the presence of the single stimuli over the final third of the test. In contrast, response rates in the T+O compound appear relatively stable and constant over the test as they diverge from response rates to the single stimuli as the test progressed. One might infer from the individual response data that additional block-randomized stimulus presentations would reveal even more summation in at least two of the four rats (O-1 and O-3) as the T+O compound continued to control a greater percentage of the total responses emitted.
These data are consistent with those of Weiss's (1971, Exp. 2) SΔ group that received training similar to rats in the present experiment but with a tone and a light as SDs. In that study, responding in the presence of the T+L compound also clearly showed greater resistance to extinction than responding to either T or L on the stimulus compounding test. Furthermore, the data presented in Figure 6 of Weiss's study are essentially comparable to the data in Figure 4 of the present experiment with respect to (1) the mean data trends, and also (2) the response trends for individual animals. This similarity is not only testament to the robust summative effects observed using this manipulation, but also is indicative of the fact that the T+O compound was controlling behavior in a manner similar to that observed when comparable experiments were conducted using tone and light.
Impediments to using ambient odor in a free-operant setting, such as the ability of odor to linger within the operant chamber by either sticking to the chamber walls, the animals' fur, or by remaining in the chamber air, were all potential problems in the current study. These issues were addressed by using amyl acetate (a highly evaporative odorant) and a high-speed flow-through design. In addition, with this preparation it was clear that discrimination on a free-operant baseline using ambient odor as a discriminative stimulus is possible as responding came under discriminative control in approximately the same amount of time required to obtain discriminative tone control (8–10 days).
However, as evidenced by the sheer number of training sessions required to obtain comparable response rates between tone and odor, achieving this objective of the current study was challenging. Relatively equal response rates in tone and odor were sought not only to demonstrate comparable response topography and comparable excitatory properties between the two stimuli (T and O) but also to provide optimal conditions for additive summation during stimulus compounding. Since this was the first time ambient odor and tone were used as discriminative stimuli on a free-operant baseline, a significant effort was required to obtain equal response rates in both tone and odor.
Odor was clearly a more salient stimulus for the rats than the tone, requiring us to work near what appeared to be the relative threshold of the odor SD using this preparation. By reducing odor intensity, providing additional sessions in the disadvantaged stimulus (tone) as well as a greater number of components within sessions of the tone stimulus and, finally, increasing the intensity of the disadvantaged stimulus, comparable response patterns emerged. In all, as many as 200 training sessions per animal were required to produce comparable response rates in the tone and in the odor components.
This excessive amount of training was hardly ideal. However, the information obtained from this study with regard to an apparent ambient odor threshold using this preparation on a free-operant baseline should reduce significantly the training time in future studies. A considerable amount of the training period can be attributed to the gradual reductions in the dilution ratio of amyl acetate until deficits in the discrimination ratio were observed. Obtaining this threshold where discrimination degraded proved to be far more elusive than originally anticipated, often requiring several days of data collection to determine if further reductions in odor concentrations had adversely affected discrimination. In future studies, less training should be required because the range of concentrations with this odor that were effective when used as discriminative stimuli is now known.
This is not to say that using odor as a discriminative stimulus in the future will be easy. Precautions will need to be taken to reduce the level of odor in the chamber during the non-odor conditions (tone and extinction). In the present study this involved using amyl acetate, fast air velocity in the delivery/evacuation of the chamber air, and careful cleaning of the operant chamber after each session. It might be impossible to eliminate all residual odorant from the test chamber, but it should be appreciated that even in studies with tone and light SDs there is often a houselight and ambient sound that could be considered analogous to a residual odor.
Regardless, this potential problem with residual odor could have influenced the data since, if odor were always present in the chamber, then, taken to the extreme, there would have been no SΔ condition signaled by the absence of T and O but rather an SΔ condition signaled by a low level of odor and the absence of tone. Some support for this concern may be evident from the length of time required in the initial odor versus no-odor training. Most studies using odor in discrimination tasks have shown that rodents learn complex discrimination tasks using a variety of different odor conditions with only a minimal number of odor exposures (Slotnick et al., 2000). In the Schellinck et al. (2001) study, for example, mice learned an odor discrimination task with only four CS+/CS− presentations, and the discrimination persisted for up to 60 days. However, in the current study, discrimination did not emerge until after hundreds of presentations of the odor stimulus.
This concern may seem reasonable, but there are competing explanations in support of the view that any residual odor was only minimally problematic, if at all. First, given the novelty of this preparation and the requirement of a trained operant response (lever press) as opposed to a natural response such as foraging, it is unsurprising that initial training took so long in comparison to other studies that do not involve an operant requirement. Second, and in agreement with the argument that odor should be a more biologically significant stimulus to rats, the amount of effort required to titrate the odor levels in order to reduce the robust response rates initially observed to the odor SD were substantial. Finally, the most compelling argument opposing the concern is the actual results of the current study. As described above, the degree of additive summation reported here using tone and odor has only been obtained using schedule parameters similar to those used in the present study when stimuli from different sensory modalities were compounded and has never been observed with two discriminative stimuli from the same sensory modality.
This study has extended the use of ambient odor (1) as an SD in the free-operant situation, and (2) through the stimulus-compounding assay by demonstrating that odor produced results similar to that of a comparably prepared SD from the visual modality when presented simultaneously with an auditory SD. Although extensive training was required in this new application, future research using ambient odor as an SD will be entered with a greater appreciation of the challenges involved, the range of intensity values for odor to be used as an effective discriminative stimulus, plus the likely odor stimulus intensities required to maintain control comparable to that of a tone SD. The additional information that could be gathered by adding a third orthogonal stimulus dimension to the powerful and informative stimulus-compounding paradigm should justify this effort. Such an orthogonal stimulus could be used to investigate further the degree to which additional sources of excitation might energize behavior, as well as how multiple conditioned inhibitors might suppress it.
Acknowledgments
This research was supported in part by NIDA Research Grant DA08651-04A2 awarded to Stanley J. Weiss. The authors are indebted to Burton M. Slotnick for his technical assistance in designing, building, and testing the olfactometer.
References
- Barr G.A, Rossi G. Conditioned place preference from ventral tegmental injection of morphine in neonatal rats. Developmental Brain Research. 1992;66:133–136. doi: 10.1016/0165-3806(92)90149-q. [DOI] [PubMed] [Google Scholar]
- Braun J.J, Marcus J. Stimulus generalization among odorants by rats. Physiology & Behavior. 1969;4:245–248. [Google Scholar]
- Darling F.M.C, Slotnick B.M. Odor-cued taste avoidance: A simple and efficient method for assessing olfactory detection, discrimination and memory in the rat. Physiology & Behavior. 1994;55:817–822. doi: 10.1016/0031-9384(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Durlach P.J, Rescorla R.A. Potentiation rather than overshadowing in flavor-aversion learning: An analysis of within-compound associations. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:175–187. [PubMed] [Google Scholar]
- Emurian H.H, Weiss S.J. Compounding discriminative stimuli controlling free-operant avoidance. Journal of the Experimental Analysis of Behavior. 1972;17:249–256. doi: 10.1901/jeab.1972.17-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Smith B.H. A computational model of the response of honey bee antennal lobe circuitry to odor mixtures: Overshadowing, blocking and unblocking can arise from lateral inhibition. Behavioural Brain Research. 1997;87:1–14. doi: 10.1016/s0166-4328(96)02271-1. [DOI] [PubMed] [Google Scholar]
- Long C.K, Allen J.D. Stimulus compounding in pigeons. Bulletin of the Psychonomic Society. 1974;4:95–97. [Google Scholar]
- Meltzer D, Hamm R.J. Response summation in the pigeon. Bulletin of the Psychonomic Society. 1976;7:515–518. [Google Scholar]
- Moncrieff R.W. The chemical senses (3rd ed.) London: L. Hill; 1967. [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Palmerino C.C, Rusiniak K.W, Garcia J. Flavor-illness aversion: The peculiar roles of odor and taste in memory for poison. Science. 1980;208:753–755. doi: 10.1126/science.7367891. [DOI] [PubMed] [Google Scholar]
- Panlilio L.V, Weiss S.J, Schindler C.W. Cocaine self-administration increased by compounding discriminative stimuli. Psychoparmacology. 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Panlilio L.V, Weiss S.J, Schindler C.W. Motivational effects of compounding discriminative stimuli associated with food and cocaine. Psychopharmacology. 1998;136:70–74. doi: 10.1007/s002130050540. [DOI] [PubMed] [Google Scholar]
- Panlilio L.V, Weiss S.J, Schindler C.W. Effects of compounding drug-related stimuli: Escalation of heroin self-administration. Journal of the Experimental Analysis of Behavior. 2000;73:211–222. doi: 10.1901/jeab.2000.73-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffmann C, Goff W.R, Bare J.K. An olfactometer for the rat. Science. 1958;128:1007–1008. doi: 10.1126/science.128.3330.1007. [DOI] [PubMed] [Google Scholar]
- Schellinck H.M, Forestell C.A, LoLordo V.M. A simple and reliable test of olfactory learning and memory in mice. Chemical Senses. 2001;26:663–672. doi: 10.1093/chemse/26.6.663. [DOI] [PubMed] [Google Scholar]
- Slotnick B.M. Olfactory stimulus control in the rat. Chemical Senses. 1984;9:157–165. [Google Scholar]
- Slotnick B, Hanford L, Hodos W. Can rats acquire an olfactory learning set? Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:399–415. doi: 10.1037//0097-7403.26.4.399. [DOI] [PubMed] [Google Scholar]
- Tapp J.T, Long C.J. A comparison of the reinforcing properties of stimulus onset for several sensory modalities. Canadian Journal of Psychology. 1968;22:449–455. doi: 10.1037/h0082784. [DOI] [PubMed] [Google Scholar]
- Tapp J.T, Mathewson D.M, D'Encarnacao P.S, Long C.J. The effect of the onset of stimuli on reactivity in the rat. Psychonomic Science. 1970;19:61–62. [Google Scholar]
- Taukulis H, St. George S. Overshadowing of environmental cues by an odor in toxicosis-based conditioning in rats. Animal Learning & Behavior. 1982;10:288–292. [Google Scholar]
- Weiss S.J. Summation of response strengths instrumentally conditioned along a composite-stimulus continuum during free-operant summation. Journal of the Experimental Analysis of behavior. 1964;68:151–155. doi: 10.1037/h0049180. [DOI] [PubMed] [Google Scholar]
- Weiss S.J. Attentional processes along a composite stimulus continuum during free-operant summation. Journal of Experimental Psychology. 1969;82:22–27. [Google Scholar]
- Weiss S.J. An effective and economical sound attenuation chamber. Journal of the Experimental Analysis of Behavior. 1970;13:151–155. doi: 10.1901/jeab.1970.13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.J. Discrimination training and stimulus compounding: Consideration of non-reinforcement and response differentiation consequences of SΔ. Journal of the Experimental Analysis of Behavior. 1971;15:387–402. doi: 10.1901/jeab.1971.15-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.J. Stimulus control of free operant avoidance: The contribution of response rate and incentive relations between multiple schedule components. Learning and Motivation. 1976;7:477–516. [Google Scholar]
- Weiss S.J. Discriminated response and incentive processes in operant conditioning: A two-factor model of stimulus control. Journal of the Experimental Analysis of Behavior. 1978;30:361–381. doi: 10.1901/jeab.1978.30-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.J, Schindler C.W, Eason R. The integration of habits maintained by food and water reinforcement through stimulus compounding. Journal of the Experimental Analysis of Behavior. 1988;50:237–247. doi: 10.1901/jeab.1988.50-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M.M. Some effects of combined SDs. Journal of the Experimental Analysis of Behavior. 1963;6:343–347. doi: 10.1901/jeab.1963.6-343. [DOI] [PMC free article] [PubMed] [Google Scholar]